Molecular Docking, Dynamics Simulations, ADMET, and DFT Calculations: Combined In Silico Approach to Screen Natural Inhibitors of 3CL and PL Proteases of SARS-CoV-2
Abstract
Considering natural compounds for the antiviral effect is another opportunity for exploring novel drug candidates for severe acute respiratory syndrome coronavirus 2. The selected natural compounds were interacted using a molecular docking approach. The 3D structures of the main protease and papain-like protease were used for the virtual screening to detect the potent inhibitor against SARS-CoV-2. The top-scored compounds were further analyzed for absorption, digestion, metabolism, excretion, and toxicity properties and density functional theory analysis. Our results indicated that glycyrrhizin exhibited better docking scores of -9.5 kcal/mol with main protease and -9.7 kcal/mol with papain-like protease. Next to glycyrrhizin, rutin showed a better docking score of -9.1 kcal/mol and -9.2 kcal/mol with 3-chymotrypsin-like and papain-like proteases. Violaxanthin and naringin occupied the subsequent position in the docking score table with 3CL and PL proteases, respectively. In addition, the crucial properties like drug likeliness and pharmacokinetics of the compounds were determined. There is no significant toxicity identified.
1. Introduction
SARS-CoV-2, the first pandemic of this century, has been detected in 216 countries, with more than 756 million confirmed cases and 68,44,267 deaths as of February 20, 2023. The alteration happening in the genome of this virus resulting in different variants is the biggest challenge among the scientific community to predict its characteristic features for drug development. The UK found the first mutant variety (called alpha) in September 2020. In May 2020, South Africa found beta, and in November 2020, Brazil found gamma. By October 2020, India had reported the delta variant, and by December 2020, Peru had registered the lambda variant. In January of 2021, Colombia found the Mu species.
The omicron variant has been reported in more than 50 countries (coronavirus disease (COVID-19) pandemic, https://www.who.int/emergencies/diseases/novel-coronavirus-2019) [1]. Practical diagnostic approaches have been developed, including RT-PCR, CT imaging, and interleukin-based serological screening [2].
Since the abrupt prerequisite for an effective therapeutic option against SARS-CoV-2 is in the relentless phase of the explosion, evaluating the prevailing antiviral agents for repurposing is obligatory. Many countries conduct in vivo and human trials with existing drugs for possible repurposing. Hence, virtual screening of antiviral medications against SARS-CoV-2 will institute a platform for the lead identification process.
Over 250 genome sequences have been deposited into GenBank (GenBank, taxid: 2697049). This β-coronavirus shares an 89% resemblance to the bat coronavirus [3, 4]. A 29 kb size, single-stranded RNA encoding 9,860 amino acids, is present. Since December 2019, researchers have identified several proteins as targets. Main protease, RNA polymerase, and spike protein have attracted outstanding attention owing to their significant role in interactions with the host (human) and the subsequent replication.
Viral proteases are endopeptidases that catalyze the cleavage of viral polyproteins [5]. Coronaviridae genomes carry two polyproteins, which get hewed and changed into nonstructural proteins (NSP) by the actions of the 3CL protease and the PL protease [6]. The NSP has a role in viral replication in host cells [7]. As a result, targeting those proteases to mimic the transformation of NSP into biologically functional proteins is worthwhile. The SARS papain-like protease cleaves ISG15 and polyUb [8, 9], whereas 3CL protease is indispensable to the viral life cycle [10].
Food and Drug Administration (FDA) of the USA has not approved any antiviral medicine to treat SARS-CoV-2 except remdesivir under emergency use authorization (EUA). The focus of research has shifted to evaluating the available antiviral drugs. An efficient, naturally occurring drug must be identified and capable of targeting ACE-2 receptors, spike protein, RdRp, or SARS-CoV-2 proteases (https://www.guidetopharmacology.org/coronavirus.jsp).
Literature studies indicate that saquinavir, remdesivir, and darunavir and two natural drugs, flavone and coumarin derivatives, were employed to inhibit 3CL protease [11]. Herbal treatment was demonstrated as effective in suppressing infectious diseases during the 2003 SARS outbreak [12]. Significant similarities can be found between SARS-CoV-2 and MERS-CoV [13, 14]. Thus, screening for antiviral compounds in herbals previously used to treat viral pneumonia will expedite the identification of a potential SARS-CoV-2 antiviral drug. In India, Andrographis paniculata was widely used to control the dengue outbreak in 2006, following the Ministry of Ayush’s recommendations. Traditionally used by the Tamil people of South India, “Nilavembu Kudineer” is an aqueous preparation of Andrographis paniculata. It was administered to dengue-infected patients for treatment and to healthy individuals as a prophylactic immune booster. Its antidengue properties have been extensively explored and demonstrated [15]. In India, papaya leaves were used to treat dengue [16]. An investigation was conducted with 49 naturally occurring metabolites derived from 23 distinct medicinal plant species. Among the total of 49 compounds that were subjected to screening, only 7 exhibited drug-like properties. The study employed molecular docking, dynamics simulation, ADME/T analysis, and DFT analysis to identify naringenin’s most promising ligand [17]. Another similar study confirmed that artificially synthesized acyl phloroglucinols showed notable inhibitory potential against SARS-CoV-2 PL protease [17].
Other plants considered include Azadirachta indica (Neem), Eclipta prostrata, Carica papaya, and Zingiber officinale, all extensively examined for their antiviral activity [18]. The preceding data concluded that natural antiviral chemicals are critical in mimicking infectious diseases such as SARS, dengue, and chikungunya. As a result, antiviral compounds are chosen for this investigation from the sources listed above.
The objectives of this study were the effective interaction of selected natural compounds with the chosen protein targets by in silico docking analysis followed by dynamics simulation analysis and conceptual density functional theory (DFT) computation. Finally, the absorption, digestion, metabolism, excretion, and toxicity (ADMET) properties were investigated to determine their drug-like properties.
2. Materials and Methods
2.1. Retrieval of Protein Receptor
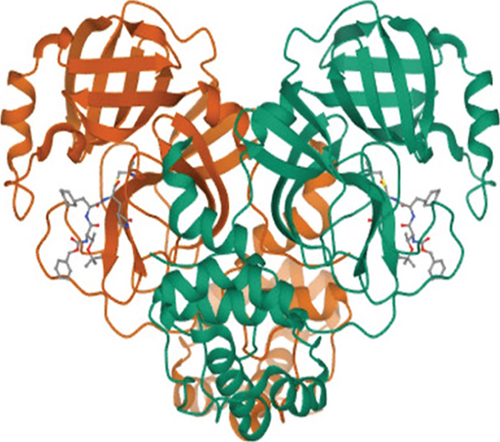
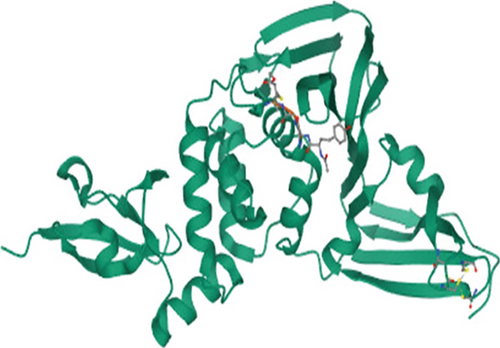
The 3D structures of 3CL protease (PDB ID: 7C8T) and PL protease (PDB ID: 6WX4) were downloaded from the Protein Data Bank based on the resolution of crystal structure (Figure 1).
2.2. Retrieval of Ligands
3D structures of antiviral compounds (Table 1) such as 6-gingerol, catechin, chlorogenic acid, coumaric acid, caricaxanthin, dasyscyphin C, glycyrrhizin, hyperoside, kaempferol, naringin, nimbaflavone, quercetin, rutin, violaxanthin, zeaxanthin, and zingiberene were downloaded from PubChem.
| Name | PubChem ID | Molecular weight | Molecular formula | Structure | Antiviral property |
|---|---|---|---|---|---|
| Caricaxanthin | 44554791 | 552.9 | C40H56O |  |
Antidengue |
| Catechin | 9064 | 290.27 | C15H14O6 |  |
Anti-influenza, anti-HBV, anti-HSV, antiadenovirus, anti-HIV, antidengue, anti-CHIKV |
| Chlorogenic acid | 1794427 | 354.31 | C16H18O9 |  |
Anti-influenza, antihepatitis, anti-HSV |
| Coumaric acid | 637542 | 164.16 | C9H8O3 |  |
Anti-HIV, anti-influenza, antienterovirus, anti-CVA |
| Dasyscyphin C | 11562458 | 504.6 | C28H40O8 |  |
Antinodavirus |
| Gingerol | 442793 | 294.4 | C17H26O4 |  |
Anti-HCV, anticorona, anti-HRSV |
| Glycyrrhizin | 14982 | 822.9 | C42H62O16 |  |
Antiherpes, anti-corona-alpha, antiflaviviruses, anti-HIV, anti-influenza A, antipolio type I |
| Hyperoside | 5281643 | 464.4 | C21H20O12 |  |
Anti-HBV |
| Kaempferol | 5280863 | 286.24 | C15H10O6 |  |
Anti-influenza, anti-JEV, anti-HIV |
| Naringin | 442428 | 580.5 | C27H32O14 |  |
Anti-ZIKV, antidengue |
| Nimbaflavone | 14492795 | 422.5 | C26H30O5 |  |
Anti-influenza |
| Quercetin | 5280343 | 302.23 | C15H10O7 |  |
Anti-influenza, antiadenovirus, antisyncytial, antirhino |
| Rutin | 5280805 | 610.5 | C27H30O16 |  |
Anti-influenza, antidengue, antihepatitis |
| Violaxanthin | 448438 | 600.9 | C40H56O4 |  |
Antidengue |
| Zeaxanthin | 5280899 | 568.9 | C40H56O2 |  |
Antidengue |
| Zingiberene | 521253 | 204.35 | C15H24 |  |
Anti-HSV |
2.3. Ligand and Protein Preparation
The ligand molecules are prepared by energy minimization using ArgusLab software [19]. The energy minimization has been done by the restricted Hartree-Fock (closed-shell). Until attaining the lowest potential energy, all the ligand molecules were energy minimized. The energy-minimized ligands were used for docking studies.
The 3D structures of chosen protein molecules were directly downloaded from PDB (PDB ID: 7C8T and PDB ID: 6WX4) and used for docking. The water molecules and ligand (inhibitor) were removed from the structure, and the essential polar hydrogen was added to the structure using Discovery Studio Visualizer (version 20.1.0.19295) developed by Dassault Systems BIOVIA Corporation. The prepared protein structure was further finalized using an online server of the Centre for Molecular and Biomolecular Informatics, Radboud University, Netherlands (https://swift.cmbi.umcn.nl/servers/html/index.html), and then saved for docking in the form of PDB format [20].
2.4. Molecular Docking
PyRx (version 0.8) was used to dock the protein receptors with the selected ligands, and the final output files were obtained as PDBQT files. Individual poses are prepared by a vina split [21]. The amino acids involved in different interactions were observed.
2.5. Conceptual DFT
DFT helps analyze a structure by molecular orbital energies [22]. It may help clarify the structure-activity relationship of compounds. DFT calculation will help link biological activities to structure [23]. The Hohenberg and Kohn theorems underpin it [24]. This work used a subset of DFT called conceptual DFT to investigate chemistry using electron density ideas [25]. The electron density of a molecule yields ten separate molecular characteristics, including the electrophilicity index, chemical potential, molecular dipole moment, and electronegativity.
2.6. Prediction of ADME Properties
All the ligands used in this study were not approved as drugs by the FDA. As a result, it is critical to understand the drug likelihood of the best ligands targeting both 3CL and PL proteases. The ligands’ ADME properties were foreseen by SwissADME (https://www.swissadme.ch). The ADME properties were observed [26] for the ligands that showed the best docking score with 3C and papain-like proteases of SARS-CoV-2.
2.7. Prediction of Toxicity
The selected best ligands with significant docking scores were subjected to toxicity prediction using the pkCSM (http://biosig.unimelb.edu.au/pkcsm/). The canonical SMILES of the target ligands, taken from the PubChem website, were utilized on the pkCSM server under toxicity mode for prediction [27].
2.8. Molecular Dynamics Simulation
The glycyrrhizin, rutin, and violaxanthin ligands were simulated using the GROMACS 5.1.2 software and the GROMOS96 54a7 force field. The study mentioned above employed traditional algorithmic techniques and methodologies. The PDBQT files of the docked complexes’ main protease and drug molecules were converted to PDB files using Discovery Studio 2020. These PDB data were subsequently refined using Swiss PDB Viewer. GROMACS generated the core protease topology file, while PRODRG2 was responsible for creating the topologies of the drug candidates. Topology and data for protein-ligand complexes were generated. Four sodium ions were introduced into a dodecahedron container with a volume of 1 nanometer to preserve electrostatic charge equilibrium, which was subsequently filled with water. The energy was reduced after 1,000 iterations using the steepest descent algorithm. The drug candidates and main protease were later constrained, allowing the solvent to diffuse without restriction. The conditions required for achieving the constant number of particles, volume, and temperature (NVT) and continuous number of particles, pressure, and temperature (NPT) equilibration were as follows. The output analogue coordinates consisted of a total of 500 steps. The computational technique employed in this study was the Particle Mesh Ewald approach, with a cut-off distance of 1.2 nm for the electrostatic interactions. Following the completion of NVT (canonical ensemble) and NPT (isothermal-isobaric ensemble) simulations, production molecular dynamics (MD) runs were conducted at a temperature of 300 K and a pressure of 1 bar, with a time step of 2 femtoseconds. A 20,000-picosecond simulation was performed [20, 28].
3. Results
3.1. Analysis of Docking with 3CL Protease
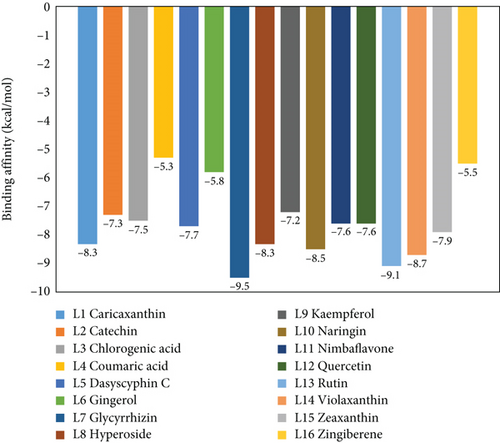
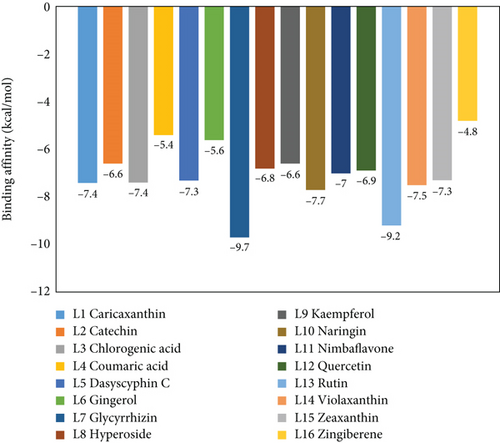
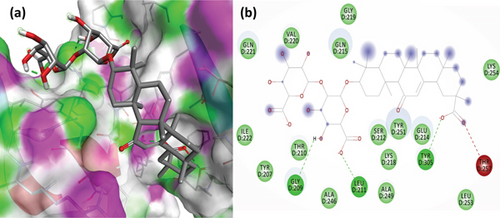
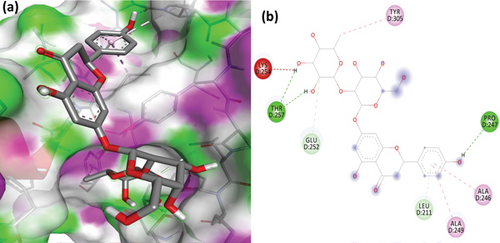
To understand the molecular interaction, all compounds were docked with the 3CL protease (PDB ID: 7C8T). Our results indicated that glycyrrhizin had a better docking value of -9.5 kcal/mol and coumaric acid had a lower docking value of -5.3 kcal/mol (Figure 2). Table 2 lists the amino acids of the 3CL protease that form chemical bonds with each ligand. The docking image (Figure 3) of glycyrrhizin with 3C protease revealed seven hydrogen bonds with CYS145, ARG131, LYS137, THR199, LEU287, ASP289, and GLU288 and one electrostatic interaction with LYS137. Compared to glycyrrhizin, rutin (Figure 4) and violaxanthin (Figure 5) demonstrated significant docking scores with 3C protease. Rutin formed six hydrogen bonds and three additional contacts, while violaxanthin formed three hydrophobic connections (Table 2).
| Ligand | Details of hydrogen bond | Other interactions | |||
|---|---|---|---|---|---|
| No. of bonds | Amino acids involved | No. of bonds | Amino acids involved | Bond | |
| Caricaxanthin | — | — | 3 | TYR154, HIS246, PHE294 | Hydrophobic bond |
| Catechin | 1 | GLU166 | 1 | CYS145 | Pi–sulfur bond |
| 1 | HIS41 | Hydrophobic bond | |||
| Chlorogenic acid | 4 | TYR239, ASP289, LEU271, GLU288 | 2 | LEU287, MET276 | Hydrophobic bond |
| Coumaric acid | 2 | TYR154, GLN306 | 2 | TYR154, PHE294 | Hydrophobic bond |
| Dasyscyphin C | 3 | LYS137, ASN238, LEU287 | — | — | — |
| Gingerol | 3 | SER144, ARG188, ASN142 | 2 | HIS41, MET165 | Hydrophobic bond |
| 1 | CYS145 | Pi–sulfur bond | |||
| Glycyrrhizin | 7 | CYS145, ARG131, LYS137, THR199, LEU287, ASP289, GLU288 | 1 | LYS137 | Electrostatic bond |
| Hyperoside | 6 | GLN189, GLN192, MET165, VAL186, LEU141, GLU166 | 1 | CYS145 | Pi–sulfur bond |
| 2 | HIS41, MET165 | Hydrophobic bond | |||
| Kaempferol | 1 | GLU166 | 4 | HIS41, LEU141, ASN142, CYS145 | Hydrophobic bond |
| Naringin | 4 | GLN110, TYR154, THR111, THR292 | — | — | — |
| Nimbaflavone | 2 | GLN192, GLN189 | 5 | CYS145, MET49, HIS41, PRO168, MET165 | Hydrophobic bond |
| Quercetin | 2 | PHE140, LEU141 | 2 | HIS41, CYS145 | Hydrophobic bond |
| 1 | CYS145 | Pi–sulfur bond | |||
| Rutin | 6 | SER144, GLN189, THR26, ASP187, GLU166, ASN142 | 1 | CYS44 | Pi–sulfur bond |
| 2 | HIS41, MET49 | Hydrophobic bond | |||
| Violaxanthin | — | — | 3 | ILE249, TYR154, PHE294 | Hydrophobic bond |
| Zeaxanthin | — | — | 2 | PRO241, HIS246 | Hydrophobic bond |
| Zingiberene | — | — | 1 | PHE294 | Hydrophobic bond |
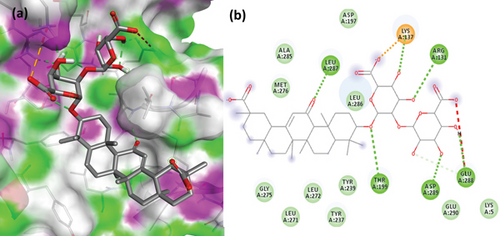
3.2. Docking Analysis with PL Protease
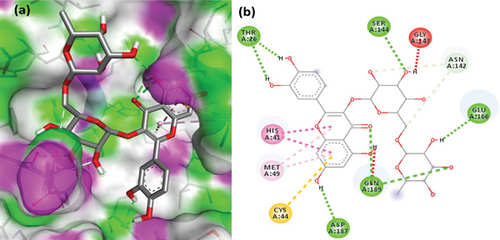
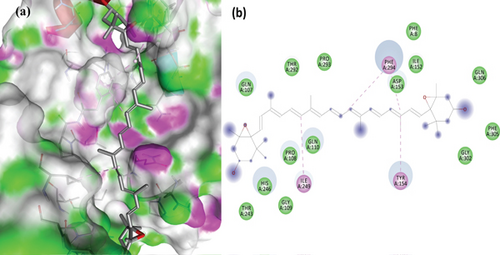
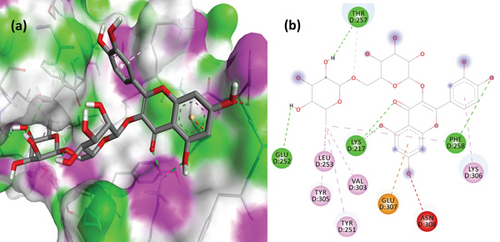
The PL protease (PDB ID: 6WX4) was docked individually with each identified ligand. Glycyrrhizin was discovered with the highest docking value of -9.7 kcal/mol (Figure 6). Zingiberene had the lowest docking value of -4.8 kcal/mol. The amino acid details of each ligand’s bonding with the protein receptor are listed in Table 3. According to the Discovery Studio Visualizer (Figure 7), glycyrrhizin forms three hydrogen bonds with PL protease via LEU211, TYR305, and GLY209. Compared to glycyrrhizin, rutin (Figure 8) and naringin (Figure 9) had significant docking scores. Rutin formed four hydrogen bonds and seven other interactions with the PL protease, whereas naringin formed four hydrogen bonds and four other interactions.
| Name of the ligand | Details of hydrogen bond | Details of other interactions | |||
|---|---|---|---|---|---|
| No. of bonds | Amino acids involved | No. of bonds | Amino acids involved | Bond | |
| Caricaxanthin | — | — | 5 | ARG65, ALA68, PRO77, PRO59, PHE69 | Hydrophobic bond |
| Catechin | 6 | TYR213, GLU214, LYS254, GLU252, SER212, THR257 | 1 | TYR251 | Hydrophobic bond |
| 1 | GLU214 | Electrostatic interaction | |||
| Chlorogenic acid | 5 | TYR213, LYS217, TYR305, LYS306, SER212 | 1 | TYR305 | Hydrophobic bond |
| Coumaric acid | 2 | TYR305, TYR251 | 1 | GLU214 | Electrostatic interaction |
| Dasyscyphin C | 5 | ARG166, GLN174, SER170, MET206, GLU203 | 1 | TYR207 | Hydrophobic bond |
| Gingerol | 4 | TYR213, GLU214, TYR305, TYR251 | 1 | GLU214 | Electrostatic interaction |
| 3 | TYR305, LYS254, TYR213 | Hydrophobic bond | |||
| Glycyrrhizin | 3 | LEU211, TYR305, GLY209 | — | — | — |
| Hyperoside | 5 | LYS217, GLY256, THR257, GLU307, ASN308 | 1 | TYR310 | Hydrophobic bond |
| 2 | GLU214, GLU307 | Electrostatic interaction | |||
| Kaempferol | 3 | THR257, GLU252, TYR251 | 1 | TYR305 | Hydrophobic bond |
| 1 | GLU214 | Electrostatic interaction | |||
| Naringin | 4 | PRO247, THR257, GLU252, LEU211 | 4 | TYR305, LEU211, ALA246, ALA249 | Hydrophobic bond |
| Nimbaflavone | 1 | GLU307 | 2 | LYS217, GLU214 | Electrostatic interaction |
| 5 | THR257, LEU253, VAL303, PHE258, TYR305 | Hydrophobic bond | |||
| Quercetin | 1 | TYR273 | 2 | TYR264, PRO248 | Hydrophobic bond |
| Rutin | 4 | LYS217, PHE258, THR257, GLU252 | 1 | GLU307 | Electrostatic interaction |
| 6 | LEU253, VAL303, TYR251, PHE258, TYR305, LYS306 | Hydrophobic bond | |||
| Violaxanthin | 1 | GLN250 | 2 | TYR251, TYR305 | Hydrophobic bond |
| Zeaxanthin | — | — | 2 | LYS279, LYS306 | Hydrophobic bond |
| Zingiberene | — | — | 4 | VAL188, ILE314, PRO316, TYR233 | Hydrophobic bond |
3.3. Prediction of ADME Properties
The docking investigation revealed that glycyrrhizin and rutin have a better binding affinity for 3CL and PL proteases, respectively. It is worth noting that glycyrrhizin has a high potential for interaction with the 3CL and PL proteases. Compared to glycyrrhizin, rutin has a high potential for interaction with both proteases. ADME properties of the top four ligands are determined by their docking score for both proteases. As an outcome, glycyrrhizin, rutin, violaxanthin, and naringin were chosen for ADME property prediction using the SwissADME server.
The SwissADME results (Table 4) indicate that none of the ligands can inhibit the cytochrome P450 system, which is a significant finding. All ligands have a negligible level of intestinal absorption rather than none. None of the ligands are capable of passing through the blood-brain barrier. Rutin and naringin are found to be water-soluble, whereas glycyrrhizin and violaxanthin are found to be insoluble and very poorly soluble in water. Compared to the other ligands, glycyrrhizin and violaxanthin do not contain heavy aromatic atoms. To be regarded as a drug-like molecule in ADMET prediction, any molecule must adhere to Lipinski’s rule of 5. Here, the final chosen molecules violate some of Lipinski’s rules, which must be evaluated before evaluating any ligand as a prospective drug-likeness compound based on its ADME features.
| Property | Glycyrrhizin | Rutin | Naringin | Violaxanthin |
|---|---|---|---|---|
| Physicochemical properties | ||||
| Heavy atoms | 58 | 43 | 41 | 44 |
| Aromatic heavy atoms | 0 | 16 | 12 | 0 |
| Rotatable bonds | 7 | 6 | 6 | 10 |
| H bond acceptors | 16 | 16 | 14 | 4 |
| H bond donors | 8 | 10 | 8 | 2 |
| TPSA | 267.04 Å2 | 269.43 Å2 | 225.06 Å2 | 65.52 Å2 |
| Lipophilicity | ||||
| Log Po/w (iLOGP) | 2.15 | 2.43 | 2.38 | 7.22 |
| Log Po/w (XLOGP3) | 2.8 | -0.33 | -0.44 | 9.76 |
| Log Po/w (WLOGP) | 2.25 | -1.69 | -1.49 | 8.97 |
| Log Po/w (MLOGP) | 0.02 | -3.89 | -2.77 | 5.37 |
| Log Po/w (SILICOS-IT) | 0.52 | -2.11 | -1.64 | 10.60 |
| Consensus Log Po/w | 1.55 | -1.12 | -0.79 | 8.39 |
| Water solubility | ||||
| Log S (ESOL) and class | -6.24 and poorly soluble | -3.3 and soluble | -2.98 and soluble | -9.05 and poorly soluble |
| Log S (Ali) and class | -8.06 and poorly soluble | -4.87 and moderately soluble | -3.82 and soluble | -11.06 and insoluble |
| Log S (SILICOS-IT) and class | -1.39 and soluble | -0.29 and soluble | -0.49 and soluble | -5.38 and moderately soluble |
| Pharmacokinetics | ||||
| GI absorption | Low | Low | Low | Low |
| BBB permeant | No | No | No | No |
| P-gp substrate | Yes | Yes | Yes | Yes |
| CYP1A2 inhibitor | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No |
| Log Kp (skin permeation) | -9.33 cm/s | -10.26 cm/s | -10.15 cm/s | -3.04 cm/s |
| Drug-likeness and violations | ||||
| Lipinski’s rule of 5 and no. of violations | No and 3 | No and 3 | No and 3 | No and 2 |
| Ghose’s filter and no. of violations | No and 3 | No and 4 | No and 4 | No and4 |
| Veber’s filter and no. of violations | No and 1 | No and 1 | No and 1 | Yes and 0 |
| Egan’s (Pharmacia) filter and no. of violations | No and 1 | No and 1 | No and 1 | No and 1 |
| Muegge’s (Bayer) filter and no. of violations | No and 1 | No and 4 | No and 3 | No and 2 |
| Bioavailability score | 0.11 | 0.17 | 0.17 | 0.17 |
| Medicinal chemistry | ||||
| PAINS | 0 alert | 1 alert | 0 alert | 0 alert |
| Synthetic accessibility score | 8.84 | 6.52 | 6.16 | 7.58 |
3.4. Conceptual DFT
The phytochemicals were optimized using the B3LYP function [29, 30] with a 6-31G (2p, d) basis in Gaussian 16 [31] to determine their molecular properties [32]. The highest occupied molecular orbital (HOMO) energy (EHOMO) and the least unoccupied molecular orbital (LUMO) energy (ELUMO) were calculated, which are critical descriptors that refer to a molecule’s ability to donate as well as accept electrons, correspondingly. We developed and examined maps reflecting the electron density in various locations of the molecules at HOMO and LUMO. The EHOMO and ELUMO values for the phytochemicals are listed in Table 5.
| Compound | Total energy (E γ) (in eV) | Molecular dipole moment (Debye) | EHOMO (eV) | ELUMO (eV) | Egap (eV) | Absolute hardness (η) | Global softness (σ) | Electronegativity (χ) | Chemical potential (μ) | Electrophilicity index (ω) |
|---|---|---|---|---|---|---|---|---|---|---|
| Glycyrrhizin | -77321.04 | 8.97 | -6.25 | -1.28 | 4.97 | 2.48 | 0.20 | -3.76 | 3.76 | 2.85 |
| Rutin | -2237.71 | 7.53 | 5.05 | 3.83 | -1.22 | -0.61 | -0.82 | 4.44 | -4.44 | -16.19 |
| Naringin | -2101.18 | 8.0019 | -5.97 | -1.30 | 4.67 | 2.33 | 0.12 | -2.33 | 2.33 | 1.16 |
| Violaxanthin | -1858.70 | 0.4315 | -4.87 | -1.69 | 3.18 | 1.59 | 0.31 | -1.59 | 1.59 | 0.79 |
Table 6 shows the electron density maps of selected phytochemicals’ molecular orbitals. We built and evaluated density maps of molecular orbitals. The energy gap (Egap) was determined like E = ELUMO–EHOMO. The energy gap is proportionate to the molecules’ reactivity [33]. As a result, with an E value of -1.22 eV, the rutin protease had the smallest energy gap. Glycyrrhizin, on the other hand, showed the largest, measuring 4.97 eV.
| Compound | DFT optimized structure | HOMO | LUMO |
|---|---|---|---|
| Glycyrrhizin |  |
 |
 |
| Rutin |  |
 |
 |
| Naringin |  |
 |
 |
| Violaxanthin |  |
 |
 |
A molecule’s molecular dipole moment can be related to its chemical reactivity [34]. Rutin protease and glycyrrhizin exhibited dipole moments greater than 7.0 Debye. Using predicted EHOMO and ELUMO scores, electronegativity and other properties of the ligands were determined. The electron density of a compound is significant because it influences the electronegativity. If the electronegativity is low, the inhibitory activity will be higher and effective [35]. Glycyrrhizin has the lowest electronegativity value of -3.76 eV, while the rutin protease has a value of 4.44 eV. Correlations are found between the values of descriptors for phytochemicals derived using conceptual DFT and the docking analysis. From this, glycyrrhizin was the highest scoring molecule with huge electronegativity than rutin, which is consistent with the docking analysis.
3.5. Prediction of Toxicity
The toxicity of the final selected ligands, glycyrrhizin, rutin, naringin, and violaxanthin, was predicted and analyzed [36]. The results (Table 7) demonstrate unequivocally that none of the four ligands contain AMES toxicity and is neither mutagenic nor carcinogenic. The maximum recommended tolerated dose in humans for all ligands is less than 0.477, which is considered very low. None of the ligands were found to be hepatotoxic or skin-sensitizing. Glycyrrhizin and violaxanthin showed no inhibitory potential on hERG genes encoding for potassium channels. The LD50 and minnow toxicity values are significantly high for all the ligands, which shows that only at very high doses, ligands may tend to cause toxicity (http://biosig.unimelb.edu.au/pkcsm/static/help/pkcsm_theory.pdf).
| Property | Glycyrrhizin | Rutin | Naringin | Violaxanthin |
|---|---|---|---|---|
| AMES toxicity (yes/no) | No | No | No | No |
| Max. tolerated dose in human (log mg/kg/day) | 0.389 | 0.452 | 0.43 | -0.384 |
| hERG I inhibitor (yes/no) | No | No | No | No |
| hERG II inhibitor (yes/no) | No | Yes | Yes | No |
| Oral rat acute toxicity (LD50) (mol/kg) | 2.48 | 2.491 | 2.495 | 2.132 |
| Oral rat chronic toxicity (LOAEL) (log mg/kg_bw/day) | 5.889 | 3.673 | 4.202 | 2.054 |
| Hepatotoxicity (yes/no) | No | No | No | No |
| Skin sensitization (yes/no) | No | No | No | No |
| T. pyriformis toxicity (log μg/L) | 0.285 | 0.285 | 0.285 | 0.308 |
| Minnow toxicity (log mM) | 5.591 | 7.677 | 6.042 | -2.492 |
3.6. Molecular Dynamics Simulation
Following the molecular docking and ADMET prediction analysis, the best three phytochemicals were subjected to research the stability of ligands with 3CL and PL proteases using a molecular dynamics simulation study. Root-mean-square deviation (RMSD), root-mean-square fluctuation (RMSF), and protein-ligand contacts were calculated. Glycyrrhizin, rutin, and violaxanthin molecules show good binding affinity and dug-likeness properties in this docking and ADMET analysis. RMSD, RMSF, Rg, and solvent-accessible surface are shown in Figures 10-12. The average RMSD value of glycyrrhizin, rutin, and violaxanthin molecules with 3CL and PL proteases shows 0.19 nm, 0.18 nm, and 0.18 nm and 0.2 nm, 0.21 nm, and 0.2 nm, respectively. The stability of the 3CL with glycyrrhizin, rutin, and violaxanthin in radius of gyration (total and around axes) shows at 2.35 Rg (nm) up to 20000 ps.
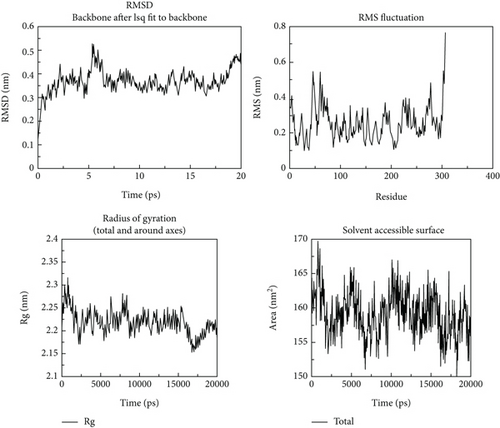
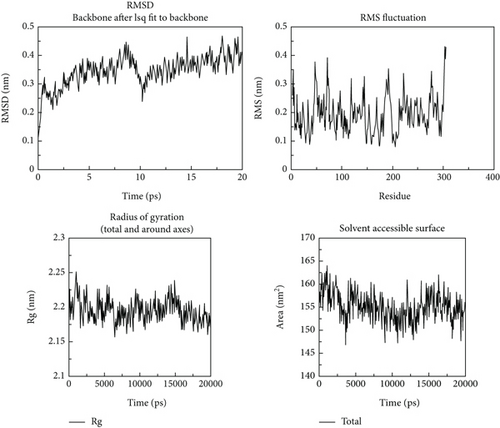
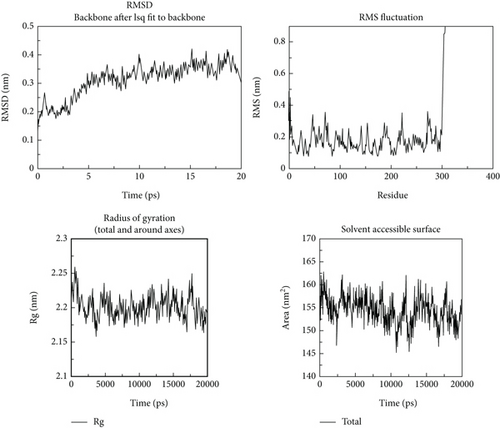
The results obtained in this investigation are consistent and comparable with the findings reported in the earlier study conducted by Jang et al. [37]. The results demonstrate the stability of the relationship between the natural metabolites and the protein structure. The results obtained for the root-mean-square deviation (RMSD) and root-mean-square fluctuation (RMSF) in our study demonstrate comparable or superior performance compared to the findings reported in earlier studies. The molecular dynamics simulation conducted for 2000 picoseconds showed encouraging interactions between the protein and the selected metabolites. In the investigation mentioned above, the compounds that were chosen had protein-based root-mean-square deviation (RMSD) values of 0.5, 0.4, and 0.3 nm, respectively (refer to Figure 3(d)). Additionally, their root-mean-square deviation (RMSF) values displayed comparable patterns. Moreover, the in vitro assessment demonstrated that this interaction has a higher degree of stability, indicating inhibitory potential. The findings closely align with our results.
4. Discussions
Virtual screening of the existing antiviral drugs and the naturally available compounds with antiviral properties is essential for the treatment. The widely used drug, remdesivir, is still in trial and is authorized to be used only for emergency management. A recent report [38, 39] stated that combining azithromycin and hydroxychloroquine significantly aided in emergency cases. The inhibition of proteases was observed due to the increase in pH. However, the adverse effects of hydroxychloroquine and azithromycin must be considered, and a side-effect-free alternative is urgently needed [39].
Indian spices are of great importance because of their traditional use as a source of medicine. Many bioactive metabolites with therapeutic applications have been identified [40]. Plants are well characterized and proven for their noteworthy basis of high-value bioactive metabolites, including alkaloids, phenols, flavonoids, coumarins, steroids, and lignans. Plants have widened the window of opportunity to discover natural compounds with therapeutic potential [41–43]. According to a study [44], the plant-derived chemicals carnosol and rosmanol may be employed as therapeutic candidates against SARS-CoV-2. Another study [45] also asserts that three natural substances, ursolic acid, carvacrol, and oleanolic acid, are possible inhibitors.
In this study, four compounds, glycyrrhizin, rutin, violaxanthin, and naringin, were finally chosen from a pool of sixteen compounds based on their docking score.
Glycyrrhizin, the compound with the highest docking score, has been reported to be a moderately potent antiviral agent against HIV and herpes simplex virus [46]. It was recently found as a possible inhibitor of HIV’s replicative mechanism [47]. Glycyrrhizin inhibits many viruses, including herpes, corona-alpha, flaviviruses, HIV, Epstein–Barr virus, influenza A virus, vaccinia, and polio type I viruses [48–53]. Our findings correlated with earlier research, demonstrating a potent 3CL and PL protease inhibitors. The drug is also effective against SARS in vitro studies and may show promise for treating COVID-19 infections. More promising, some derivatives of glycyrrhizin are shown to have a much higher antiviral activity than glycyrrhizin itself [54]. The in silico results of this paper are similar to a recent study [55] that has clearly explained the suitability of glycyrrhizin against the 3CL protease of SARS-CoV-2 through in vitro assays, and EC50 value was found to be 0.44 mg/mL.
Additionally, conceptual DFT analyses demonstrate that all molecular characteristics indicate that this compound is a potent inhibitor. The combination of glycyrrhizin and boswellic acid demonstrated efficacy in reducing death rates, accelerating the healing process, and enhancing prognosis or reducing clinical status scores on a 7-point scale. The laboratory findings indicate notable disparities in the serum levels of C-reactive protein (CRP) and the percentage of lymphocytes between the placebo group and the intervention group, providing evidence to support the efficacy of GR+BA in promoting improvement. The combination above has qualities of safety and affordability, rendering it a potential candidate for addressing mild to moderate infections caused by SARS-CoV-2 or its variations associated with COVID-19 [56]. Glycyrrhizin had superior inhibitory effectiveness against SARS-CoV-2 (EC50 = 300 mg/L) during the processes of viral adsorption and penetration, in comparison to other antiviral agents such as ribavirin, 6-aziridine, pyrazofurin, and mycophenolic acid, while also demonstrating a higher selectivity index [57].
Rutin has the second-highest docking score. It is a glycosidic derivative of a flavonoid available in natural resources. According to a study [58], the compound’s 50% effective concentration (EC50) against the cucumber mosaic virus was 394.78 g/mL. A prior work [59] demonstrated that sodium rutin sulfate could be a sulfated rutin analogue that can inhibit HIV-1. Additionally, rutin has been shown [45] to significantly inhibit the avian influenza strain H5N1 in the Madin-Darby canine kidney.
Additionally, there is evidence [60] that rutin significantly inhibits the viral replication cycle during canine distemper virus infection during adsorption and diffusion. The current results of the molecular docking study demonstrate the compound’s antiviral activity and suggest that it may be a significant inhibitor of both 3CL and PL proteases. It has been studied [61] that rutin can inhibit 3CL protease. The findings of the conceptual DFT calculation also indicate that it can be a probable inhibitor next to glycyrrhizin.
Violaxanthin is a naturally occurring orange-coloured xanthophyll pigment found in plants. In silico analysis demonstrated a moderate antidengue potential for this compound [62]. There is scant evidence for violaxanthin’s antiviral properties; however, there is evidence for its anti-inflammatory, antiproliferative, and antioxidant properties. Thus, more research and in vitro testing are required to demonstrate its antiviral efficacy.
The fourth ligand with an exceptionally high docking score, naringin, a flavanone 7-O-glycoside, is found in citrus fruits, particularly grapefruits. Naringin was found to have antiadsorption properties against dengue virus—type 2 in a study [63], with an IC50 of 168.2 g/mL and a corresponding selectivity index of 1.3. Another study [64] reported that naringin interacted successfully with the chikungunya virus’s nsp2, inhibiting viral replication at the initiation stage. Naringin also suppresses human rotavirus (Wa-1 strain) [65, 66]. The docking research results indicate that it can interact strongly with 3CL and PL proteases. Using conceptual DFT calculations, violaxanthin and naringin are placed third and fourth in potency.
Molecular dynamics simulation validated the conversational stability of glycyrrhizin, rutin, and violaxanthin molecules with the main protease. The RMSD of these ligands showed minor fluctuation with each protein conformation at 20 ns. Also, the binding nature of glycyrrhizin, rutin, and violaxanthin ligands with 3CL did not produce any conformational stability of the protein. Furthermore, the RMSF analysis shows that the best ligands were firmly bound to the active site of the 3CL protein and did not change the loop sections. The gyration radius was also examined to illustrate the protein’s structural alterations. Figures 10–12 show the time development of the Rg of 3CL subjected to applied electric fields of various strengths and directions. The value of Rg rises with the power of the applied electric field E ≤ 0.3 V/nm. When 1BBL is exposed to electric fields, the importance of Rg becomes increasingly exceptional E ≥ 0.5 V/nm as the intensity of the electric field increases due to the protein’s extension along the electric field. The protein-ligand association was stable during MD modelling, with slight backbone changes.
According to the toxicity prediction, glycyrrhizin and violaxanthin exhibited no toxicity with a higher LD50 value. Additionally, rutin and naringin inhibited the hERG II gene. The chosen ligands are prospective antiviral medicines due to their effective interaction with proteases. As per the results of ADME property prediction, glycyrrhizin is the best choice for an antiviral molecule with specific breaches of Lipinski’s rule. Additionally, no substantial toxicity was detected using toxicological prediction. Interactions with SARS-CoV-2 proteases in silico demonstrate their ability to work against various viruses. While glycyrrhizin, rutin, and naringin all exhibit promising antiviral effects, their drug-likeness violations should not be overlooked when considering them as drug lead compounds. As a result, glycyrrhizin is the best antiviral drug and may be a probable alternative treatment option.
5. Conclusion
Repurposing existing medications and utilizing natural antiviral compounds can be the best scientific option until the development of a new drug. The primary target of most viral infections, including HIV, dengue, SARS, influenza, and n-COVID-19, is a protease. The in silico screening of sixteen natural antiviral medicines for their capacity to inhibit the SARS-CoV-2 protease suggests that glycyrrhizin and rutin may be deemed more powerful antiviral agents than others. Additionally, their ADME and toxicology features indicate their potential as anti-SARS-CoV-2 agents. Also, molecular dynamics simulation analysis reveals the strong interaction of top ligands with the protein targets. As a result, additional research and trials are necessary to establish them as antiviral agents.
Consent
All authors agree to the publishing of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Authors’ Contributions
Sugumar Mohanasundaram was responsible for the methodology and investigation and wrote the original draft. Porkodi Karthikeyan was responsible for the software and supervision and wrote, reviewed, and edited the manuscript. Venkatesan Sampath was responsible for the conceptualization and supervision. Anbazhagan. M was responsible for the methodology and wrote, reviewed, edited, and validated the manuscript. Sundramurthy Venkatesa Prabhu was responsible for the conceptualization and supervision and wrote, reviewed, and edited the manuscript. Jamal M. Khaled was responsible for the funding acquisition and validation. Muthu Thiruvengadam was responsible for the conceptualization and supervision.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-457-2).
Open Research
Data Availability
The data used to support the findings of this study are included in the article.