Chemical Reaction Effects on the Flow of Titanium Dioxide–Water in a Mixed Convective Nanofluid along an Inclined Plate in a Porous Medium
Abstract
An inclined plate is being approached by a mixed convective boundary layer nanofluid flow of titanium dioxide–water through a porous medium. A numerical analysis has been done to investigate the effects of chemical reactions on the considered nanofluid flow. With the aid of a system of governing partial differential equations, a set of nonlinear ordinary differential equations for taking into consideration nanofluid flow have been derived using appropriate matching transformations. The Runge–Kutta method, as well as the Nachtsheim and Swigert Shooting method, is applied to numerically resolve the group of subsequent nondimensionalized equations. The associated numerical result was then successfully compared with the available published literature in a few unique, limited circumstances. Based on the considered nanofluid flow characteristics for heat as well as mass transfer, the effects of the Schmidt number, the permeability, and the chemical reaction parameters of the titanium dioxide–water nanofluid flow have been examined, assessed, and presented for velocity in conjunction with the local skin friction coefficient, temperature in conjunction with the local Nusselt number, as well as concentration in conjunction with the local Sherwood number. Numerical results reveal that the local Sherwood number Sh, as well as the local Nusselt number Nux, increase, whereas the local skin friction coefficient Cf decreases due to the increase in the chemical reaction parameter Krp.
1. Introduction
A number of engineering purposes, as well as industrial procedures that necessitate combined heat transfer and mass transfer in the flow sector, have recently made the impact of heat and mass transfer on fluids crucial. Due to the chemical reaction involved in various mass transfer techniques, heat transfer concerns become necessary due to the nature of the process. Porous solids drying, the petroleum industry, geothermal reservoirs, the extrusion process, enriched oil recovery, wire and fiber coating, thermal insulation, food processing, the design of various heat exchangers, and nuclear reactor cooling are additional significant practical applications in the flow field through porous medium. Many academics have been engaged in the development of complex states into linear states due to its broad variety of major physical applications.
In the existence of a magnetic field, Yohannes and Shankar [1] have observed the effects of viscosity dissipation, chemical reaction, and Soret in nanofluids flowing through porous media. The governing equation for fluid flow has been solved using the Keller box method, and numerical results for different convective heat and mass transfer parameters have been presented. Haile and Shankar [2] have stated that MHD boundary layer nanofluid flow concerns heat with mass transfer via porous media by accounting for the effects of chemical reaction, viscosity dissipation, and thermal radiation. They were referred to as aluminum (Al2O3)–water and copper (Cu)–water nanofluids, and it was discovered that the magnetic field’s strength causes a drop in the velocity field. Through the work of Pal and Mandal [3] in the porous medium, the influence of chemical reaction with thermal radiation, together with mass transfer boundary layer flow in relation to the nanofluids over and done with shrinking/stretching sheet, has been explored for mixed convection.
Roy et al. [4] have computationally thought through the diffusion thermo and thermal diffusion effect on mass transfer flow based on considering fluid through an infinitely inclined vertical plate on the approach to a porous medium. They provide a graphic explanation of the physical significant properties for the different values of the related factors. The possibility exists to expand this research to include magnetohydrodynamic and viscous dissipation effects for different forms of nanofluid flow. The existence of chemical reaction impacts, Hari et al. [5] have examined and evaluated MHD mixed convection taking place in fluid flow in a porous medium with heat generation, as well as radiation, and toward a vertical plate. The velocity profile overshoots at the plate surface as the magnetic field strength increases, and it eventually converges to the boundary that they discovered. The convective heat and mass transfer features employing the copper–water and aluminum–water nanofluid flows in a porous medium, in addition to the unique impacts of the chemical reaction, have been statistically explored by Yirga and Tesfay [6]. Pal et al. [7] have numerically investigated the issue of constant magnetohydrodynamic boundary layer flow of a Casson nanofluid across a vertical stretching surface with nonlinear stretching velocity and suction. On the subject of heat and mass transmission in Casson nanofluid, considerations have been made of the combined effects of thermal radiation, Ohmic dissipation, thermophoresis, and Brownian motion by them. Krishnamurthya et al. [8] have investigated and addressed the effects of MHD Williamson nanofluid flow on radiation, melting, and chemical reaction via the porous medium.
Pandey and Kumar [9] have focused on the interaction between the significant chemical reactions and thermal radiation effects occurring in the MHD boundary layer flow of nanofluid. Khan et al. [10] have described the influence of uniform fluid suction on the non-Newtonian fluid along with the influence of heat radiation and chemical reactions on the unsteady flow through a vertically stretched porous plate. Through the entire boundary layer, they have discovered that the temperature profiles are greater based on the heat source parameter and lower based on the heat sink parameter. However, Sambasivudu [11] has concentrated on the combined effects of dissipation, thermo-diffusion, and Hall current on the hydromagnetic fluid flow over a stretching surface, including thermal and mass transfer features. The mixed convective MHD Poiseuille fluid flow based on nanofluid in a porous media has been explored by Aman et al. [12] along with influences in reference to gold nanoparticles, taking into account the unique qualities of thermal radiation, thermal diffusion, and chemical reaction. Under the action of a uniform transverse magnetic field normal to two vertical plates, Krishna et al. [13] have deliberated the oscillatory flow of an unsteady, electrically conducting, incompressible, magnetohydrodynamic, second-grade fluid through a saturated, porous medium with a heat source together with a chemical reaction. Taking into consideration Hall effects, Krishna et al. [14] have comprehensively observed the Joule, as well as Soret effects of MHD, mixed convective flow of electrically conducting fluids together with an incompressible viscous fluid via an infinite vertical porous plate.
Govardhan et al. [15] have investigated a numerical analysis on the flow of nanofluid across a stretched surface, together with the impacts of chemical reactions and viscous dissipation. The authors discovered that, based on the assumed nanofluid flow, the magnetic parameters have diminishing impacts on the velocity field while having reverse influences on the concentration and temperature fields. Hymavathi and Sakuntala [16] have considered the heat and the impacts of mass transfer based on magnetohydrodynamic visco-elastic flow through an exponentially extending sheet toward the porous medium with the assistance of chemical reaction in addition to the impact of thermal radiation. The MHD flow of an electrically conducting second-grade fluid through a porous media across a semi-infinite vertical stretching sheet has been studied by Krishna et al. [17] using thermophoresis, thermal radiation, and convective boundary conditions. The Darcy–Forchheimer flow of a magneto-couple stress fluid across an inclined exponentially extending sheet with various thermal boundary conditions has been studied theoretically by Das et al. [18]. In order to numerically solve the transformed system of nonlinear ODEs, they used the Runge–Kutta fourth-order numerical integration method based on the shooting technique. With the incorporation of numerous heat as well as mass transfer characteristics with viscous dissipation relating to copper–water nanofluid flow, Uddin [19] has computationally observed mixed convective nanofluid flow along an inclined plate in a porous medium.
Keeping in mind the abovementioned and specialized literature inquiry, we tolerate that the scrutiny of chemical reaction effects for the mixed convective flow of a laminar, steady, viscous, incompressible titanium dioxide–water nanofluid along an inclined plate in a porous medium has not been established yet. In order to fill the gap in the existing literature, chemical reaction effects on the flow of titanium dioxide–water in a mixed convective nanofluid with viscous dissipation have been considered and investigated along an inclined plate in a porous medium due to a wide range of applications in various fields like geothermal operations, petroleum industries, and boundary layer resistors in aerodynamics. The analytical and numerical results are presented along with a discussion of how various significant nondimensional parameters affect the distributions of hydrodynamic, thermal, and concentration boundary layers in velocity, temperature, and concentration, as well as other important engineering terms like friction factor, local Nusselt number, and Sherwood number.
2. Computational Analysis Together with Governing Equations, including Chemical Reaction
The chemical reaction effects of mixed convection on nanofluid flow with boundary conditions along an inclined plate in a porous medium are being taken into account when there is a viscous dissipation present. The titanium dioxide (TiO2) particle is thought to be the nanoparticle, while water is thought to be the base fluid. Additionally, local thermal equilibrium exists between the adopting nanoparticles and the base fluid. The accompanying Figure 1 displays the coordinate system for the investigation field as well as the physical description of the nanofluid flow.
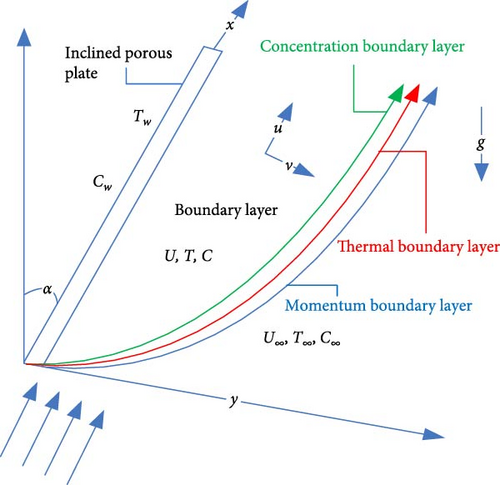
The x-axis, which indicates the flow direction, runs parallel to the plate, while the y-axis runs perpendicular to the plate in the direction of the flow. Additionally, u and v, respectively, are the velocity components in the x and y directions. In comparison to the y-direction, the radiation heat flux in the x-direction is insignificant.
An inclined plate with an inclination α is parallel to the flow field’s uniform external velocity, which is U∞. The sign g stands for acceleration-induced gravity. Nanofluid has a temperature and concentration of T and C within the boundary layer, but the temperature and concentration of the plate’s wall, Tw and Cw, are higher than the ambient conditions of T∞ and C∞.
| Particles | Thermophysical characteristics | ||||
|---|---|---|---|---|---|
| ρ (kg/m3) | Cp (J/kg.K) | k (W/m.K) | β (1/K) | σ (S/m) | |
| Water (H2O) | 997.1 | 4,179 | 0.613 | 21 × 10−5 | 0.05 |
| Copper (Cu) | 8,933 | 385 | 401 | 1.67 × 10−5 | 5.96 × 107 |
| Titanium dioxide (TiO2) | 4,250 | 686.2 | 8.9528 | 0.90 × 10−5 | 1 × 10−12 |
| Aluminum oxide (Al2O3) | 3,970 | 765 | 40 | 0.85 × 10−5 | 1 × 10−10 |
3. Techniques of Numerical Results for the Deliberated Nanofluid Flow
Using the Nachtsheim and Swigert [25] approach for discovering undefined initial conditions of dimensionless momentum, energy, and concentration equations with the aid of FORTRAN codes, the system is solved in terms of the acquired boundary value problem, such as through Equations (13), (14), and (15), and in conjunction with the associated boundary conditions Equations (16) and (17). The transformed boundary value system is retransformed as an initial value problem on its path to the initial value system after the detection of the undefined beginning circumstances.
Using sixth-order Runge–Kutta techniques, the obtained system is numerically resolved. Furthermore, the practical numerical approaches of Nachtsheim and Swigert [25] are considered, as are Al-Shimmary [26] sixth-order Runge–Kutta tactics. Moreover, the postprocessing software TECPLOT is used to present the numerical results graphically in addition to tabular form.
4. Comparison of Numerical Results
Comparisons are prepared to utilize the current numerical results, with the aid of exploring the obtained velocity results in consideration of several values for the various nanofluids flow as copper (Cu)–water, titanium dioxide (TiO2)–water, aluminum oxide (Al2O3)–water, and the outcomes acquired via Uddin [19] and Bachok et al. [20].
The corresponding numerical results for the first solution show a better outcome using Bachok et al. [20], as demonstrated in Figure 2 for various nanofluids on the velocity field with the velocity ratio parameter = −0.3, nanoparticle volume fraction = 0.1, and the Prandtl number Pr = 6.2. As a result, this favorable conventional comparison is motivated as a means of expanding the numerical approach of Nachtsheim and Swigert [25] in relation to the current work.
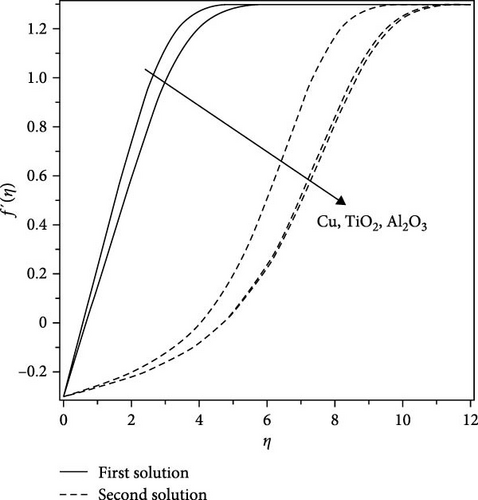
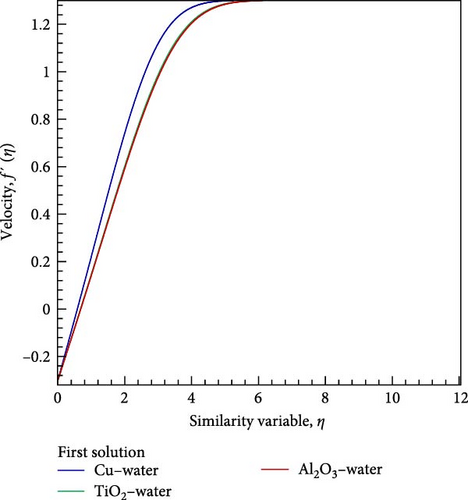
Furthermore, the acquired numerical result based on nanofluid flow has been graphically presented in the next section.
5. Results and Discussion of Significant Parameters
With the aid of different values for nondimensional parameters, thorough numerical results are worked out in relation to titanium dioxide (TiO2) and water (H2O) nanofluid flow to show flow characteristics based on the considered nanofluid. For the purpose of examining flow characteristics, the effects of the Schmidt number Sc, the permeability parameter K, and the chemical reaction parameter Krp, are all investigated. Except where otherwise specified, the values in consideration of nondimensional parameters such as Pr = 6.2, ϕ = 0.02, Ec = 0.1, Rit = 1, Ric = 1, K = 0.5, Sc = 0.60, Krp = 0.5, α = 300, and U∞/ν = 1.0 are considered.
For the sake of velocity in conjunction with the local skin friction coefficient Cf, temperature in conjunction with the local Nusselt number Nux, as well as concentration in conjunction with the local Sherwood number Sh of the flow fields, the number of factors that have contributed in addition to the engineering terms of interest are depicted graphically.
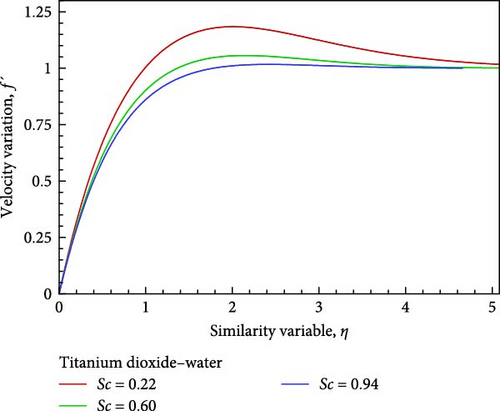
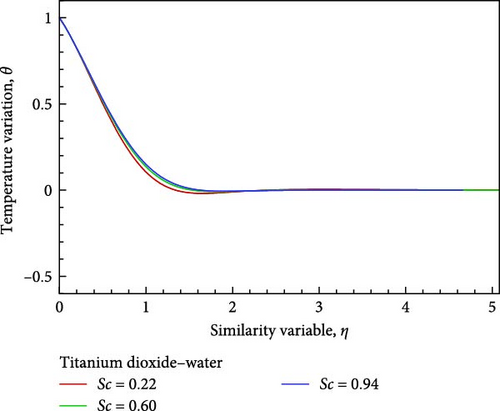
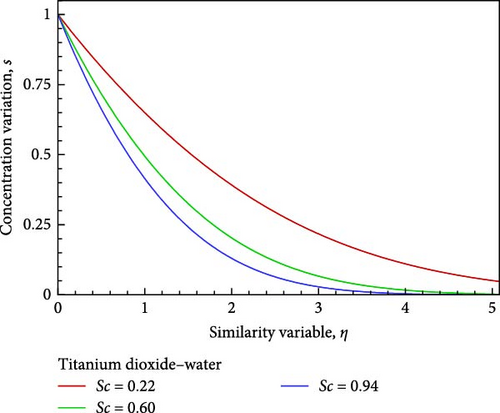
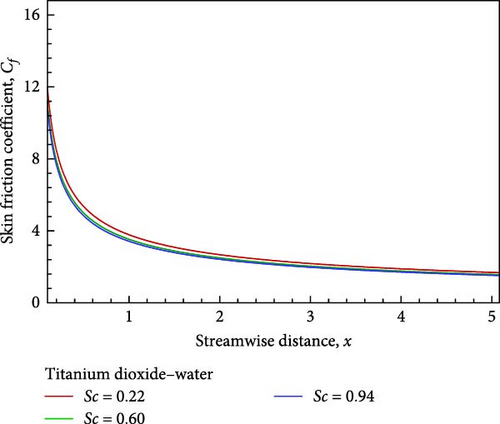
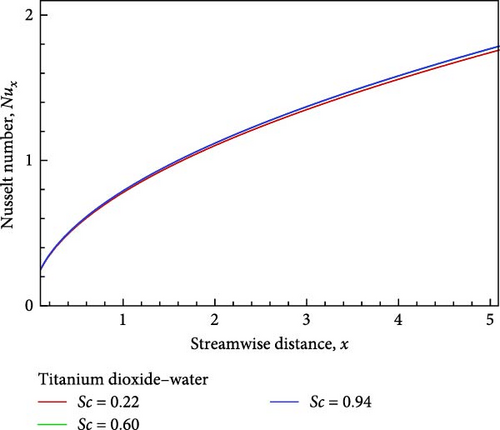
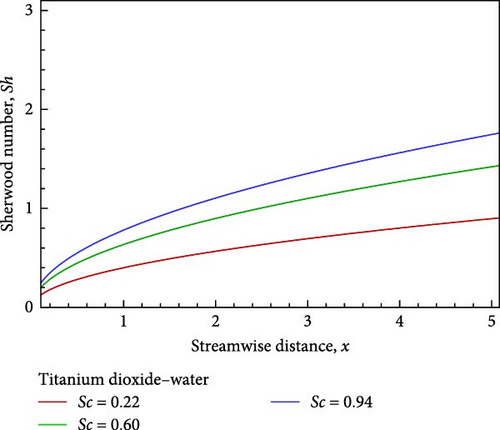
For the titanium dioxide (TiO2)–water (H2O) nanofluid, the flow field and the fluctuation of the Schmidt number Sc, which correspond to hydrogen (H2) as Sc = 0.22, water vapor (H2O) as 0.60, and benzene (C6H6) as 0.94, respectively, are shown in Figures 3(a), 3(b), 3(c), 3(d), 3(e), and 3(f). According to Figure 3(a), increasing the Schmidt number Sc generates an increase in kinematic viscosity but decreases the velocity with respect to nanofluid flow. The temperature of the flow increases next to the wall, then declines, and finally converges to the boundary condition, as illustrated in Figure 3(b), because the ambient temperature on the nanofluid flow subject is lower than the wall temperature. With the aid of Figure 3(c), it really is observed that when the Schmidt number Sc increases, the mass diffusivity associated with the investigated nanofluid flow drops, and as a result, the concentration of the field of flow lowers. Prasad et al. [27] found a comparable outcome. The local skin friction coefficient Cf decreases by way of Sc rises, as seen in Figure 3(d). But in relation to the rise in Sc, the local Sherwood number Sh and the local Nusselt number Nux also rise as a result of rising heat and mass flux, respectively. Figures 3(f) and 3(e) provide examples of this.
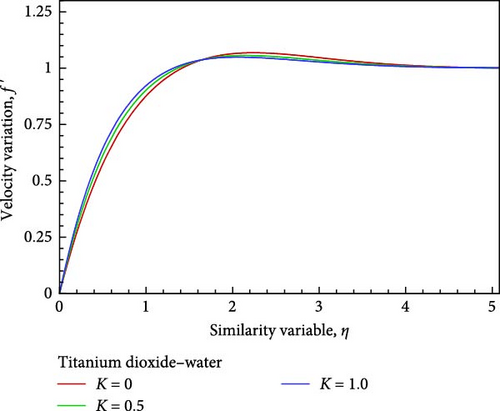
The effects of the permeability parameter K, such as K = 1, 0.5, as well as 0, on the momentum, temperature, and concentration boundary layers are depicted in Figures 4(a), 4(b), 4(c), 4(d), 4(e), and 4(f), together with the local skin friction coefficient Cf, the local Nusselt number Nux, and much more so the local Sherwood number Sh. Figure 4(a) illustrates how the velocity in proportion to the flow field within the velocity boundary layer grows near the plate, decreases in the next, and finally converges in the direction of the boundary condition as the permeability of the porous medium increases.

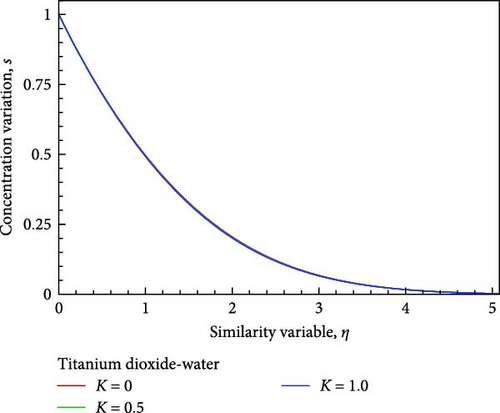
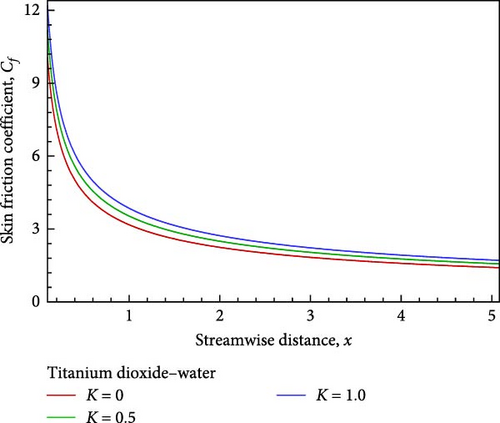
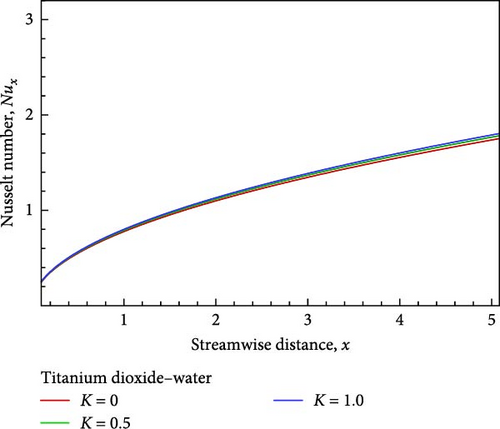
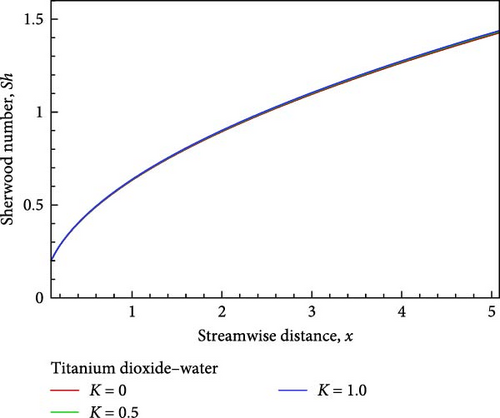
With the help of Figure 4(b), we can observe the temperature variation. Near the wall, the temperature, as well as the thickness of the thermal boundary layer in proportion to the flow field decrease, but then grows and converges as the porous medium parameter’s permeability grows. On the other hand, as shown in Figure 4(c), the concentration of the flow field gradually decreases insignificantly in relation to the increase in permeability based on the porous medium parameter. The influence of the permeability associated with the porous medium on the local skin friction coefficient, the local Nusselt number, and the local Sherwood number are displayed in Figures 4(d), 4(e), and 4(f) in relation to the streamwise distance x. The local Nusselt number, the local Sherwood number, and the local skin friction coefficients rise as the permeability parameter with regard to the porous medium increases, as shown in Figures 4(d), 4(e), and 4(f).
Using velocity in collaboration with temperature, concentration, and nanofluid flow, the impacts of different values of the chemical reaction parameter Krp, such as Krp = 0, 0.5, and 1, are exposed in Figures 5(a), 5(b), 5(c), 5(d), 5(e), and 5(f). As shown in Figures 5(a) and 5(b), the chemical reaction parameter rises as a result of the corresponding system consuming the chemical, which causes the velocity and temperature of the nanofluid flow to reduce and increase toward the wall before decreasing and convergent. A decrease in chemical molecule diffusivity caused by higher values of chemical reaction Krp in the nanofluid flow field would result in a drop in the concentration of the nanofluid flow on one occasion, the chemical reaction parameter Krp of the nanofluid flow field is increased by way of observed in Figure 5(c).
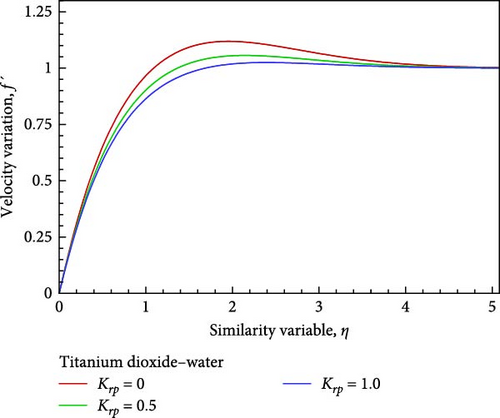
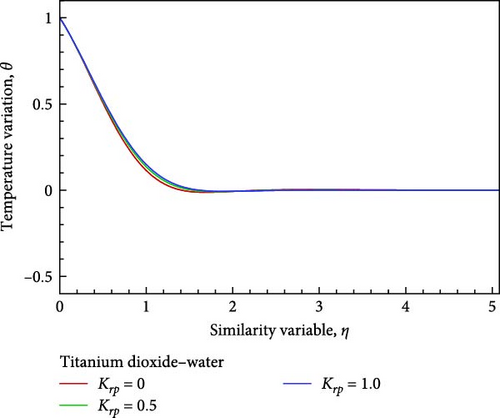
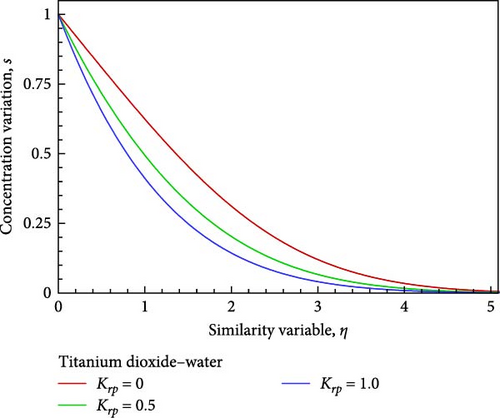
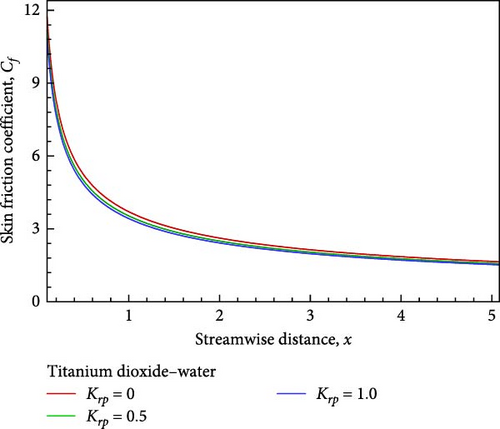
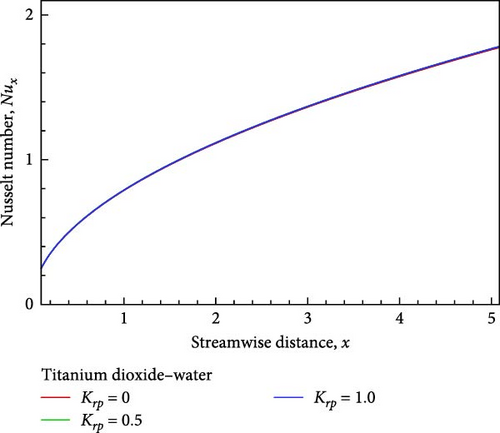
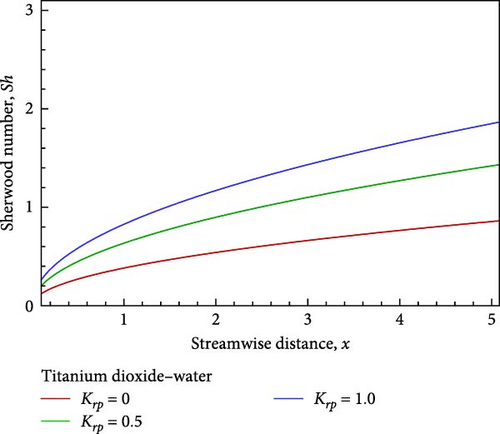
Additionally, as shown in Figure 5(c), the rising reaction parameter causes the concentration of the nanofluid flow to drop in altogether respect points of the nanofluid flow field. The local Sherwood number Sh is raised by way of a result of an increase in the chemical reaction parameter, as seen in Figures 5(d), 5(e), and 5(f), while the local skin friction coefficient Cf decreases in addition to the local Nusselt number Nux changing insignificantly.
6. Conclusion
For the sake of a recent study, a computational exploration of the mixed convective nanofluid of titanium dioxide (TiO2)– water (H2O) flow with chemical reaction impacts along an inclined plate via the porous medium is conducted with respect to viscous dissipation. As the Schmidt number Sc increases, the temperature rises, falls, and eventually converges as the velocity and concentration decrease. Meanwhile, Cf declines and Nux and Sh increase. While the temperature first drops near the wall before rising and convergent, the velocity increases near the plate, decreases in the next, and finally converges in conjunction with the concentration falls slightly, the Cf drops, but Nux and Sh rise as a result of the rising K. As the flow of nanofluid falls in velocity and concentration, it also rises in temperature near the wall before descending and converging, along with a decrease in Cf and in the meantime, Nux and Sh rise as a consequence of the rising Krp.
In order to understand how nanofluid flow behaves in complicated geometries, the engineering and industrial sectors, academics, experimentalists, and simulation specialists will be able to use the findings of this inquiry, together with information from physics, to their advantage. The present study has a broad variety of major applications; therefore, more work may be done to expand the characteristics of the nanofluid flow across more complicated geometries. The limitations of the current work can be developed for future research in the areas of unsteady as well as turbulent flow, variable permeability in porous media, temperature-dependent features of thermal conductivities, viscosity, or density, and 3D flow by taking into account the findings of the current study.
Nomenclature
-
- C:
-
- Species, mass fraction, or concentration (kg/m3)
-
- Cw:
-
- Concentration at the wall (kg/m3)
-
- C∞:
-
- Ambient concentration (kg/m3)
-
- Cf:
-
- Local skin friction coefficient
-
- Cp:
-
- Specific heat at constant pressure (J/(kg.K))
-
- D:
-
- Mass diffusivity (J/(kg.K))
-
- Ec:
-
- Eckert number
-
- f:
-
- Dimensionless stream function
-
- g:
-
- Acceleration due to gravity (m/s2)
-
- Grt:
-
- Local thermal Grashof number
-
- Grc:
-
- Local mass Grashof number
-
- K:
-
- Permeability parameter
-
- kpp:
-
- Permeability of the porous medium
-
- krp:
-
- Chemical reaction effect
-
- Krp:
-
- Chemical reaction parameter
-
- Knf:
-
- Thermal conductivity of the nanofluid (W/(m.K))
-
- Nux:
-
- Local Nusselt number
-
- Pr:
-
- Prandtl number
-
- Rit:
-
- Local Richardson number for thermal
-
- Ric:
-
- Local Richardson number for mass
-
- Rex:
-
- Local Reynolds number
-
- Sc:
-
- Schmidt number
-
- Sh:
-
- Local Sherwood number
-
- T:
-
- Temperature in the boundary layer (K)
-
- Tw:
-
- Temperature at the wall (K)
-
- T∞:
-
- Ambient temperature (K)
-
- U∞:
-
- Free stream velocity (m/s)
-
- u:
-
- Streamwise velocity component (m/s)
-
- v:
-
- Normal velocity component (m/s)
-
- x:
-
- Coordinate along the inclined plate (m)
-
- y:
-
- Coordinate normal the inclined plate (m)
Greek Symbols
-
- α:
-
- Angle of inclination (deg)
-
- αnf:
-
- Thermal diffusivity of the nanofluid (m2/s)
-
- (βt)nf:
-
- Volumetric coefficient of thermal expansion
-
- (βc)nf:
-
- Volumetric coefficient of concentration expansion
-
- η:
-
- Similarity variable
-
- ρnf:
-
- Density of the nanofluid (kg/m3)
-
- vnf:
-
- Kinematic viscosity of the nanofluid (Pa.s)
-
- ϕ:
-
- Nanoparticle volume fraction (%)
-
- θ:
-
- Dimensionless temperature
-
- qw:
-
- Heat flux (W/m2)
-
- qm:
-
- Mass flux (kg/m2.s)
-
- s:
-
- Dimensionless concentration
-
- τw:
-
- Local shear stress
-
- μnf:
-
- Dynamic viscosity of the nanofluid (kg/ms)
-
- ψ:
-
- Stream function
Subscripts
-
- w:
-
- Condition at the wall
-
- ∞:
-
- Condition at the free stream
-
- nf:
-
- Nanofluid
-
- bf:
-
- Base fluid
-
- np:
-
- Nanoparticle
Superscript
-
- “ ′ ”:
-
- Differentiation with respect to η.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




