The Evaluation for Expandable Applications of Tislelizumab in First-Line Treatment for Advanced Gastric Cancer
Abstract
Programmed death receptor-1 monoclonal antibodies (PD-1 mAbs) have been applied in the treatment of different kinds of malignant tumors. However, a streamlined and expedited evaluation method for certain tumor types without approved indications is currently lacking in terms of their expandable applications. In this study, a novel evaluation method for the expandability of PD-1 mAb was established for the first time. Clinical trial data of PD-1 mAb in first-line treatment for advanced gastric cancer were collected for comparison. For the first time, the clinical trial outcomes were analyzed through the entropy weight method and the technique for order preference by similarity to ideal solution (TOPSIS) method to evaluate the effectiveness and safety. The accessibility was assessed using the World Health Organization/Health Action International (WHO/HAI) standard survey method. Combining the results of effectiveness, safety, and accessibility, the recommendation for expandability of PD-1 mAb was provided. Tislelizumab ranks seventh in effectiveness, higher than the chemotherapy group and the pembrolizumab group, and ranks fourth in safety evaluation and first in the combination chemotherapy groups. The annual drug cost of tislelizumab is 0.497 times the annual household income for urban residents of Shaanxi Province. 56.67% of medical institutions are equipped with tislelizumab in Shaanxi Province. These results indicate the promising efficacy and safety profile of tislelizumab in combination with chemotherapy as a first-line treatment option for advanced gastric cancer. Notably, tislelizumab emerges as a more accessible alternative to sintilimab and boasts greater affordability compared to nivolumab and pembrolizumab. Consequently, tislelizumab should be considered a viable option for expandable application in first-line treatment of advanced gastric cancer, contingent upon clinical necessity.
1. Introduction
The incidence and mortality rates of gastric cancer both rank among the top five globally, making it one of the most common malignant tumors [1]. The primary treatment modalities for advanced gastric cancer have evolved from initial regimens based on 5-fluorouracil to the incorporation of platinum-based drugs, and now, with the continuous emergence of novel chemotherapy drugs. However, these treatments have not yet extended the overall survival (OS) of gastric cancer patients to more than one year [2]. Immunotherapy has brought new hope for gastric cancer patients, with the CheckMate-649 trial establishing the position of PD-1 inhibitors, in combination with chemotherapy, as a first-line treatment for advanced gastric cancer [3]. Currently, programmed death receptor-1 monoclonal antibodies (PD-1 mAbs) such as nivolumab have received approval for the first-line treatment of advanced gastric cancer.
However, due to the unaffordability or unavailability of nivolumab and pembrolizumab for patients in some regions, other similar drugs such as tislelizumab may sometimes be used as substitutes. Tislelizumab is a new drug developed by BeiGene, Ltd., in December 2019. The overlap between its Fab segment/PD-1 binding interface and PD-1/PD-ligand 1 (PD-L1) binding interface reaches as high as 82%, enabling a more thorough blockade of PD-1/PD-L1 interaction. This significantly reduces the probability of off-target effects. Tislelizumab has a slow dissociation rate, higher affinity with PD-1, and can exert a prolonged effect. As a humanized mAb, the variable region of tislelizumab is derived from mice, while the constant region is from humans. Tislelizumab has low IC50 and EC50 values, indicating potent inhibition of cancer cell activity [4]. Moreover, its extended half-life contributes to enhanced antitumor activity [5]. So far, tislelizumab has registered indications worldwide [6]. It has been approved for several indications in China, including advanced melanoma, non–small-cell lung cancer, and esophageal squamous cell carcinoma (ESCC). In 2023, the drug received approval from the European Medicines Agency for use in ESCC in the European Union. Building on this momentum, tislelizumab received approval from the Food and Drug Administration in the United States to treat adult patients with unresectable or metastatic ESCC who have progressed after prior systemic chemotherapy. Regarding gastric cancer treatment, there have been an increasing number of clinical trials conducted on tislelizumab. For example, in the BGB-A317-205 trial, tislelizumab combined with chemotherapy has demonstrated durable clinical benefits and manageable tolerability [7]. For patients with advanced gastric cancer receiving first-line platinum-based chemotherapy combined with fluoropyrimidines, the overall response rate (ORR) was 46.7% and the median OS was 15.4 months. The treatment-emergent adverse events (TEAE) observed mainly included weakness, thrombocytopenia, nausea, and vomiting, among others. No new significant adverse reactions were reported. In addition, a real-world study also proved that tislelizumab plus chemotherapy has good efficacy and safety for patients with locally advanced gastric cancer. In this study, neoadjuvant chemotherapy plus tislelizumab significantly improved the efficacy and R0 resection rate of locally advanced gastric cancer without increasing the incidence of perioperative complications, particularly in esophagogastric junction carcinoma [8]. Although tislelizumab has shown potential for the treatment of advanced gastric cancer, the current research evidence is not yet sufficient. Using alternatives without adequate evidence may lead to uncertain treatment outcomes and an increased risk of adverse reactions. This poses irreparable losses for both the medical professionals and patients involved.
To ensure medical safety and safeguard the rights of medical professionals and patients, it is of certain urgency and necessity for medical institutions to establish standardized management protocols and implementation procedures for the expandable applications of drugs. Expandability of drugs refers to the process where physicians, under special circumstances where there is no better treatment available, establish a management system within their institution to regulate the use of drugs for which the drug label is not explicit but has evidence-based medicine proof. This process involves physicians implementing clinical drug treatment after obtaining informed consent from the patient. The expanded drug application should, to some extent, promote the accessibility of effective drugs [9]. The scope of expandability includes indications, dosage, duration of treatment, administration routes, or specific populations.
Expanded drug applications are common in medical practice, particularly among special populations such as cancer patients. However, existing evidence-based evaluation methods are time-consuming and require a high level of research literacy evaluators. It often leads to situations where patients urgently need medication, but systematic comprehensive evaluations are challenging to complete in a timely manner. Currently, the Phase II clinical trial of tislelizumab in the first-line treatment for advanced gastric cancer has been completed. In special circumstances, such as a lack of accessibility to other similar drugs, can tislelizumab be expanded?
In this article, to address practical clinical concerns and present a preliminary assessment of the expandability of tislelizumab as a first-line treatment for advanced gastric cancer, various aspects, including effectiveness, safety, and accessibility, were evaluated. Based on these evaluations, a rapid clinical selection and rational application method for PD-1 mAb was established for the first time, utilizing the combination of the entropy weight method and the technique for order preference by similarity to ideal solution (TOPSIS) method. This approach aims to enhance the rationality of drug usage in clinical practice.
2. Methods
2.1. Evaluation Method
Principle: The expanded value of tislelizumab in the first-line treatment was evaluated for advanced gastric cancer from the dimensions of effectiveness, safety, and accessibility. Basis: Clinical trial data reported in the literature, drug labels, and relevant guidelines. Schematic diagram of the assessment process for the expandable application is illustrated in Figure 1.
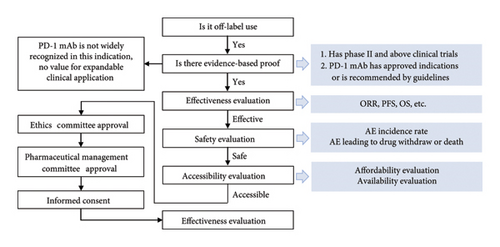
2.1.1. Effectiveness and Safety Evaluation Methods
Clinical trial results of PD-1 mAb in the first-line treatment for advanced gastric cancer were retrieved from the literature for effectiveness and safety assessments. Due to the limited availability of Phase II and above clinical trial data for tislelizumab, making a high-quality network meta-analysis was not feasible. Therefore, we introduce a novel effectiveness and safety evaluation method that combines both entropy weight method and TOPSIS method.
The entropy weight method is a multicriteria comprehensive evaluation approach that leverages the concept of entropy in information theory to determine weights for evaluation indicators. Its strengths lie in simplicity (eschewing complex mathematical models), objectivity (eliminating subjective biases in weight assignment), and comprehensiveness (considering both correlations and disparities among indicators, ideal for intricate multicriteria evaluations). However, it can be time-consuming for large datasets and primarily suits discrete data, with limited capacity for continuous data. The EWM finds application in economic evaluations, environmental assessments, and project selections. TOPSIS, proposed by C. L. Hwang and K. Yoon in 1981, is a multicriteria decision analysis technique that ranks alternatives based on their proximity to an ideal (best) solution and distance from a negative ideal (worst) solution. The closer an alternative is to the ideal and further from the negative ideal, the better it is considered. TOPSIS advantages include simplicity and intuitive operation, comprehensive treatment of interactions and trade-offs among criteria, and robustness to minor data errors. Nevertheless, it requires normalization of data into dimensionless relative indicators, which may introduce uncertainty and subjectivity. TOPSIS results are sensitive to weight settings and often assume static ideal and negative ideal solutions, which may vary in real-world decisions. The method is widely used in market research, environmental evaluations, and policy formulation [10, 11].
- 1.
Imputation of missing value: For missing data, the median was used for imputation.
- 2.
Data forward/reverse processing: (a) Combination therapy data are positive indicators and require no processing, (b) safety data are reverse indicators and need to be reverse-transformed using the “data processing–generated variable” reverse function to convert them into positive indicators.
- 3.
Data standardization: Data standardization was performed to address dimensional issues. The processing method in this study is normalization, compressing all data between 0 and 1.
- 4.
Entropy weight method for weight calculation: Weight calculation using the entropy weight method, multiplying the weights by the data to obtain new data.
- 5.
TOPSIS method calculation: Using the TOPSIS method to calculate and obtain the rankings for each research protocol.
Currently, only PD-1 mAb combination therapy with chemotherapy obtained approval indications in the first-line treatment of advanced gastric cancer. In clinical trials related to tislelizumab, only the combination therapy with XELOX has reached the Phase II clinical trial stage. Based on the principles of “has Phase II and above clinical trials” and “PD-1 mAb has approved indications or is recommended by guidelines,” the focus of effectiveness and safety evaluation is specifically directed toward PD-1 mAb in combination with chemotherapy.
2.1.2. Accessibility Evaluation Methods
Accessibility is usually divided into affordability and availability, and specific methods are as follows.
Affordability evaluation: Affordability focuses on PD-1 mAb with retrievable Phase II and above clinical trial results. The dosage and usage information are obtained from the drug label, and the annual treatment cost of the drug is calculated. The WHO/HAI standard survey method is used for evaluation. Considering the relatively long treatment period for anticancer drugs and the fact that medical expenses for patients in China are generally borne by the entire family, this study refines the WHO/HAI standard survey method by calculating the annual drug cost of the disease as a multiple of the annual income of urban and rural residents. A multiple < 1 indicates affordability, while ≥ 1 indicates unaffordability [12].
Availability evaluation: Availability evaluation uses simple random sampling. Considering that the first chemotherapy regimen for advanced gastric cancer patients is generally formulated in tertiary hospitals, we take tertiary hospitals in urban areas of Shaanxi Province as an example. All tertiary hospitals in urban areas of Shaanxi Province are sorted and coded alphabetically. Each hospital is assigned a random number between 0 and 1 instead of the hospital code. The numbers are then sorted from the largest to the smallest, and the top 30 hospitals are selected for the study. Telephone consultations are conducted to investigate the drug procurement situation. A procurement rate > 80% indicates good availability, 50%–80% indicates relatively good availability, and < 50% indicates low availability [13].
2.2. Literature Retrieval Methods
After consulting drug labels and relevant guidelines, nivolumab, pembrolizumab, and sintilimab in combination with chemotherapy have been approved for first-line treatment indications or recommended by guidelines for advanced gastric cancer. Therefore, these drugs are considered as comparative agents for tislelizumab. Clinical studies involving interventions for the first-line treatment of advanced gastric cancer with tislelizumab, nivolumab, pembrolizumab, or sintilimab were searched from databases such as Cochrane Library, PubMed, Embase, Web of Science, and others. For trials with multiple publications of the same data, the reference containing most comprehensive data was retained. The search was conducted up to August 31, 2023. The search terms included gastroesophageal junction carcinoma, advanced gastric cancer, first-line therapy, tislelizumab, nivolumab, pembrolizumab, and sintilimab. The schematic diagram of the literature search process is illustrated in Figure 2.
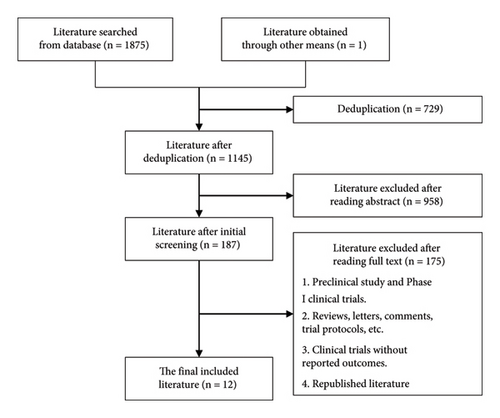
2.3. Inclusion and Exclusion Criteria
Inclusion criteria include the following: (1) Phase II or above clinical trials or real-world studies; (2) primary treatment involving PD-1 mAb or their combination in the first-line treatment for advanced gastric cancer; and (3) primary endpoint includes OS, progression-free survival (PFS), ORR, adverse event (AE) incidence rate, and immune-related adverse events (irAE) incidence rate.
Exclusion criteria include the following: (1) clinical trials in which the experimental or control group received nonpharmacological interventions such as radiotherapy; (2) clinical trials with undisclosed or inaccessible results; (3) the literature published in non-Chinese or non-English languages, such as Japanese or German; (4) republished clinical trials; (5) clinical trials that did not report or had inaccessible outcomes; and (6) reviews, letters, comments, and trial protocols.
3. Results
3.1. Basic Information
After the literature search and consultation of guidelines and drug labels, the PD-1 mAb currently used in the first-line treatment for advanced gastric cancer mainly include nivolumab, pembrolizumab, sintilimab, and tislelizumab. Among them, nivolumab and pembrolizumab have been recommended for the first-line treatment of advanced gastric cancer by both the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO) guidelines. Although PD-1 mAb have the same mechanism of action, blocking the binding of PD-1 to PD-L1 to eliminate the inhibition of tumor cell activity on T-cells and enhancing the ability of T cells to kill tumor cells, there are differences in the nature of different PD-1 mAbs (Table 1). Before undergoing expandability evaluation, they should not be simply substituted in clinical use.
| Name | Source | EC50(nmol/L) | IC50 | Vd (L) | CL (mL/d) | T1/2(d) |
|---|---|---|---|---|---|---|
| Nivolumab | Fully humanized | 0.64 | 2.92 nmol/L | 6.80 | 197 | 25.0 |
| Pembrolizumab | Humanized | 0.07 | 0.60 nmol/L | 6.10 | 212 | 21.6 |
| Sintilimab | Fully humanized | 2.20 | 4.49 μg/mL | 4.71 | 239 | 13.7 |
| Tislelizumab | Humanized | 0.12 | 0.50 nmol/L | 5.24 | 164 | 26.0 |
- Abbreviations: CL, plasma clearance; EC50, concentration for 50% of maximal effect; IC50, median inhibition concentration; T1/2, half-life; Vd, apparent volume of distribution.
3.2. Effectiveness Evaluation
The Phase II and above clinical trial results of tislelizumab, nivolumab, pembrolizumab, and sintilimab in the first-line treatment for advanced gastric cancer were obtained through literature studies [14–24]. Relevant clinical trial information and effectiveness data were summarized (as shown in Table 2). The trial results were processed using the SPSSAU project, and the weights of various effectiveness indicators were determined through the entropy weight method (Table 3). The effectiveness of tislelizumab was evaluated using the TOPSIS method (Table 4). The results show that in the effectiveness evaluation process, the weights of OS and PFS are higher than the objective response rate, which is consistent with clinical reality, indicating that this method has a certain reference value. Through the TOPSIS method for effectiveness ranking, it was found that although the effectiveness of tislelizumab in combination with chemotherapy is not as good as nivolumab in combination with chemotherapy and sintilimab in combination with chemotherapy, it is higher in ranking than pembrolizumab in combination with chemotherapy and chemotherapy alone, indicating its effectiveness in the first-line treatment of advanced gastric cancer.
| Trials | Drug | Phase | Region | Patient selection | n | Arms (regimen) | OS | PFS | ORR | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Med | HR | p | Med | HR | p | % | p | ||||||||
| Monotherapy | |||||||||||||||
| BGB-A317 (001 & 102) | Tisl | I/II | China | TMB-high | 11 | Tisl | 12.9 | / | 0.18 | 2.8 | / | 0.240 | 36.4 | 0.014 | [14] |
| TMB-low | 52 | Tisl | 5.2 | 2.0 | 5.8 | ||||||||||
| HA+ | 30 | Tisl | 4.2 | / | 0.0072 | 1.9 | / | 0.018 | 20.0 | 0.055 | |||||
| HA− | 44 | Tisl | 11.6 | 2.1 | 4.6 | ||||||||||
| KEYNOTE-062 | Pemb | III | Global | CPS ≥ 1 | 256 | Pemb | 10.6 | 0.91 | / | 2.0 | 1.66 | / | 15.0 | / | [15] |
| HER2-neg | 250 | XP/FP | 11.1 | 6.4 | 37.2 | ||||||||||
| CPS ≥ 10 | 92 | Pemb | 17.4 | 0.69 | / | 2.9 | 1.10 | / | 25.0 | / | |||||
| HER2-neg | 90 | XP/FP | 10.8 | 6.1 | 38.0 | ||||||||||
| Combined with chemotherapy | |||||||||||||||
| BGB-A317-205 | Tisl | II | China | HER2-neg | 15 | Tisl + XELOX | 15.4 | / | / | 6.1 | / | / | 46.7 | / | [16] |
| KEYNOTE-062 | Pemb | III | Global | CPS ≥ 1 | 257 | Pemb + XP/FP | 12.5 | 0.85 | 0.05 | 6.9 | 0.84 | 0.04 | 48.6 | / | [15] |
| HER2-neg | 250 | XP/FP | 11.1 | 6.4 | 37.2 | ||||||||||
| CPS ≥ 10 | 99 | Pemb + XP/FP | 12.3 | 0.85 | 0.16 | 5.7 | 0.73 | / | 53.0 | / | |||||
| HER2-neg | 90 | XP/FP | 10.8 | 6.1 | 38.0 | ||||||||||
| CheckMate-649 | Nivo | III | Global | CPS ≥ 5 | 473 | XELOX/FOLFOX + Nivo | 14.4 | 0.71 | < 0.0001 | 7.7 | 0.68 | < 0.0001 | 60 | / | [17] |
| HER2-neg | 482 | XELOX/FOLFOX | 11.1 | 6.0 | 45 | ||||||||||
| CPS ≥ 1 | 641 | XELOX/FOLFOX + Nivo | 14.0 | 0.77 | < 0.0001 | 7.5 | 0.74 | / | 60 | / | |||||
| HER2-neg | 655 | XELOX/FOLFOX | 11.3 | 6.9 | 46 | ||||||||||
| All | 789 | XELOX/FOLFOX + Nivo | 13.8 | 0.80 | 0.0002 | 7.7 | 0.77 | / | 58 | ||||||
| HER2-neg | 792 | XELOX/FOLFOX | 11.6 | 6.9 | 46 | ||||||||||
| ATTRACTION-4 | Nivo | III | Asia | All | 38 | XELOX/SOX + Nivo | 17.5 | 0.90 | 0.2570 | 10.5 | 0.68 | 0.0007 | 57.5 | 0.0088 | [18] |
| HER2-neg | / | XELOX/SOX | 17.2 | 8.3 | 47.8 | ||||||||||
| ORIENT-16 | Sint | III | China | CPS ≥ 5 | 197 | XELOX + Sint | 18.4 | 0.660 | 0.0023 | 7.7 | 0.628 | 0.0002 | / | / | [19] |
| HER2-neg | 200 | XELOX | 12.9 | 5.8 | / | ||||||||||
| All | 327 | XELOX + Sint | 15.2 | 0.766 | 0.0090 | 7.1 | 0.636 | < 0.0001 | 58.2 | / | |||||
| HER2-neg | 323 | XELOX | 12.3 | 5.7 | 48.4 | ||||||||||
| Combined with anti-HER2 targeted therapy | |||||||||||||||
| BGB-A317-ZW25-101 | Tisl | Ib/II | Asia | HER2-pos | 33 | Tisl + ZW25 + CAPOX | / | / | / | 10.9 | / | / | 72.7 | / | [20] |
| KEYNOTE-811 | Pemb | III | Global | HER2-pos | 133 | FP/XELOX + Tmab + Pemb | / | / | / | / | / | / | 74.4 | 0.00006 | [21] |
| 131 | FP/XELOX + Tmab | 51.9 | |||||||||||||
| Combined with CTLA-4 inhibitor | |||||||||||||||
| CheckMate-649 | Nivo | III | Global | CPS ≥ 5 | / | Nivo + Ipi | 11.2 | 0.89 | 0.2302 | 2.8 | 1.42 | / | 27 | / | [17] |
| / | XELOX/FOLFOX | 11.6 | 6.3 | 47 | |||||||||||
| All | / | Nivo + Ipi | 11.7 | 0.91 | / | 2.8 | 1.66 | / | 23 | / | |||||
| / | XELOX/FOLFOX | 11.8 | 7.1 | 47 | |||||||||||
| Combined with tyrosine kinase inhibitor (TKI) | |||||||||||||||
| BGB-900-104 | Tisl | I/II | China | All | 24 | Sitr + Tisl | / | / | / | 3.4 | / | / | 12.5 | / | [22] |
| EPOC1706 | Pemb | II | Japan | All | 29 | Lenva + Pemb | 12.3 | / | / | 5.6 | / | / | 44 | / | [23, 24] |
- Abbreviations: CPS, combined positive score; HR, hazard ratio; Ipi, ipilimumab; Lenva, lenvatinib; Med, median; Nivo, nivolumab; Pemb, pembrolizumab; Sint, sintilimab; Sitr, sitravatinib; Tisl, tislelizumab; Tmab, trastuzumab; TMB, tumor mutation burden (≥ 8 mut/Mb are defined as TMB high and < 8 mut/Mb are defined as TMB low); ZW25, zanidatamab.
| Clinical outcomes | Information entropy | Utility value | Weight coefficient (%) |
|---|---|---|---|
| OS | 0.823 | 0.177 | 40.75 |
| PFS | 0.8456 | 0.1544 | 35.54 |
| ORR | 0.897 | 0.103 | 23.71 |
| Trials | Phase | Region | Patient selection | Arms (regimen) | Positive ideal solution distance | Negative ideal solution distance | Relative approach degree | Sort |
|---|---|---|---|---|---|---|---|---|
| BGB-A317-205 | II | China | HER2-neg | Tisl + chemo | 0.450 | 0.209 | 0.317 | 7 |
| KEYNOTE-062 | III | Global | CPS ≥ 1 | Pemb + chemo | 0.517 | 0.096 | 0.157 | 9 |
| HER2-neg | ||||||||
| CPS ≥ 10 | Pemb + chemo | 0.555 | 0.112 | 0.168 | 8 | |||
| HER2-neg | ||||||||
| CheckMate-649 | III | Global | CPS ≥ 5 | Nivo + chemo | 0.338 | 0.313 | 0.480 | 3 |
| HER2-neg | ||||||||
| CPS ≥ 1 | Nivo + chemo | 0.368 | 0.295 | 0.444 | 5 | |||
| HER2-neg | ||||||||
| All | Nivo + chemo | 0.372 | 0.269 | 0.420 | 6 | |||
| HER2-neg | ||||||||
| ATTRACTION-4 | III | Asia | All | Nivo + chemo | 0.075 | 0.533 | 0.877 | 1 |
| HER2-neg | ||||||||
| ORIENT-16 | III | China | CPS ≥ 5 | Sint + chemo | 0.212 | 0.474 | 0.691 | 2 |
| HER2-neg | ||||||||
| All | Sint + chemo | 0.332 | 0.301 | 0.475 | 4 | |||
| HER2-neg | Chemo | 0.579 | 0.030 | 0.050 | 10 | |||
- Abbreviations: CPS, combined positive score; Nivo, nivolumab; Pemb, pembrolizumab; Sint, sintilimab; Tisl, tislelizumab.
3.3. Safety Evaluation
The safety results of clinical trials for tislelizumab, nivolumab, pembrolizumab, and sintilimab used in the first-line treatment of advanced gastric cancer are presented in Table 5 [15–25]. Similar to the effectiveness evaluation, safety was assessed using the entropy weight method and the TOPSIS method. The results indicate that the safety of tislelizumab in combination with chemotherapy is superior to that of nivolumab or sintilimab in combination with chemotherapy, demonstrating the favorable safety profile of tislelizumab (Tables 6 and 7).
| Trials | Arms (regimen) | n | TrAE | irAE | TEAE | SAE | AE leading to interruption | AE leading to death | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | ≥ 3 | All | ≥ 3 | All | ≥ 3 | |||||||
| BGB-A317-102 | Tisl | 300 | / | 99 (33) | / | 33 (11) | / | 119 (40) | 76 (25) | 25 (8) | 12 (9) | [25] |
| KEYNOTE-062 | Pemb | 254 | 138 (54) | 43 (17) | 54 (21) | 15 (6) | / | / | / | 4 (2) | 1 (0.4) | [15] |
| Pemb + chemo | 250 | 235 (94) | 183 (73) | 60 (24) | 14 (6) | / | / | / | 11 (4) | 1 (0.4) | ||
| Chemo | 244 | 224 (92) | 169 (70) | 19 (8) | 4 (2) | / | / | / | 2 (1) | 1 (0.4) | ||
| BGB-A317-205 | Tisl + chemo | 15 | 13 (87) | / | / | / | 15 (100) | 10 (67) | 5 (33) | / | 0 (0) | [16] |
| CheckMate-649 | Nivo + chemo | 782 | 738 (94) | 466 (60) | / | / | / | / | 172 (22) | 284 (36) | 16 (2) | [17] |
| Chemo | 767 | 679 (89) | 341 (45) | / | / | / | / | 93 (12) | 181 (24) | 4 (0.5) | ||
| ATTRACTION-4 | Nivo + chemo | 39 | 39 (100) | 24 (62) | / | / | / | / | 16 (41) | 7 (18) | / | [18] |
| ORIENT-16 | Sint + chemo | 328 | 319 (97) | 196 (60) | / | / | / | / | 86 (26) | 38 (12) | 6 (2) | [19] |
| Chemo | 320 | 308 (96) | 168 (53) | / | / | / | / | 70 (22) | 25 (8) | 2 (0.6) | ||
| BGB-A317-ZW25-101 | Tisl + ZW25 chemo | 33 | 33 (100) | 20 (61) | 9 (27) | 7 (21) | 33 (100) | 24 (73) | / | / | 2 (6) | [20] |
| KEYNOTE-811 | Pemb + Tmab + chemo | 217 | / | / | 73 (33.6) | 21 (10) | 211 (97) | 124 (57) | 68 (31) | 53 (24) | 7 (3) | [21] |
| Tmab + chemo | 216 | / | / | 45 (20.8) | 7 (3) | 212 (98) | 124 (57) | 83 (38) | 56 (26) | 10 (5) | ||
| BGB-900-104 | Sitr + Tisl | 24 | / | / | / | / | 23 (96) | 12 (50) | 11 (46) | 5 (21) | / | [22] |
| EPOC1706 | Lenva + Pemb | 29 | / | 13 (44.8) | / | / | / | / | / | / | / | [23, 24] |
- Abbreviations: AE, adverse events; irAE, immune-related adverse events; Lenva, lenvatinib; Nivo, nivolumab; Pemb, pembrolizumab; SAE, serious adverse events; Sint, sintilimab; Sitr, sitravatinib; TEAE, treatment-emergent adverse events; Tisl, tislelizumab; Tmab, trastuzumab; TrAE, treatment-related adverse events; ZW25, zanidatamab.
| Clinical outcomes | Information entropy | Utility value | Weight coefficient (%) |
|---|---|---|---|
| TrAE-All | 0.7541 | 0.2459 | 31.55 |
| TrAE-≥ 3 | 0.8915 | 0.1085 | 13.92 |
| irAE-All | 0.9569 | 0.0431 | 5.53 |
| irAE-≥ 3 | 0.9597 | 0.0403 | 5.17 |
| TEAE-All | 0.8853 | 0.1147 | 14.72 |
| TEAE-≥ 3 | 0.9367 | 0.0633 | 8.13 |
| SAE | 0.9406 | 0.0594 | 7.62 |
| AE leading to interruption | 0.9459 | 0.0541 | 6.94 |
| AE leading to death | 0.9501 | 0.0499 | 6.41 |
- Abbreviations: AE, adverse events; irAE, immune-related adverse events; SAE, serious adverse events; TEAE, treatment-emergent adverse events; TrAE, treatment-related adverse events.
| Trials | Arms (regimen) | Positive ideal solution distance | Negative ideal solution distance | Relative approach degree | Sort |
|---|---|---|---|---|---|
| BGB-A317-102 | Tisl | 0.305 | 0.175 | 0.364 | 3 |
| KEYNOTE-062 | Pemb | 0.111 | 0.373 | 0.771 | 1 |
| KEYNOTE-062 | Pemb + chemo | 0.327 | 0.142 | 0.303 | 7 |
| BGB-A317-205 | Tisl + chemo | 0.3 | 0.142 | 0.321 | 4 |
| CheckMate-649 | Nivo + chemo | 0.324 | 0.133 | 0.291 | 8 |
| ATTRACTION-4 | Nivo + chemo | 0.359 | 0.108 | 0.232 | 10 |
| ORIENT-16 | Sint + chemo | 0.335 | 0.131 | 0.281 | 9 |
| BGB-A317-ZW25-101 | Tisl + ZW25 | 0.383 | 0.079 | 0.171 | 11 |
| KEYNOTE-811 | Pemb + Tmab + chemo | 0.314 | 0.14 | 0.309 | 6 |
| BGB-900-104 | Sitr + Tisl | 0.311 | 0.184 | 0.372 | 2 |
| EPOC1706 | Lenva + Pemb | 0.308 | 0.14 | 0.313 | 5 |
- Abbreviations: Lenva, lenvatinib; Nivo, nivolumab; Pemb, pembrolizumab; Sint, sintilimab; Sitr, sitravatinib; Tisl, tislelizumab; Tmab, trastuzumab; ZW25, zanidatamab.
3.4. Accessibility Evaluation
The annual household income for urban and rural residents is calculated based on the “Shaanxi Statistical Yearbook 2021.” The annual household income for urban residents is 37,868 × 2.62 = 99,214.16 yuan, and for rural residents is 13,316 × 2.62 = 34,887.92 yuan. Sintilimab and tislelizumab are affordable for urban residents but not for rural residents. On the other hand, nivolumab and pembrolizumab are not affordable for both urban and rural residents (Table 8). Therefore, we primarily investigated the availability of sintilimab and tislelizumab in urban tertiary hospitals. In 62 tertiary hospitals in urban areas of Shaanxi Province, China, 30 hospitals were randomly sampled to inquire about their drug availability (Table 9).
| Indexes | Tisl | Nivo | Pemb | Sint |
|---|---|---|---|---|
| Annual drug cost (yuan) | 49,300 | 110,652 | 143,344 | 36,720 |
| WHO/HAI (urban residents) | 0.497 | 1.115 | 1.445 | 0.370 |
| WHO/HAI (rural residents) | 1.413 | 3.172 | 4.109 | 1.053 |
- Abbreviations: Nivo, nivolumab; Pemb, pembrolizumab; Sint, sintilima; Tisl, tislelizumab.
| Regions | Medical institutions | Tisl equipped | Tisl provision rate (%) | Sint equipped | Sint provision rate (%) |
|---|---|---|---|---|---|
| Xi’an | 17 | 10 | 58.82 | 6 | 35.29 |
| Xianyang | 2 | 2 | 100.00 | 2 | 100.00 |
| Yulin | 2 | 1 | 50.00 | 0 | 0.00 |
| Weinan | 1 | 0 | 0.00 | 1 | 100.00 |
| Baoji | 3 | 2 | 66.67 | 1 | 33.33 |
| Hanzhong | 1 | 0 | 0.00 | 1 | 100.00 |
| Tongchuan | 2 | 2 | 100.00 | 0 | 0.00 |
| Yan’An | 1 | 0 | 0.00 | 1 | 100.00 |
| Ankang | 1 | 0 | 0.00 | 0 | 0.00 |
| Total | 30 | 17 | 56.67 | 12 | 40.00 |
- Abbreviations: Sint, sintilimab; Tisl, tislelizumab.
4. Discussion
Based on the positive results from previous Phase II or Phase III clinical trials, nivolumab and pembrolizumab, whether used as monotherapy or in combination, have obtained approval indications or recommendations in guidelines. This indicates that immunotherapy has demonstrated clear efficacy in the first-line treatment of advanced gastric cancer. These findings provide a basis for the expanded use of tislelizumab in the first-line treatment of advanced gastric cancer. In the BGB-A317-205 trial, tislelizumab in combination with the XELOX regimen was studied in Chinese HER2-negative patients. The ORIENT-16 trial investigated the treatment efficacy of the XELOX regimen for all Chinese HER2-negative patients, matching the study scope, population, and chemotherapy regimen of the BGB-A317-205 trial most closely. Therefore, in the absence of sufficient trial data, assuming similar trial conditions among different regimens of PD-1 mAbs in combination with chemotherapy, the chemotherapy group from the ORIENT-16 trial is used as a control, and comparisons are made with each group of regimens. Although tislelizumab is not superior in effectiveness compared to nivolumab and sintilimab, it ranks higher than the chemotherapy group and the pembrolizumab group. Since the BGB-A317-205 trial has not reached the median OS at this stage, its ranking may further improve after the trial is completed. Therefore, it is considered that tislelizumab in combination with chemotherapy for the first-line treatment of advanced gastric cancer is effective.
In terms of safety, the safety data from all clinical trials were comprehensively evaluated using the entropy weight method and the TOPSIS method. Although tislelizumab in combination with chemotherapy ranks fourth in safety evaluation results, the top three are the monotherapy groups of two PD-1 mAbs (including tislelizumab) and the combination of tislelizumab with sitravatinib. In the combination chemotherapy regimens, it ranks first. Therefore, it can be considered that tislelizumab demonstrates good safety in the first-line treatment of advanced gastric cancer and may be superior to the other three PD-1 mAbs.
From the perspectives of affordability and accessibility, an accessibility assessment was conducted using the WHO/HAI standard survey method. The calculation results of the multiple of annual drug treatment expenses to the annual household income of urban and rural residents prove that tislelizumab is affordable among urban residents. Internationally, there is no strict unified standard for the availability of drugs. Generally, a rate > 80% is considered good, 50%∼80% indicates relatively good availability, and < 50% suggests low availability. The results of the accessibility assessment show that tislelizumab has good accessibility.
However, this study has certain limitations. In terms of effectiveness and safety, there is a lack of high-quality systematic comprehensive evaluations and meta-analytical results for tislelizumab in the first-line treatment of advanced gastric cancer. This study mainly relies on the results of Phase II clinical trials. Under the assumption of default trial conditions being the same, missing data were imputed using the median, and evaluations were conducted using the entropy weight method and the TOPSIS method. The evaluation results can only be qualitatively referenced, and ongoing attention should be given to higher-quality evidence. When analyzing affordability using the WHO/HAI standard survey method, the annual average income of urban and rural residents was used, but in reality, families with better living conditions may spend more on medical expenses than this value. In addition, families with lower wages but higher savings may be categorized as unaffordable. The accessibility assessment only investigated tertiary hospitals in Shaanxi Province, and its results cannot represent a broader situation.
In summary, tislelizumab in the first-line treatment of advanced gastric cancer has some effectiveness and good safety, with better accessibility for urban residents. When sintilimab is inaccessible or nivolumab, pembrolizumab is unaffordable, taking into account the actual conditions and considering the above limitations, with the informed consent of the patient and approval from the hospital’s pharmacy management committee and ethics committee, tislelizumab can be used in an expandable manner.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported financially by the Technology Development Incubation Fund of Shaanxi Provincial People’s Hospital (no. 2023YJY-54), National Health Commission Institute of Hospital Management, Research Project on Continuous Improvement of Hospital Management and Evidence-Based Medical Quality Management, Key Project (no. YLZLXZ23K003), and Norman Bethune Charity Foundation, Yaodongshenzhou-Pharmaceutical Research Capacity Building Project, General Project (no. Z04JKM2023E040).
Acknowledgments
This work was supported financially by the Technology Development Incubation Fund of Shaanxi Provincial People’s Hospital (no. 2023YJY-54), National Health Commission Institute of Hospital Management, Research Project on Continuous Improvement of Hospital Management and Evidence-Based Medical Quality Management, Key Project (no. YLZLXZ23K003), and Norman Bethune Charity Foundation, Yaodongshenzhou-Pharmaceutical Research Capacity Building Project, General Project (no. Z04JKM2023E040).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the authors on request.




