Investigation of Eggshell Membrane Removal With Two Different Treatment Methods
Thermal and Chemical
Abstract
Chicken eggshells (ESs) consist of 96%–97% calcium carbonate, while about 3%–4% consists of organic substances, mainly in the form of protein-based membranes and occluded organic matter. Recently, ESs have been studied as a filler in polymer composite materials, which represents an innovative solution to address ES valorization. In this study, thermal and chemical treatments were investigated for membrane removal since the membrane may alter various properties when the ES fillers are added to the composite material. A nanoindentation method was used to measure changes in the ES mechanical properties. Scanning electron microscopy (SEM), thermogravimetric analysis (TGA), and Fourier-transform infrared (FT-IR) spectroscopy were used to characterize the ES membrane removal through chemical treatment. The results showed that even at a heating temperature of 100°C, the ES mechanical properties were negatively affected, while a low concentration of bleach solution (25% bleach solution and 10 min of holding time) was able to remove the membrane without reducing the ES mechanical properties. The chemical treatment method offers a means for ES membrane removal while conserving the quality of the mineral fraction (calcite; CaCO3).
1. Introduction
Environmental awareness, high cost of petroleum feed-stocks, rising prices due to global instability, and independence from petroleum-based derivatives are vital reasons why industries are tending to shift their products from petroleum to bio-based materials and composites. Eggshells (ESs) can be used as a bio-based filler in composite materials, similar to the utilization of mineral limestone (calcium carbonate) as fillers in polymer composites [1]. A key source of large quantities of ESs worldwide is from egg-breaking plants [2, 3]. Developing novel applications and repurposing industry ES by-products can be a win–win strategy for egg-breaking plants and the biomaterials industry. Business owners who manufacture composite materials can potentially replace the use of expensive polymers with low-cost bio-based filler materials such as ESs [1, 4].
ES consists of a rich source (96%–97%) of calcium that exists as calcium carbonate (CaCO3) as the main mineral phase with minor amounts of calcium triphosphate and magnesium carbonate, while 3%–4% consists of organic substances of which about 3.3% are proteins and 1.6% is water [5]. From the inner to outer portions of the ES, the structure consists of six layers; two inner membranes of interlaced fibers, a mammillary layer (knobs), and a spongy palisade layer (columnar crystals) both composed of calcite (CaCO3), and both held together by an occluded organic matrix and an outermost layer referred to as the cuticle [6–8]. The membrane fibers consist of proteins, glycoproteins, and collagens (types I, V, and X) [9], while the organic matrix is composed of proteins, glycoproteins, and proteoglycans [10]. The cuticle consists of glycoproteins, polysaccharides, lipids, and inorganic phosphorus, including hydroxyapatite crystals [10]. As of 2024, 900 different proteins have been identified as constituents of the ES matrix [11]. Recently, several applications for ES use have been reported, such as food additives for both human and animal nutrition [12, 13], soil amendment [14], cosmetics [12, 15], catalysts and sorbents [16–19], and as fillers in polymers [20, 21]. The addition of ES or calcium carbonate fillers to polymer composites can lower fabrication costs since a portion of the expensive polymer fraction in the composite formulation is replaced with low-cost ES filler [20, 22, 23]. For instance, polylactic acid (PLA) composites reinforced with CaCO3 have a wide range of applications in different industries [20], such as biomedical applications for the production of tissue scaffolds [24] and the building industry for the fabrication of gypsum boards [25].
In recent articles, the effect of different particle sizes and ES filler content on the mechanical properties of bio-polymer composites was evaluated. For example, Betancourt and Cree [26] studied the effect of ES particle content (5–20 wt.%) on the mechanical properties of PLA composites manufactured by injection molding. According to the results, the maximum tensile strength of the PLA composites was observed by adding 5 wt. % ES to PLA, but increasing the ES content reduced the tensile strength. However, increasing the content of ESs improved the tensile modulus where the maximum tensile modulus was observed when 20 wt.% ES was added to PLA. Furthermore, composites containing smaller (32 µm) particle ES fillers had improved tensile strengths versus composites with larger (63 µm) particle fillers. In a related study, Boronat et al. [1] investigated the effect of ESs as a filler on the mechanical properties of bio-based green polyethylene (bio-PE) polymer fabricated by the injection molding method. The results showed that ES particles (20 wt.% ES with a 25 µm particle size) strongly improved the mechanical properties of bio-PE, such as hardness, flexural modulus, and tensile modulus.
Several techniques for the removal of the ES organic membrane from the calcium carbonate layer have been reported. Cusack and Fraser [27] investigated different methods for membrane removal, such as plasma etching, sodium hypochlorite (bleach agent), and hydrochloric acid (HCl) treatments. Initially, the ESs were cleaned by rinsing with water to remove any liquid egg remnants. The study showed the organic membrane was removed when volatilized for 4 h in a plasma etching machine. In another method, ESs were placed in sodium hypochlorite for 20 min or HCl for 5 min, followed by water rinsing. Scanning electron microscopy (SEM) was used to verify if plasma etching and sodium hypochlorite treatments were effective for ES membrane removal. In a related study, Cree and Rutter [4] investigated the effect of different bleach concentrations and holding times for the ES membrane removal. According to this study, the ES membrane was removed by 10% bleach treatment for 48 h or 50% bleach for 10 min. In another study, Aina et al. [28] investigated the effect of acetic acid and nitric acid applications to remove the surface-accessible ES membrane. The ES membrane separation occurred after 17 min of ES soaking in the 1 molar (M) aqueous solution of acetic acid and nitric acid, respectively. The separation was done by peeling off the membrane from the ES by hand, which may be difficult to achieve in an industrial plant. MacNeil [29] investigated the mechanical treatment for removing the ES membrane in another study. In this method, ESs were placed in a tank filled with water. Then, a turbulent force was added by a stirrer, and the ES membrane was claimed to be totally removed from the ES. This method requires a considerable amount of water that requires water recycling, which adds additional production costs, especially at the industrial scale. In another study, Tsuboi and Koga [30] studied the effect of heat treatment for membrane removal. According to the SEM results, holes were observed on the outer surface of the ESs at 300°C. These holes were related to the release of water vapor during the heating period. In a related study, Cree and Pliya [3] studied the effect of elevated temperature on ES powder with particle size of 63 µm. To understand the effect of increased temperature on the ES properties, ESs were heated to 150, 300, 450, and 600°C. The results showed that by raising the temperature, color changes were observed for the ESs, which were related to decomposition at elevated temperatures. Furthermore, according to SEM images, there was no ES membrane after 450°C. Thermogravimetric analysis (TGA) results at 500°C showed 3%–4% weight loss, which was attributed to the available organic ES membrane. In another study, Stevenson [31] studied ES membrane removal through enzyme treatment. In this method, ESs were placed in a plastic petri dish containing 15 ml of a 2.5% solution of protease and 0.1 M Tris buffer. The mixture was then incubated at 37°C for 36–60 h, which reveals that the membrane hydrolysis was overall complete within 36–48 h.
Determination of the ES mechanical properties is a vital property of the ES since it will influence the overall properties of the final polymer composite product [1, 32]. Previously, the nonheat-treated ES tensile modulus and hardness of ESs were measured by a Berkovich nanoindentation device [32, 33]. Other studies showed that the mechanical properties of tensile modulus and hardness decreased significantly for mineral limestone rocks when submitted to elevated temperature [34, 35]. For example, one study evaluated the effect of high temperatures on the mineral limestone’s tensile strength and hardness. The results showed that the hardness values decreased for limestone at ~200°C, and by increasing the temperature, the tensile strength reduced starting at 400°C [34]. In a similar study, the maximum tensile modulus of mineral limestone was observed for the nonheat-treated sample, while at 100°C, the tensile modulus began to decrease [35].
In this study, two different methods of thermal (heat) and chemical bleach agent treatments on ES membrane removal were investigated. SEM, TGA, and Fourier-transform infrared (FT-IR) spectroscopy were used to study the membrane removal. Furthermore, the nanoindentation method was used to measure the effect of treatment methods on the ES tensile modulus and hardness mechanical properties. This study will determine which of the two methods is more effective at removing the organic membrane from the ES that has the least effect on the mechanical properties of the ES. The results from this study will demonstrate that the use of a dilute bleach solution is feasible for effective membrane removal, which offers a low-cost strategy for ES membrane removal and valorization of biogenic calcium carbonate.
2. Materials and Methods
2.1. Preparation of Materials
White chicken ESs were obtained from Harman Eggs (a Star Egg Company Limited product), a local producer in Saskatchewan, Canada. The ESs were washed with distilled water to remove any initial residue and dried at 105°C for 24 h. After initial drying, ESs containing membranes were firstly crushed manually in a mortar and pestle. Then, ESs were ground by a high-speed multifunction grinder (HC-300) and sieved to a particle size of 63 µm. To investigate the effect of heat treatment on the ES membrane removal and also the effect of temperature on the mechanical properties of ESs, specimens were heat treated at 100, 150, 300, and 450°C using a heating rate of 10°C/min in an air atmosphere for 1 h using a Lindberg/Blue M box furnace. One dried sample was not heat treated and used as the control (at 23°C). The powdered sample weights were 5 g.
Furthermore, to investigate the effectiveness of the chemical bleach agent treatment for removal of the ES membrane, a household chlorinated Clorox bleach (5% sodium hypochlorite) was used which contained 5 wt.% sodium hypochlorite. For the chemical treatment procedure, 5 g of ground ES powder was added to 25% and 50% of the bleach solution for 10 min as a holding time in the solution. Since the original household bleach is not pure sodium hypochlorite, to produce 25% bleach solution, 25 ml of household bleach was mixed with 100 ml of distilled water. A stirrer was used in the solutions to maintain solution homogeneity. The samples were rinsed with distilled water five times to remove any bleach residue.
2.2. Mechanical Tests
2.3. SEM
The ES sample morphology and surfaces were observed by SEM using a JEOL JSM-6010 LV (Tokyo, Japan) with an operating voltage of 10 kV. Prior to the analysis, the samples were placed on metal stubs and coated with a thin layer of gold to improve electrical conductivity.
2.4. TGA
A TA Instrument Q50 TGA system was used for the TGA of the samples to characterize the trends in thermal stability for untreated and treated samples. The heating rate was 10°C per minute from 25 to 480°C in a nitrogen (N2) gas atmosphere.
2.5. FT-IR Spectroscopy
FT-IR spectra of the samples were recorded after mixing with pure spectroscopic grade KBr in the ratio of 1:10 wt./wt. by Bio-RAD FTS-40 spectrophotometer (Bio-Rad Laboratories, Inc., Philadelphia, PA, USA). DRIFT (diffuse reflectance infrared Fourier transform) spectra were obtained in reflectance mode at 295 K with a resolution of 4 cm−1 over a spectral range 500–4000 cm−1.
3. Results and Discussion
The following section addresses the objective of this study by highlighting the mechanical and physicochemical properties of ES biomass that was treated chemically and thermally to remove the organic membrane of the shell. The ES properties were evaluated by complementary methods: nanoindentation, spectroscopy (FT-IR, SEM), and thermal analysis (TGA) to evaluate the role of treatment (chemical vs. thermal methods), as outlined below.
3.1. Thermal Treatment
3.1.1. Mechanical Properties
Figure 1 shows the experimental data collected for the ES tensile modulus values at 23°C and after being submitted to 100 and 150°C. These findings were compared with the literature results achieved for the tensile modulus of mineral limestone submitted to high temperatures. The maximum tensile modulus observed for the ESs and the mineral limestone was at 23°C. By increasing the temperature, the structure of both ESs and limestone samples changed, which caused a reduction in their stiffness. The maximum tensile modulus of the ESs reached 43.4 GPa at 23°C but decreased by 9.67% and 22.58% at 100 and 150°C, respectively. Measurement of the tensile modulus was possible for the samples heat-treated up to 150°C. However, the tensile modulus could not be measured by the instrument/Berkovich method above 150°C since the samples were too weak and fragile. According to Figure 1, the tensile modulus values for the limestone samples from 23 to 150°C were higher than the ES samples. For example, at 23°C and at 100°C, the tensile modulus of the ES was lower by 40.66% and 45.02%, respectively, compared to the mineral limestone. The limestone tensile modulus drops sharply around 200°C, according to its steep slope, whereas the ES tensile modulus also reduced abruptly after 150°C due to its fragility. The differences can be explained by the structure of mineral limestone and ES. Mineral limestone is composed mainly of calcium carbonate and contains different ratios of other chemical substances, such as calcium oxide (CaO) and magnesium oxide, which are mineral-rigid materials. In contrast, the ES consists of calcium carbonate and a porous organic membrane, which is fragile compared to rigid mineral substances [35]. Furthermore, although the crystal form of both limestone and ES are CaCO3, the structure of CaCO3 for these two substances is different. The CaCO3 structure of the limestone is rhombohedral, while the CaCO3 of the ES has a columnar structure [39–41], which gives them different characteristics.

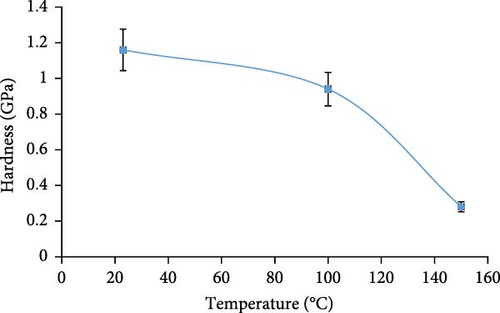
Figure 2 shows the change in hardness values of the ES with temperature. The ES hardness reached a maximum of 1.16 GPa at 23°C and decreased at greater temperatures. At 150°C, the hardness of the ES decreased by 75.9% compared to the unheated sample at 23°C. Similar to the tensile modulus results, the hardness values could not be measured using the equipment/Berkovich method due to the ES fragility and weakened structure above 150°C. According to Figure 2, the reduction of the ES hardness becomes significant above 100°C, which is similar to the reduction of the ES tensile modulus in Figure 1. In a related study, Ozguven and Ozcelik [34] studied the effect of temperature on the hardness of mineral limestone. However, the authors applied a different method, where the reported trends were similar to those obtained herein.
3.1.2. SEM Images
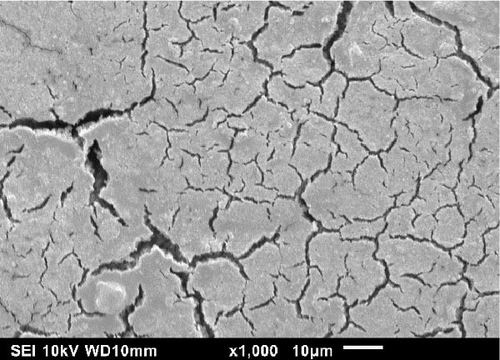
SEM images of the ES exterior and interior heated at 23, 150, 300, and 450°C are shown in Figures 3 and 4, respectively. According to Figure 3, the ES exterior structure changed from being strong and brittle at 23°C to being more fragile and weaker at higher temperatures, as shown by the cracked surface (cf., Figure 3c,d). According to the results, an increased temperature contributes to the deterioration of the ES mechanical properties, such as tensile modulus and hardness, where similar results were observed for mineral limestone [34, 35]. By increasing the temperature, the mechanical properties of limestone were reduced, and the reduction was sharper up to 200°C. A less pronounced reduction was observed from 200 to 600°C. This phenomenon is justified by the internal thermal stresses due to the thermal expansion of the CaCO3 crystals during heating, which generates microcracks [35]. By increasing the temperature beyond 200°C, there is a rapid emergence and expansion of microcracks within the CaCO3 crystals, which was observed on the exterior of the ES, as shown in Figure 3c,d. At lower temperatures of 23 and 150°C, Figure 3a,b, respectively, the effects of internal thermal stresses are reduced, and the reduction of mechanical properties is less extreme compared to higher temperatures [35]. These microcracks are also attributed to the removal of the occluded organic matrix within the columnar crystals of the palisade layer at elevated temperatures. According to the measured ES mechanical properties and SEM results, the heat treatment method is not a feasible solution for organic membrane removal, including its utility as a filler in a polymer composite material.



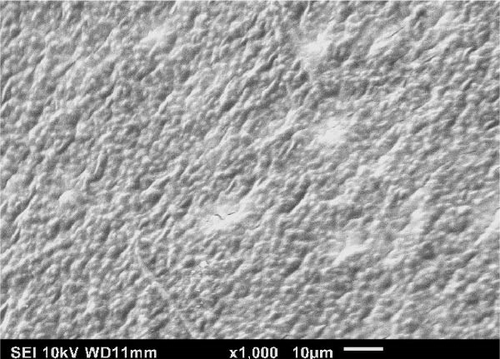



In Figure 4a, at 23°C the interior surface appears intact, while at 150°C (cf., Figure 4b), there are some holes that appear on the surface, which helps confirm the changes in fragility and weakness of the structure [4, 42]. According to Figure 4b, upon increasing the temperature to 150°C, the first inner membrane layer of the ES begins to breakdown, as depicted by the larger holes. Increasing the temperature to 300°C, the inner membrane is totally removed, and the outer membrane is visible (cf., Figure 4c) in the form of a fibrous network. The outer membrane is entirely removed at 450°C (cf., Figure 4d), and the knob structures related to the palisade CaCO3 structure layer are observed [7, 8]. Columnar calcium carbonate in the form of CaCO3 crystal structures develops from different nucleation sites, which form into knob structures [43, 44]. Therefore, the SEM results showed that the two interior ES membranes were removed at higher temperatures. In a related study, Cree and Rutter [4] reported that ES membrane removal started at 300°C and continued up to 450°C. However, at 450°C, although the knob structures remain together, it is likely that the organic substances have combusted since the mechanical properties studied were reduced at elevated temperatures. Further work is required to understand this behavior.
3.2. Chemical Treatment
According to literature reviews, different bleach solution at variable concentrations and holding times were considered to remove the ES membrane [4, 27]. In this study, the reduction of bleach solution concentration (from 50% to 25%) was investigated to determine whether the removal of the ES membrane at a lower concentration was feasible. Furthermore, the mechanical properties of chemically treated ESs were compared with thermally treated ones to gain insight into which treatment method had a less negative effect on the ES’s mechanical properties.
3.2.1. SEM Images
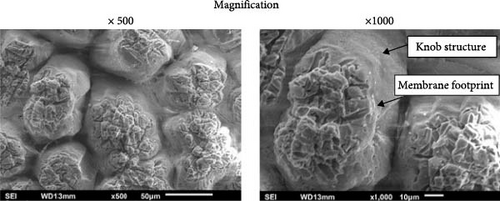
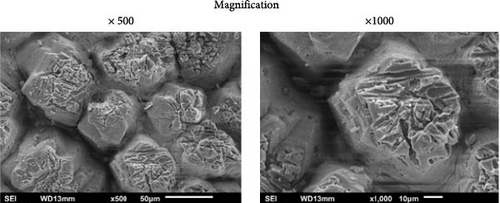
SEM images of the ESs treated with a 25% bleach solution for 10 min holding time and a sample treated with a 50% bleach solution for 10 min holding time in two different magnifications are shown in Figure 5. Comparing the SEM image for a common ES (untreated ES at 23°C), as shown in Figure 4a, a partial fibrous structure of the ES membrane was observed for untreated ES (without applying a bleach agent) surrounded with an intact surface of the ES membrane. Furthermore, according to a previous study by Cree and Rutter [4], the outer ES membrane was removed with a 50% bleach solution and 10 min holding time, which was also observed herein, as shown in Figure 5b. A reduced bleach solution concentration of 25% and 10 min holding time was considered, as shown by the SEM images in Figure 5a. The results showed that the ES membrane was removed by reducing the bleach solution from 50% to 25%. In Figure 5a,b, a footprint is observed on the knob structures of the ES. The knob structure is the calcium carbonate material, and the footprint is related to the removal of the membrane fibrous structure (Figure 4c) after treatment with the chemical bleach agent solution. Although the protein-based fibrous membrane was susceptible to the bleach solution, the knob structures in Figure 5a–c appear strongly linked together, which suggests the low concentration of bleach solution did not affect all the organic substance types present throughout the shell of the egg. In addition, the untreated and chemically treated ESs did not have a significant difference in their mechanical properties, as listed in Table 1.
| Mechanical property | Untreated ES | Chemically treated ES |
|---|---|---|
| Tensile modulus (GPa) | 43.46 ± 2.60 | 42.84 ± 3.24 |
| Hardness (GPa) | 1.16 ± 0.12 | 1.10 ± 0.86 |
- Abbreviation: ES, eggshell.
3.2.2. TGA Results
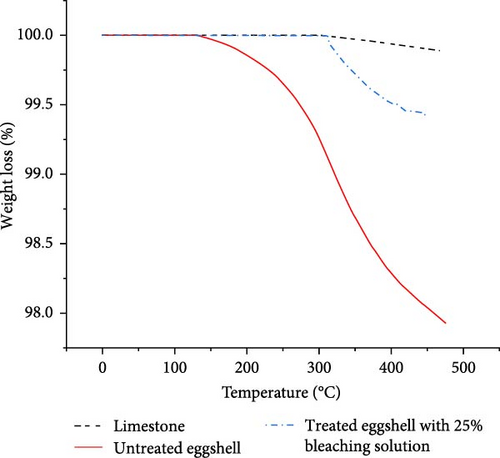
Figure 6 shows TGA results for mineral limestone, untreated ES, and ES treated with 25% bleach solution. The limestone result was added as a reference for pure calcium carbonate. According to Figure 6, the ES treated with 25% bleach solution had a similar TGA profile for limestone versus the untreated ES. The limestone had a lower weight loss (%) compared to the ES treated with 25% bleach solution, possibly due to their different limestone structures. The rhombohedral structure of limestone may have a higher strength resistance at an elevated temperature beyond 350°C compared to the columnar structure of the ES. The untreated ES had a higher weight loss (%) due to the organic membrane structure and the occluded organic matter. By removing the membrane with a 25% bleach solution, the weight loss (%) was reduced, as noted in Figure 6. For example, at 450°C, the untreated ES and 25% bleach-treated ES had weight losses of 2.09% and 0.52%, respectively. The weight loss profile begins around 100°C for the untreated ES that contains a membrane structure, which continues to 480°C. Similar results were observed for the reduction of ES mechanical properties as the temperature increased. For the ES treated with a 25% bleach solution, the slope of weight loss (%) was not steep over the range of 150–450°C since the ES membrane was removed by the chemical treatment [4, 43]. The greater weight loss of the ES treated with a 25% bleach solution, when compared to the limestone, relates to the weaker CaCO3 structure of ES versus the limestone rhombohedral structure. It appears the treated ES with a 25% bleach solution displays a decomposition of CaCO3 that begins near 330°C and continues to higher temperatures until the transition to CaO occurs. The TGA results showed that the ES membrane was removed by a 25% bleach solution. The effect of chemical treatment is revealed by the SEM images.
3.2.3. FT-IR Spectral Results
The FT-IR spectrum was used to analyze the functional groups of the untreated ES and the ES treated with 25% bleach solution, as shown in Figure 7. The ES membrane is an organic material that includes collagen, alkynes, alkanes, amines, and amides of proteins [4]. To determine whether the ES membrane and the occluded organic matter were removed with a 25% bleach solution, the broad absorption bands between 1651 and 2961 cm−1 are related to the carbonyl groups, amides, and alkanes [4, 44, 45]. According to Figure 7a, the untreated ES has IR bands in the range of 1651–2961 cm−1 related to the organic membrane, along with the functional groups of the occluded organic matter [4]. After applying a 25% bleach solution, the IR were either removed or were less intense in the range of 1651–2961 cm−1 (Figure 7b), which provides support that membrane removal was achieved.
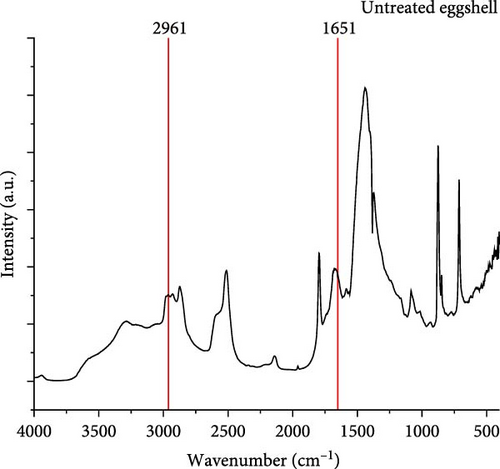
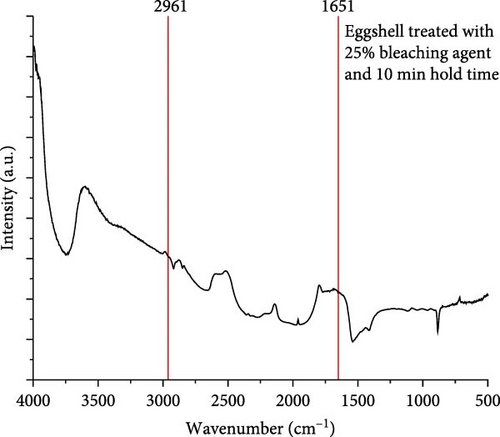
The broad absorption band near 3500 cm−1 relates to water molecules [46, 47]. This relates to the observation of absorbed moisture in the sample due to the ─OH vibration [4]. According to Figure 7, this peak is more intense for the treated ES versus the untreated ES since the bleach agent acts as an oxidative agent, which can make more hydroxyl bonds with available atmospheric moisture [4, 27].
3.2.4. Mechanical Properties
Table 1 shows a comparison between the tensile modulus and hardness properties measured using the nanoindentation technique of untreated ESs and chemically treated ESs with 25% bleach solution and 10 min holding time at 23°C.
According to Table 1, the mechanical properties (tensile modulus and hardness) of untreated ESs and chemically treated ESs were similar (within 1%). The tensile modulus and hardness properties for the two groups, chemically treated and untreated ESs, had t-values of 0.44 and 0.1, respectively. Since t-values were not greater than the pooled standard error, this suggests there is no significant difference between the mechanical properties of the chemically treated and untreated ESs [48]. According to SEM images, the ES membrane acts as a protective layer and keeps the knob structures together as a solid structure. After the ES membrane removal, the knobs act individually, and the stress concentrations were higher [4, 39]. Therefore, the mechanical properties for chemically treated ESs were lower than untreated ESs due to the total fibrous membrane removal and the organic substances to a lesser extent. These results showed that chemically treated ESs with a low concentration of bleach is a better method in terms of preserving the mineral strength properties and for effective removal of the ES membrane versus thermal (heat) treatment.
4. Conclusions
This study aimed to investigate ES membrane removal through two different methods of thermal and chemical treatments. During the investigation of the thermal treatment method, and according to the SEM images, the ES membrane was removed near 450°C, and the ES structure deteriorated due to the increased temperature. Furthermore, the results showed that the ES tensile modulus and hardness values were reduced incrementally with increased temperature. The thermal treatment was determined to be an unsuitable treatment method for removal of the membrane, if preservation of the ES mechanical properties is required. On the other hand, the effect of chemical treatment by application of a low concentration of bleach agent may be more promising over thermal techniques. The SEM images, TGA, and FT-IR results showed that with 25% bleach solution and 10 min holding time, the ES membrane was successfully removed. The mechanical properties of chemically treated ESs were similar to those of untreated ESs at 23°C. Overall, the results showed that the chemical treatment method can be considered for ES membrane removal whilst maintaining the integrity of the calcium carbonate structure. This work demonstrated the feasibility of using a low concentration of bleach solution for membrane removal, which can have potential applications in the polymer industry as a filler in composite materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Anahita Homavand: contributed to conceptualization, methodology, investigation, data curation, writing–original draft preparation, and visualization. Anahita Homavand, Duncan E. Cree, and Lee D. Wilson: contributed to formal analysis and writing–review and editing. Duncan E. Cree and Lee D. Wilson: contributed to resources, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the financial support provided by the Egg Farmers of Canada (EFC).
Acknowledgments
The authors would like to acknowledge the in-kind support of the industrial partners, Agriculture and Agri-Food Canada, Egg Solutions EPIC Inc., Star Egg Company Limited, and Burnbrae Farms Ltd. This work was carried out on Treaty 6 Territory and the Homeland of the Métis. As such, we pay our respect to the First Nations and Métis ancestors of this place and reaffirm our relationship with one another.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




