Study of Treatment and Patient Factors of Hemoporfin-Photodynamic Therapy for Port-Wine Stains
Abstract
Background. Port-wine stains (PWS) affect a substantial number of people, and despite the use of pulsed dye laser as the gold standard therapy, some patients fail to respond, and new modalities are needed. Recently, hemoporfin-photodynamic therapy (hemoporfin-PDT) has shown good efficacy and safety in treating PWS, with increasing evidence. Objectives. To evaluate the efficacy and safety of hemoporfin-PDT in the treatment of PWS and to analyze the factors that influence efficacy. Methods. The clinical data of 215 patients (79 men and 136 women aged 3–71 years) from a single center were retrospectively analyzed, out of which 173 were valid for efficacy analysis and 129 for safety analysis. Efficacy was rated as excellent (≥75% improvement), good (≥50% to <75% improvement), fair (≥25% to <50%), and poor (<25% improvement) by two blinded dermatologists. The patient-assessed efficacy was collected based on the aforementioned criteria using an electronic questionnaire. The association of efficacy with possible influential factors was investigated, namely, age, sex, history of previous treatment, combined scars caused by previous treatments, lesion site, lesion type, lesion size, degree of lip involvement, and number of hemoporfin-PDT sessions. Adverse events were investigated to evaluate the safety profile. Results. Excellent, good, fair, and poor efficacy was achieved in 78 (45.1%), 38 (22.0%), 36 (20.8%), and 21 (12.1%) patients, respectively, after a variable number of sessions of hemoporfin-PDT. More treatment sessions (p < 0.001), age ≥18 years (p = 0.037), no previous treatments (p = 0.020), and head/neck location (p = 0.009) were associated with better outcomes. Pain, edema, exudation/crusting, and hyperpigmentation were common adverse events, with satisfactory recovery. Scarring occurred in 2.3% of the patients. Conclusions. For treating PWS with hemoporfin-PDT, more treatment sessions and head/neck location are predictors of better outcomes, whereas previous treatment history is a predictor of poorer outcomes.
1. Introduction
Port-wine stains (PWS) are a type of congenital capillary malformation that affects an estimated 0.3% of all newborns [1]. The lesions can occur anywhere but typically appear on the face and upper limbs. Over time, the lesions may evolve from scarlet patches to thick purple plaques and even nodules if left untreated. Studies have shown a huge impact of this disorder on the psychological well-being of affected people and their families [2, 3]. Pulsed dye laser (PDL) therapy is by far the gold standard treatment. However, in many clinical studies, the proportion of patients who achieved >75% improvement after treatment with PDL was <20% [4]. The mechanism behind PDL is selective photothermolysis, during which hemoglobin absorbs the photo energy from the laser and converts it into heat energy, which coagulates and destroys blood vessel walls [5]. This process is influenced by the diameter of the vessels; therefore, one of the suggested mechanisms of PDL resistance is differences in vessel diameter [6]. Thus, more effective treatment modalities need to be developed. In the 1990s, photodynamic therapy (PDT) was introduced to treat PWS. First-generation PDT used hematoporphyrin derivatives as photosensitizers, which caused prolonged photosensitivity lasting 1–2 months. Recently, a second generation of photosensitizers with faster metabolism and fewer side effects, called hemoporfin, also known as hematoporphyrin monomethyl ether has emerged and was officially approved in China in 2016 for the treatment of PWS [7]. In PDT, photosensitizers can be absorbed by the endothelial cells of abnormal blood vessels and subsequently generate oxygen-derived free radicals and other phototoxic substances under appropriate light irradiation, which leads to vessel destruction. Compared with PDL, the efficacy of PDT may be less affected by the vessel diameter. Another advantage of PDT is that it can treat large lesions evenly. In contrast, PDL devices tend to result in a network of residues in patients with large lesions owing to the small spot size and the energy loss at the periphery of the spot. This issue may increase the number of treatments. There has been growing evidence supporting the efficacy and safety of hemoporfin-PDT in treating PWS, and some recent studies have evaluated the factors influencing efficacy, but with inconsistent findings. In our study, we retrospectively analyzed the clinical data of PWS patients treated with hemoporfin-PDT in the Dermatology Department of Peking Union Medical College Hospital. We also summarized the existing literature, with the hope of providing evidence to support efficacy and to identify factors influencing efficacy and evaluate the safety of hemoporfin-PDT as a treatment modality for PWS.
2. Materials and Methods
2.1. Study Design
This single-center retrospective study assessed the efficacy and safety of hemoporfin-PDT in PWS patients at Peking Union Medical College Hospital from March 2017 to December 2022. Inclusion criteria encompassed all ages of hemoporfin-PDT-treated PWS patients with complete medical records, including treatment data, photographs, and patient information. Exclusion criteria consisted of patients previously undergoing hemoporfin-PDT at other medical institutions. Data were obtained from electronic medical records and electronic questionnaires. The study received approval from the Ethics Committee of Peking Union Medical College Hospital (No. I-23PJ718).
2.2. Treatment Regimen
The non-lesion skin was covered with double-layered black cloth or a blanket, while the PWS area remained exposed. Hemoporfin (Shanghai Fudan-Zhangjiang Bio Pharmaceutical Co. Ltd., Shanghai, China), a photosensitizer, was intravenously injected at a dose of 5 mg/kg using an injection pump. After 10 minutes of drug injection, a perpendicular 532 nm light-emitting diode (LED) light (LED Therapeutic Machine, LED-IE, Wuhan YaGe Optic and Electronic Technique Co., Ltd., Wuhan, Hubei, China) was applied at a distance of 15 cm from the PWS lesion for 20–23 minutes. The LED device had an output power density of 80–85 mW/cm2 and a cumulative energy density of 78–105 J/cm2. Pediatric patients (<18 years) received a cumulative energy of 5–10 J/cm2 lower than adults (≥18 years). Skin temperature was regulated by a built-in fan in the LED device. Pain control involved the administration of oral acetaminophen or pregabalin 30 minutes prior to treatment.
2.3. Efficacy Evaluation and Its Influential Factors
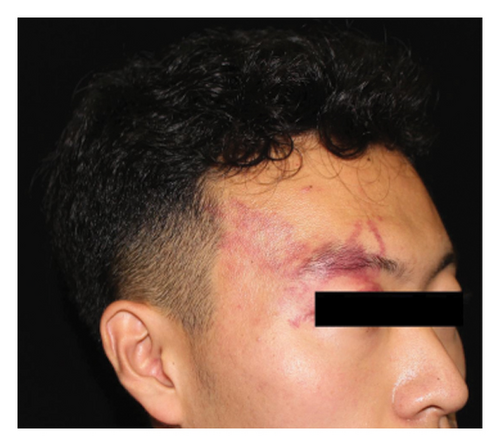
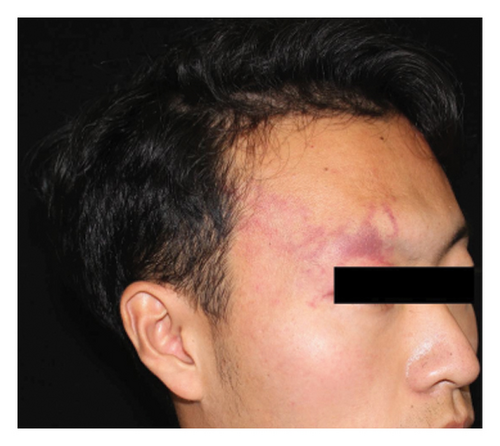
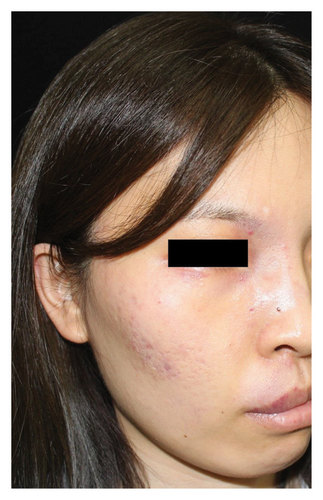
Efficacy evaluation included clinician-assessed efficacy, patient-assessed efficacy, and the impact on cosmetic outcome with the use of a concealer. For the clinician-assessed efficacy, eligible patients were those with at least two treatment sessions and one set of follow-up photographs. It involved standardized photographs rated by two independent dermatologists, assessing lesion clearance as excellent (≥75% improvement), good (≥50% to <75% improvement), fair (≥25% to <50% improvement), or poor (<25% improvement) (Figure 1). Clinical clearance was defined as ≥90% improvement. Inconsistent ratings were resolved through discussion and re-evaluation. Patient-assessed efficacy was obtained through electronic questionnaires using the same rating categories. The questionnaire also asked whether the treatment improved the cosmetic outcome, with the use of a concealer (if any). Factors influencing clinician-assessed efficacy were examined, including age, gender, previous treatment history (yes or no), combined scars caused by previous treatments (yes or no), the lesion site (head/neck or extremities/trunk), the lesion type (pink/red, purple, and thickened/nodular), lesion size (≤10 × 10 cm2 or >10 × 10 cm2), degree of lip involvement (no involvement, involved but without deformation, and lip deformation), and number of hemoporfin-PDT sessions (one, two, three, or more).
2.4. Safety Evaluation
All patients were included in the safety evaluation. For patients with missing electronic medical record data, an electronic questionnaire was used to gather information on pain, edema, exudation and crusting, pigmentation change, infection, and scarring. Pain scores were assessed using a numerical rating scale ranging from 0 to 10 (0 indicating no pain and 10 indicating the worst possible pain). Factors analyzed for potential influence on scarring included age at first treatment, gender, previous treatment history (yes or no), combined scars caused by previous treatments (yes or no), the lesion site (head/neck and extremities/trunk), the lesion type (pink/red, purple, and thickened/nodular), lesion size (≤10 × 10 cm2 or > 10 × 10 cm2), number of hemoporfin-PDT sessions (one, two, three, or more), and treatment-related adverse events (exudation/crusting, blister, and infection). Infection diagnosis relies on patient’s clinical presentation. If post-operation there is local yellow discharge or crusting, with/without odor, increased pain, swelling, or fever, it suggests a local infection.
2.5. Statistical Analysis
Quantitative data were presented as counts, mean ± standard deviation for normally distributed data and median (range) for nonnormally distributed data. Qualitative data were presented as counts and percentages for each category. Univariate and multivariate analyses were conducted to identify factors influencing efficacy or safety. Univariate analysis employed the Mann–Whitney U test, Spearman’s rank correlation analysis, or Pearson’s chi-squared/Fisher’s exact tests as appropriate. Multivariate analysis utilized a binary logistic regression model. Two-sided tests were performed, and a significance level of p < 0.05 was applied. Data analysis was conducted using SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. General Information
During the study period, 219 patients with PWS were initially treated at our hospital. Excluding four patients who received prior hemoporfin-PDT, 215 patients were included in the analysis. Of these, 79 (36.7%) were men and 136 (63.3%) were women, with a median age of 24 years (range: 3–71 years) at the first treatment. The majority of patients had head/neck involvement (189, 87.9%), with 50 (23.3%) in the medial area, 35 (16.3%) in the lateral area, and 104 (48.4%) showing a mixed pattern. Lesion types included 75 (34.9%) pink/red, 75 (34.9%) purple, and 65 (30.2%) thickened/nodular lesions. Lip involvement was present in 92 (42.8%) patients, with 30 (14.0%) experiencing lip deformation. Lesion size was ≤10 × 10 cm2 in 82 (38.1%) patients. Prior to hemoporfin-PDT, 119 (55.3%) patients had received PDL, 19 (8.8%) had received the radiation therapy, 24 (11.2%) had received first-generation PDT, and 3 (1.4%) had received cryotherapy. A total of 71 (33.0%) patients underwent only hemoporfin-PDT without any prior therapy. The number of hemoporfin-PDT sessions ranged from 1 to 8, with 40 (18.6%) patients undergoing one session, 53 (24.7%) undergoing two sessions, 48 (22.3%) undergoing three sessions, 31 (14.4%) undergoing four sessions, and 43 (20.0%) undergoing five or more sessions (Table 1).
| Characteristics | Result |
|---|---|
| Gender | Male: 79 (36.7%) |
| Female: 136 (63.3%) | |
| Age, median (range) | 24 (3–71) |
| Age profile (years), n (%) | <18: 61 (28.4%) |
| ≥18: 154 (71.6%) | |
| Lesion site, n (%) | Head/neck (medial): 50 (23.2%) |
| Head/neck (lateral): 35 (16.3%) | |
| Head/neck (mixed): 104 (48.4%) | |
| Extremities/trunk: 26 (12.1%) | |
| Lesion type, n (%) | Pink/red: 75 (34.9%) |
| Purple: 75 (34.9%) | |
| Thickened/nodular: 65 (30.2%) | |
| Lesion size, n (%) | ≤10 × 10 cm2: 82 (38.1%) |
| >10 × 10 cm2: 133 (61.9%) | |
| Lip involvement, n (%) | No involvement: 123 (57.2%) |
| Involved but without deformation: 62 (28.8%) | |
| Involved with deformation: 30 (14.0%) | |
| Previous treatments, n (%) | PDL: 119 (55.3%) |
| Radiation therapy: 19 (8.8%) | |
| 1st generation PDT: 24 (11.2%) | |
| Cryotherapy: 3 (1.4%) | |
| None: 71 (33.0%) | |
| Unknown: 11 (5.1%) | |
| Session number, n (%) | One: 40 (18.6%) |
| Two: 53 (24.7%) | |
| Three: 48 (22.3%) | |
| Four: 31 (14.4%) | |
| Five or more: 43 (20.0%) | |
- PDL, pulsed dye laser; PDT, photodynamic therapy.
3.2. Efficacy and Its Influencing Factors
Out of the eligible cases, 173 underwent clinician-assessed efficacy analysis. The distribution of efficacy ratings after varying sessions was as follows: excellent (78, 45.1%), good (38, 22.0%), fair (36, 20.8%), and poor (21, 12.1%). Overall, 40 patients (18.6%) achieved clinical clearance, typically requiring an average of 2.6 sessions for excellent outcomes. Among the subset of 72 patients evaluated for the first three sessions, improvement rates were as follows: first session—excellent (2, 2.8%), good (18, 25.0%), fair (29, 40.3%), and poor (23, 31.9%); second session—excellent (12, 16.7%), good (30, 41.7%), fair (25, 34.7%), and poor (5, 6.9%); and third session—excellent (30, 41.7%), good (27, 37.5%), fair (14, 19.4%), and poor (1, 1.4%). Univariate analysis indicated that greater treatment sessions (p < 0.001), age ≥18 years (p = 0.022), and head/neck location (p = 0.026) were associated with better outcomes. Multivariate analysis confirmed the significance of more treatment sessions (p < 0.001), age ≥18 years (p = 0.037), absence of previous treatment (p = 0.020), and head/neck location (p = 0.009) in predicting improved outcomes. No significant associations were found between outcomes and gender, pre-existing scars caused by previous treatments, the lesion type, the lesion size, or degree of lip involvement (Table 2). Representative photographs illustrating the effects of hemoporfin-PDT are presented in Figure 2. In the patient-assessed efficacy analysis, 129 valid questionnaires were collected. The reported improvement rates were as follows: excellent (44, 34.1%), good (40, 31.0%), fair (32, 24.8%), and poor (13, 10.1%), which closely aligned with clinician ratings. Among these patients, 42/129 (32.6%) regularly used concealer, with 37 (88.1%) finding it easier to cover the lesions after treatment.
| Characteristics | n | Poor/fair, n (%) | Good/excellent, n (%) | Univariate analysis p | Multivariate analysis p |
|---|---|---|---|---|---|
| Total | 173 | 57 (32.9%) | 116 (67.1%) | ||
| Age (years) | |||||
| <18 | 53 | 24 (45.3%) | 29 (54.7%) | 0.022 | 0.037 |
| ≥18 | 120 | 33 (27.5%) | 87 (72.5%) | ||
| Gender | |||||
| Male | 68 | 27 (39.7%) | 41 (60.3%) | 0.128 | 0.104 |
| Female | 105 | 30 (28.6%) | 75 (71.4%) | ||
| Session number | |||||
| 1 | 88 | 43 (48.9%) | 45 (51.1%) | <0.001 | <0.001 |
| 2 | 41 | 10 (24.4%) | 31 (75.6%) | ||
| ≥3 | 44 | 4 (9.1%) | 40 (90.9%) | ||
| History of previous treatment (n = 169) | |||||
| No | 61 | 15 (24.6%) | 46 (75.4%) | 0.076 | 0.020 |
| Yes | 108 | 41 (38.0%) | 67 (62.0%) | ||
| Scarring caused by previous treatments | |||||
| No | 129 | 43 (33.3%) | 86 (66.7%) | 0.854 | 0.999 |
| Yes | 44 | 14 (31.8%) | 30 (68.2%) | ||
| Lesion site | |||||
| Head/neck | 153 | 46 (30.1%) | 107 (69.9%) | 0.026 | 0.009 |
| Extremities/trunk | 20 | 11 (55.0%) | 9 (45.0%) | ||
| Lesion type | |||||
| Pink/red | 61 | 24 (39.3%) | 37 (60.7%) | 0.158 | 0.823 |
| Purple | 60 | 19 (31.7%) | 41 (68.3%) | ||
| Thickened/nodular | 52 | 14 (26.9%) | 38 (73.1%) | ||
| Lesion size | |||||
| ≤10 × 10 cm2 | 65 | 24 (36.9%) | 41 (63.1%) | 0.388 | 0.158 |
| >10 × 10 cm2 | 108 | 33 (30.6%) | 75 (69.4%) | ||
| Degree of lip involvement | |||||
| No involvement | 96 | 36 (37.5%) | 60 (62.5%) | 0.139 | 0.812 |
| Involved but without deformation | 52 | 15 (28.8%) | 37 (71.2%) | ||
| Lip deformation | 25 | 6 (24.0%) | 19 (76.0%) |
- A significance level of p < 0.05 is applied, and significant p values are bolded.
3.3. Safety
Data from 129 patients were included in the safety evaluation. All patients experienced pain, with a median numerical rating scale score of 7 immediately after treatment. The score gradually decreased to 6, 5, 3, and 0 on the first, second, and third days and 2 weeks after treatment, respectively.
Edema occurred in 103/129 (79.8%) patients, with the majority (72.8%) experiencing the worst edema within the first 24 hours, but for some (26.2%), it peaked at 2-3 days. Complete resolution of edema occurred within 3 days for 43.7% of patients, while it resolved in 4–7 days for 45.6% of patients, and it persisted for more than 1 week for 10.7% of patients. Severity ratings were mild (1.9%), moderate (28.2%), and severe (69.9%). Among the 42 patients with the neck edema, 14.3% reported breathing discomfort, but it did not reach the level of asphyxiation concern.
Exudation and crusting were common, affecting 60.4% of patients, primarily within the first 2 weeks after treatment. Crusting persisted for over 1 week in most cases. Blisters occurred in 20.9% of patients, appearing as early as 24 hours after treatment and developing further in the following week. The majority (85.2%) had fewer than 10 blisters, which took more than 1 week to resolve completely.
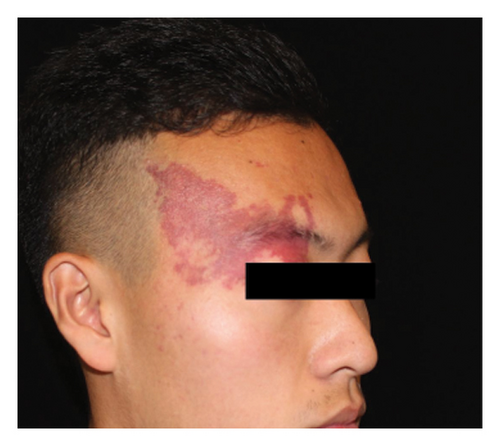
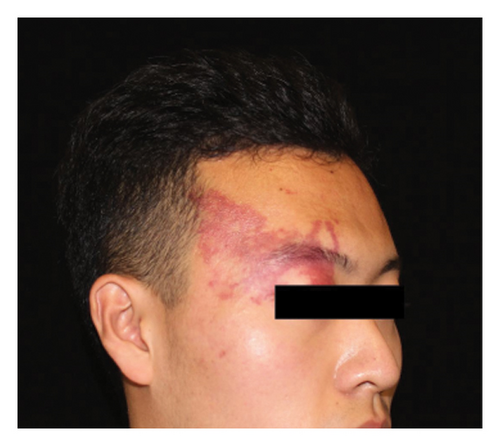
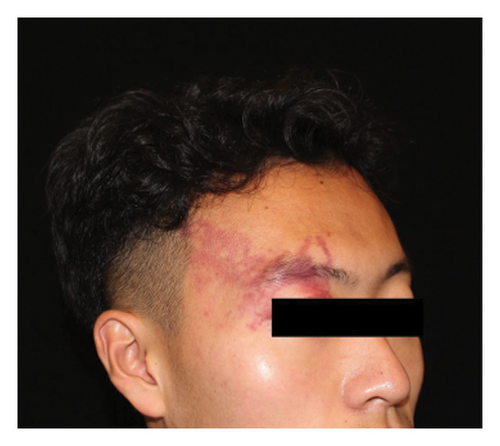
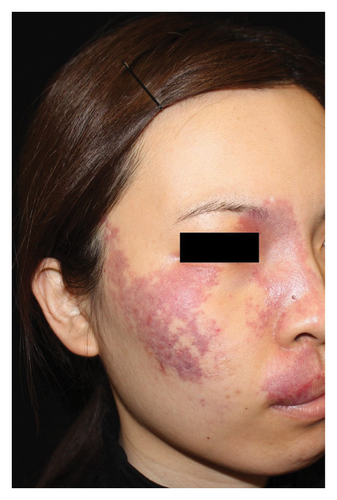
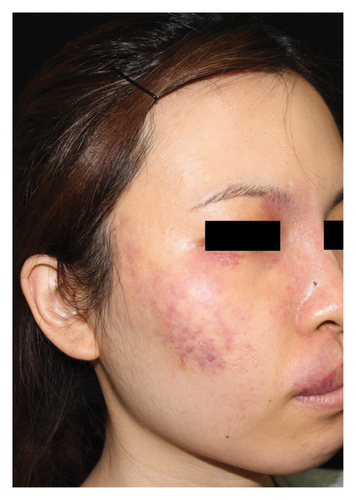
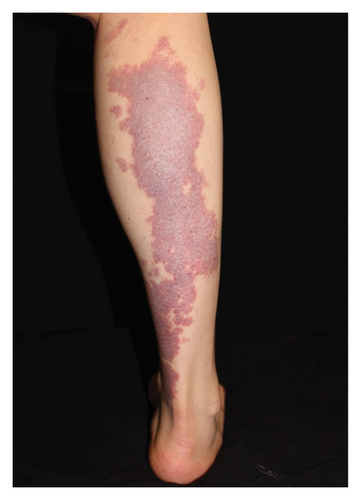
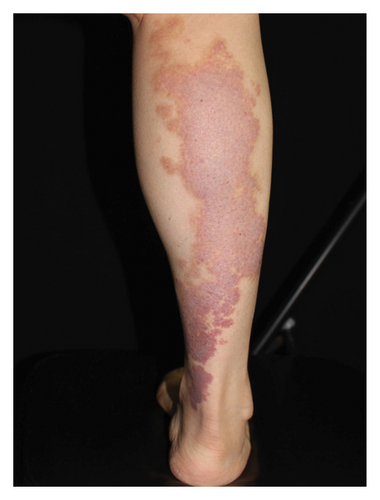
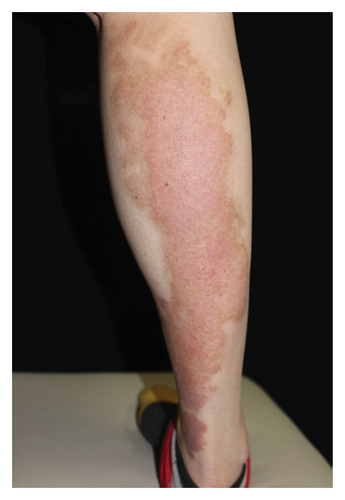
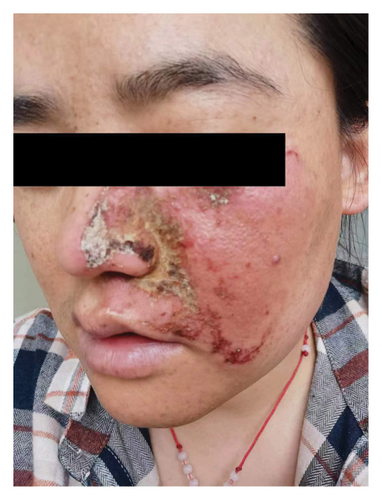
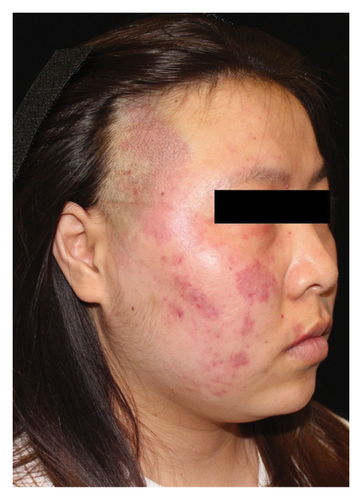
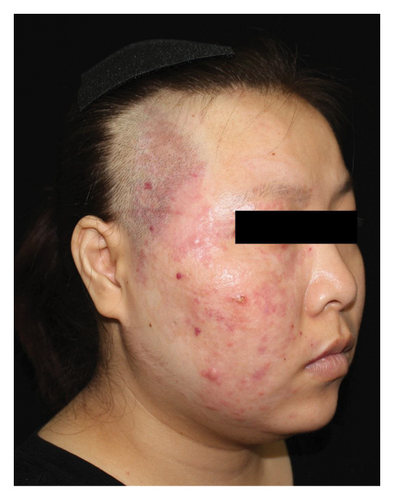
Hyperpigmentation was reported by 68.2% of patients, resolving fully within 6 months for 86.4% of them. No hypopigmentation was observed. Infection (Figure 3(a)) occurred in 5.4% of patients, all within the first week. Most patients did not undergo bacterial smear/culture, and the infection was controlled under empirical treatment with local (mupirocin) or systemic (cephalosporin) antibiotics against Gram-positive cocci. Scarring (Figures 3(b) and 3(c)) was observed in 2.3% of patients, and no associated factors could be determined by binary logistic regression model (results not shown). No anaphylaxis, laboratory abnormalities, or photosensitivity reactions were observed.
4. Discussion
In 2016, hemoporfin-PDT was approved for PWS treatment by the Chinese State Food and Drug Administration, providing an important alternative to PDL. In our retrospective study of 215 patients, hemoporfin-PDT demonstrated high effectiveness, with 45.1% of patients achieving >75% improvement after a variable number of sessions, which was higher than that reported with PDL at < 20% [4]. Comparisons with PDL were not within the study’s scope, but previous studies have shown better improvement with hemoporfin-PDT after one or two sessions [8–10]. Nevertheless, PDL remains the gold standard treatment for PWS, and larger prospective studies are needed to determine the superiority of hemoporfin-PDT. Investigating the combination of hemoporfin-PDT and PDL in future studies may enhance outcomes due to their different mechanisms of action.
In the present study, the overall efficacy improved with higher numbers of sessions, which is highly consistent with results in previous studies [11–16]. The majority of patients achieved excellent/good improvement after the third session. Therefore, we recommend that for patients with an unsatisfactory response after the initial session, physicians should continue the treatment and re-evaluate the efficacy after the third session. Importantly, it remains a challenge to improve the efficacy of therapy for a subgroup of patients who fail to respond well to both hemoporfin-PDT and PDL regardless of the number of treatments.
Previously, two studies have found that younger age is associated with better efficacy [12, 13]. These studies primarily focused on pediatric patients, with limited adult cases. Despite the expectation of reduced treatment efficacy due to factors such as thicker stratum corneum and lesion hypertrophy with age, our study found a positive association between age ≥18 years and improved treatment outcomes. Several factors may explain these contrasting results. Firstly, the relatively small proportion of patients under the age of 18 in our population may have an impact on the representativeness of this particular demographic group. Secondly, the absence of general anesthesia during treatment may have affected children’s compliance with light exposure, while adults could maintain a fixed position. Children’s light avoidance behaviors may have influenced treatment efficacy. Additionally, higher treatment energy levels were generally administered to adults, potentially contributing to their relatively better outcomes. Future studies should be conducted to evaluate the efficacy and safety of using different energy levels in pediatric patients, in order to guide the improvement of treatment outcomes in this population. Importantly, although our study suggests better efficacy in adults, it does not imply delaying treatment until adulthood. Due to the significant impact of port-wine stains on social and psychological well-being, early proactive intervention is recommended. And for these reasons, we cannot conclude that age ≥18 is a positive predictor for a better outcome.
In our study, a history of previous treatment was associated with poorer outcomes, similar to findings in a previous study [12]. The reasons for this finding might be that capillaries that remain after previous treatments are deeper vessels that are difficult to reach with radiation and resistant to treatment, and there may be connective tissue proliferation caused by previous treatments that interferes with light penetration. Interestingly, some preliminary studies have also found that patients who are resistant to PDL treatment (defined as having received at least five sessions of PDL treatment with no improvement after the last one or two sessions) may benefit from hemoporfin-PDT [11, 17]. Despite diminished efficacy in patients with prior treatments compared to treatment-naïve individuals, hemoporfin-PDT may still serve as a viable therapeutic option for mitigating skin lesions.
Lesion site and type have been significant factors in assessing the efficacy of hemoporfin-PDT. Our study observed better outcomes for head/neck region lesions, while extremities/trunk lesions had less favorable results, consistent with previous findings [14, 16]. Additionally, some authors found that lesions on the lateral face were associated with better treatment outcomes compared with lesions on the medial face [12, 13]. The thicker stratum corneum of the skin on the extremities, trunk, and the central face, as well as their deeper distribution of blood vessels and the larger diameter of the blood vessels, may lead to a relatively poorer therapeutic response in these anatomical areas [13, 14]. In the present study, we found no association between the lesion type and outcome, similar to findings in another study [15], indicating that hemoporfin-PDT might be suitable for all lesion types. Lip hypertrophy [15] and larger lesion size [16] were predictors of negative outcomes in some studies; however, these results were not found in our study. Determining the influential factors for efficacy is at the frontier of research in PWS therapy, with much debate. More studies are needed to facilitate a better understanding of this topic.
The study found hemoporfin-PDT to have a good safety profile for PWS. Pain during treatment is common but can be managed with oral acetaminophen or pregabalin. General anesthesia may increase comfort and compliance but raises concerns about timely termination when patients report unusual pain. The special feature of hemoporfin-PDT treatment compared with PDL is the systematic use of photosensitizers. Therefore, allergic reactions and photosensitivity are of particular concern. No allergic reactions or photosensitivity was observed in this study, likely owing to the proximity in the structure of hemoporfin and natural hematoporphyrin, as well as proper light protection. Scarring, an adverse event of high concern, has been observed with both PDL and hemoporfin-PDT. According to the literature, the incidence of postoperative scarring in PDL is approximately 5% [18]. In previous studies, the incidence of postoperative scarring after hemoporfin-PDT ranged from 0 to 10.1% [7, 8, 11–17, 19], and in our study, the incidence was 2.3%. To prevent scarring, our practice employs various measures. Patients experiencing significant swelling or bleaching after treatment received a 0.3−0.5 mg/kg body weight dose of glucocorticoids for 5–7 days to reduce edema and exudation. Antibiotics are administered to patients with heavy crusting to prevent secondary infection. Appropriate energy reduction in subsequent sessions is crucial for patients with severe post-treatment adverse events. It is important to note that hemoporfin-PDT should generally be avoided for anatomical sites with low blood supply and poor tissue elasticity, such as the ears, hands, and feet, which may be better managed with PDL.
In conclusion, hemoporfin-PDT is an effective and safe treatment for PWS. More treatment sessions, no previous treatment history, and lesions in the head/neck region are predictors of positive outcomes. Most adverse events associated with this treatment are mild and resolve quickly, and only a small number of patients experience scarring, with proper post-treatment management. The limitations of this study are the retrospective design and subjective evaluation methods, with potential observational bias. Besides, the relatively small number of patients under 18 years old or with extremities/trunk involvement may limit the generalizability of the study’s conclusions to these specific populations. Future research endeavors should focus on conducting prospective studies using objective evaluation methods to assess the efficacy and safety of hemoporfin-PDT in the treatment of PWS, and new treatment strategies need to be developed for patients who are resistant to both PDL and hemoporfin-PDT.
Ethical Approval
The study was performed under the 1975 Declaration of Helsinki guidelines and was approved by the Ethics Committee of our institution (No. I-23PJ718).
Consent
The patients in this manuscript have given written informed consent to publication of their case details.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Xiaofei Mao and Hao Feng are co-first authors.
Acknowledgments
The authors would like to thank Wei Wang, Wenhong Xie, Ping Chen, and other members of their nursing team for assisting with the treatment. The authors would also like to thank Jane Charbonneau, DVM, from Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the English text of a draft of this manuscript. This study was supported by the National Key R&D Program of China (2022YFC3601800), National High Level Hospital Clinical Research Funding (2022-PUMCH-B-092), Beijing Natural Science Foundation (7242109), and National Key Clinical Specialty Project of China.
Open Research
Data Availability
Data are available on request from the authors.