Design and Application of Flexible Sensors in Human–Machine Interaction
Abstract
To improve the electrical conductivity, mechanical properties, and antibacterial properties of conventional hydrogels while simplifying their preparation steps for better application in wearable, flexible sensors and biomimetic electronic skins. Polyvinyl alcohol (PVA) hydrogels were doped with an ionic liquid based on zinc chloride to synthesize improved hydrogels using the freeze-thawing method. It is found that the addition of ionic liquid based on zinc chloride to the hydrogel resulted in a significant increase in electrical conductivity. However, an excessive amount of these liquids led to a reduction in their mechanical properties. The results reveal that the balance between conductivity and mechanical properties can be achieved by controlling the concentration of the ionic liquid based on zinc chloride. The higher the ionic liquid concentration based on zinc chloride in the composite hydrogels, the better the conductivity performance. The addition of an ionic liquid based on zinc chloride resulted in a significant improvement in the conductivity performance of the hydrogels. Furthermore, excellent mechanical properties are maintained even at a mass ratio of 1 : 10 between ionic liquids based on zinc chloride and PVA hydrogels, and composite hydrogels exhibit excellent antibacterial properties.
1. Introduction
With the emergence of the metaverse, electronic human–machine interaction (HMI) attracts more and more attention as a novel form of HMI, even VR/AR, human–machine systems, and robotics [1, 2]. As a viable alternative, hydrogel electronic skin exhibits excellent characteristics, such as flexibility [3], scalability [4], stretchability [5], and biocompatibility [6]. Therefore, hydrogel electronic skin exhibits vast potential for applications in electronic HMI. Hydrogels are cross-linked 3D hydrophilic networks. The formation of hydrogels primarily occurs through physical cross-linking, chemical cross-linking, and chemical–physical cross-linking. Polyvinyl alcohol (PVA) hydrogels exhibit inherent mechanical properties, excellent biocompatibility, and resemblances to organic organisms in its properties, and it assumes a vital and irreplaceable role in domains such as wearable, flexible sensors; bionic electronic skin; and flexible electrodes [7–10].
However, most of the current reports have mainly focused on the anti-freezing performance of conductive hydrogels [11]. The current conductive hydrogel sensors face three major challenges: the contradiction of mechanical stability and self-healing, lack of environmental adaptability and multifunctionality, and a single data acquisition channel, which seriously limit their application as e-skin [12]. How to simultaneously achieve high transparency, adhesion, antibacterial, anti-freezing, anti-drying, and biocompatibility properties through a simple method remains a challenge [13].
What’s more, traditional synthesized hydrogels have been constrained in their development and application [14, 15] due to their loose internal 3D network structure [16] and large and uniform pore sizes. Meanwhile, most PVA-based bionic electronic skins cannot be adapted to the demands of complex environments, such as HMI devices, innovative rehabilitation medicine, and underwater encryption [17]. Therefore, the exploration of high-performance hydrogel materials with remarkable tensile strength and electrical conductivity has received considerable scientific interest owing to the escalating societal demands for their application in diverse real-world scenarios. Wu et al. [18] investigated the improvement of Zn ionic liquid/PVA composite hydrogels by incorporating metal ions, but the challenge of achieving a balance between mechanical properties and conductivity remains.
In addition, traditional conductive hydrogels using pure water as the dispersion medium are easy to become hard, brittle, and nonconductive at low temperatures owing to the freezing of water, which greatly restricts their practical applications. Meanwhile, at high or even room temperatures, the flexibility, stretchability, and conductivity of these hydrogels will be severely impaired due to the evaporation of water. Therefore, endowing conductive hydrogels with environmental resistance has attracted extensive interest from researchers. At present, the introduction of organic solvents such as glycerol through direct addition or solvent replacement is an effective method to prepare hydrogel with long-term stability and frost resistance [19].
The mechanical properties of hydrogels are more dependent on the number and duration of freeze-thawing cycles [20–23], and mutual cross-linking networks were constructed between ionic liquids and PVA by hydrogen bonding [24] action. They simplify the conventional PVA hydrogel formation process by reducing the reliance on multiple freeze and thaw cycles [25, 26]. Above all, low-cost flexible sensors have a wide range of applications in biomaterials and biointerfaces. In terms of biomaterials, such as organic semiconductors, carbon nanotubes and nanofibers. These materials not only have excellent mechanical flexibility but also have excellent sensing properties. In the field of biointerfaces, flexible sensors can be used to monitor physiological parameters such as heart rate, respiration, and electromyogram (EMG) signals to provide support for personalized medicine and remote health monitoring [27, 28]. In this work, the introduction of metal ionic liquid doping into PVA is employed to enhance the conductivity, mechanical properties, and antibacterial properties of this hydrogel. Furthermore, the balance between mechanical and conductive properties of Zn ionic liquid/PVA composite hydrogels was achieved by varying the content of metal ionic liquids.
In summary, the novelty of conductive hydrogels from PVA and ionic liquids lies in the synergistic combination of the unique properties of these materials. The design ideas focus on enhancing conductivity, ensuring biocompatibility, allowing tunability, and enabling responsiveness to environmental stimuli for various applications, particularly in bioelectronics and sensing technologies.
2. Materials and Methods
2.1. Materials
The materials are as follows: PVA (polymerization degree 1799, > 99%, Hefei BASF Biotechnology Co., Ltd., Anhui, China), choline chloride (C5H14CINO, 98%, Shanghai McLean Biochemical Technology Co., Shanghai, China), zinc chloride (ZnCl2, 98%, Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China), Luria-Bertani (LB) agar (Sanhe Luqiao QC Technology Co., Ltd., Beijing, China), and LB broth (Sanhe Luqiao QC Technology Co., Ltd., Beijing, China). Deionized water was produced by a laboratory water purification system (Eco-S15UV, Shanghai Hetai Instruments Co., Ltd., Shanghai, China).
2.2. Preparation of Zn Ionic Liquid/PVA Composite Hydrogels
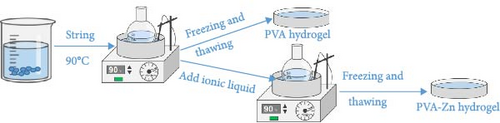
Firstly, 2.5 g of PVA and 22.5 ml of deionized water were weighed in a round bottom flask and heated in a water bath at 90°C for 5 h. The mixture was stirred thoroughly until it became clear and transparent. Resulting in a 10 wt% PVA aqueous solution, this solution was poured into molds. Secondly, weigh zinc chloride and choline chloride with a molar ratio of 2 : 1 in a round bottom flask, and heat them in an oil bath at 130°C for 3 h until the solution becomes clear and transparent. Next, Zn ionic liquid/PVA composite hydrogels with different concentrations (including 5%, 7.5%, 10%, 50%, and 100%) were prepared by dissolving predetermined ionic liquid content in the deionized water which were heated in a water bath at 90°C for 5 h at a specific ratio until it becomes colorless and transparent. Record it as PVA-Zn-x (x = 1, 2, 3, 4, 5) gel. Then, the prepared aqueous solution was poured into molds, and the freeze-thawing method was repeated twice to obtain the desired hydrogels. Finally, the hydrogels were stored in deionized water for subsequent testing (Figure 1). All the samples were summarized at Table 1.
| Sample number | PVA (g) | Zn ionic liquid/(g) | Deionized water (ml) |
|---|---|---|---|
| PVA gel | 2.5 | 0 | 22.5 |
| PVA-Zn-1 gel | 2.5 | 0.25 | 22.5 |
| PVA-Zn-2 gel | 2.5 | 0.5 | 22.5 |
| PVA-Zn-3 gel | 2.5 | 2.5 | 22.5 |
| PVA-Zn-4 gel | 2.5 | 3.33 | 22.5 |
| PVA-Zn-5 gel | 2.5 | 5 | 22.5 |
2.3. Material Testing
2.3.1. Mechanical Properties
The tensile properties of hydrogels were tested by a universal testing machine (WDW-20, Zhejiang No.1 Testing Instrument Manufacturing Co., Ltd., China). Before the test, hydrogel samples were prepared in the form of dumbbells with a length of 20 mm, width of 10 mm, and thickness of 1.5 mm. The sample was pulled to fracture at a rate of 30 mm/min. For compression experiments, hydrogel samples made in a mold with a height of 8 mm and a diameter of 4 mm were compressed at a rate of 10 mm/min until the compression was complete. All experiments were conducted at room temperature.
2.3.2. Conductivity
ρ is the resistivity, S is the cross-sectional area, R is the resistance value, and L is the length of the resistance. conductivity = 1/resistivity.
2.3.3. General Sensing Performance of Hydrogels
The composite hydrogels with various identification codes were affixed to the forehead, neck, and fingers. Hydrogels were connected to the digital bridge using heated tweezers to measure the resistance values.
2.3.4. Advanced Sensing Applications of Hydrogel
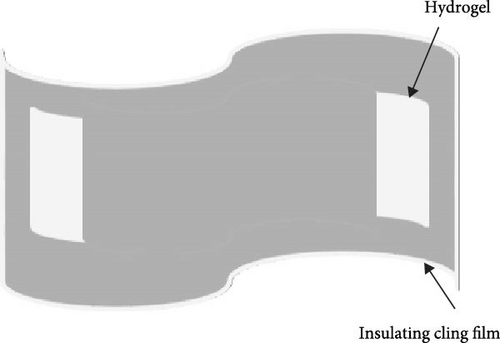
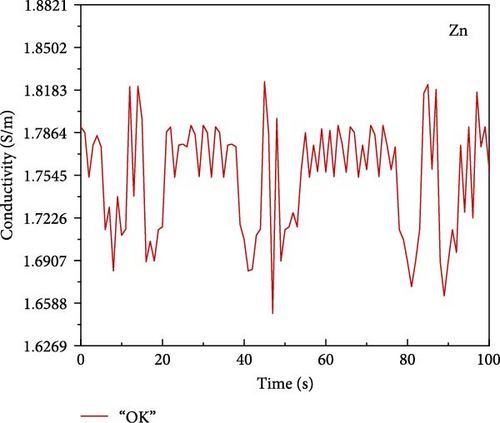
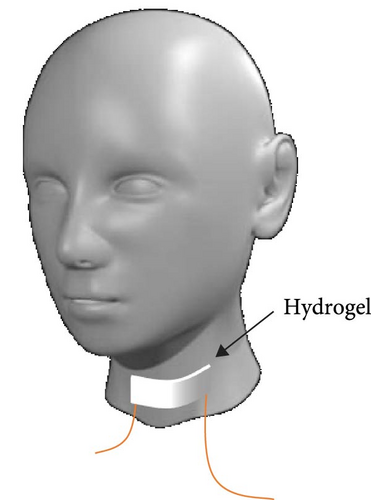
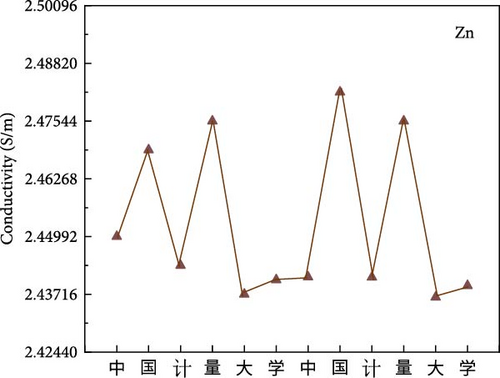
A simple writing board is created by sandwiching the hydrogel between insulating cling film. The conductivity variation curve of the hydrogel while writing “OK” three times on the simple writing board. In addition, performing LCR measurements with an insulated conductive tape applied to the throat and the utterance of “中国计量大学 (China Jilaing University)” by the tester elicits subtle muscular changes in the throat region. The change in the conductivity curve of the hydrogel after the tester vocalizes.
2.3.5. Antibacterial Properties of Supramolecular Hydrogel Electronic Skin
The supramolecular hydrogel electronic skin also has unique antibacterial properties due to the antibacterial properties of Zn. In the antibacterial experiment, 2.5 g of LB broth was taken and dissolved in 100 ml of distilled water, followed by heating and boiling. The resulting liquid culture medium was then subjected to high-pressure sterilization. Then, 2.5 g of LB broth was placed in 100 ml of distilled water. After that, add 1.6 g of agar, and heat it to boiling to dissolve. The resulting mixture is subjected to high-pressure sterilization for a solid culture medium. Last, inoculate Escherichia coli culture onto the sterilized liquid culture medium, and incubate it under constant agitation at 160 rpm and 37°C for 12 h to obtain E. coli broth.
Disinfect all required items, and place them in the laminar flow hood for 30 min under UV light. Prepare a water bath at 55–60°C for the solid culture medium. Pour 30 ml of the solid culture medium into a petri dish, and use a marker pen to label the dish with information such as time and sample name. Allow the medium to stand undisturbed for half an hour until it solidifies. Once solidified, use a pipette to transfer 200 μL of concentrated E. coli broth with a 4,000 CFU/ml concentration into the petri dish. Use a sterile spreader to distribute the broth evenly on the culture medium by making circular motions and allowing the broth to air-dry. Then, place the rectangular samples onto the petri dish, invert it, and incubate it in a 35°C incubator for 18 h. Observe regularly during the incubation period. Finally, analyze the antibacterial effect of the materials.
3. Results and Discussion
3.1. Analysis of Conductivity Results
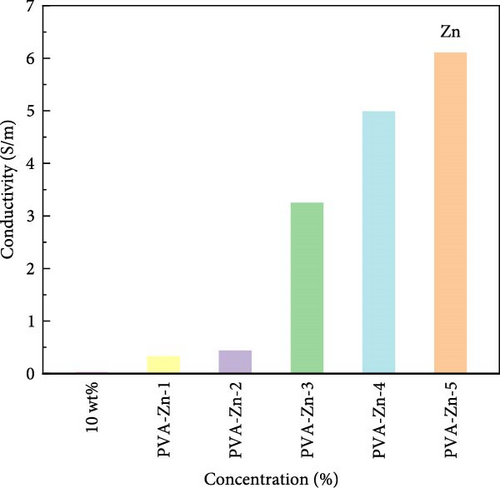
PVA is a polymer compound with hydrogen bond cross-linking ability [29], enabling it to form a three-dimensional network structure. It is a semiconductor material whose conductivity lies between conductors and insulators. The LCR digital bridge is used to measure the resistance of hydrogels, and the change of conductivity can be indirectly reflected by the resistance. The conductivity of PVA hydrogel displays the effect of different ionic liquids based on zinc chloride on the conductivity of 10 wt% PVA hydrogel (Figure 2). The experimental results indicate that the higher concentrations of ionic liquid based on zinc chloride, the higher the conductivity of Zn ionic liquid/PVA composite hydrogels. The conductivity of the control group is 0.032 S/m. After adding an ionic liquid based on zinc chloride, the conductivity of PVA hydrogel has been significantly improved. Notably, the conductivity of PVA-Zn-5 gel is 189.75 times higher than that of the unmodified hydrogel. This is attributed to the fact that after the thermal initiation radical polymerization of ionic liquids in the system, a portion of the ionic liquid still retains an intact hydrogen bond network in the system, providing a pathway for electronic migration within the gel. Additionally, the liberated choline chloride can ionize into a large number of cations and anions, and the conductivity of the hydrogel is enhanced. Furthermore, metal ions exhibit good conductivity [30]. As a result, hydrogels doped with metal ion liquids experience a significant enhancement in conductivity.
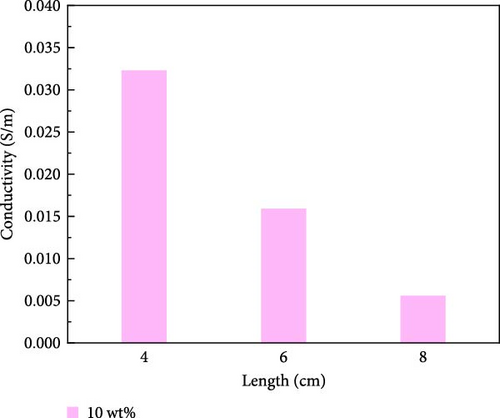
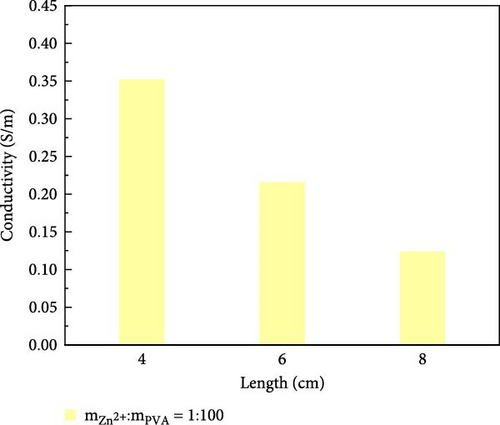
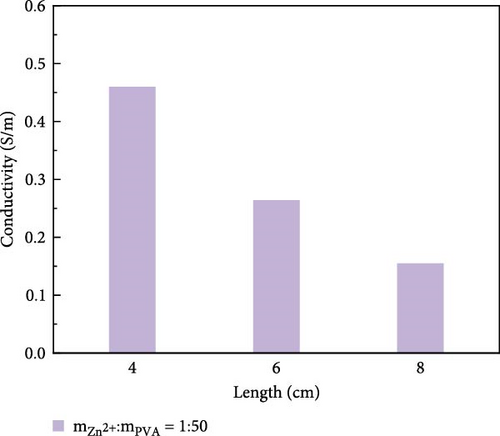
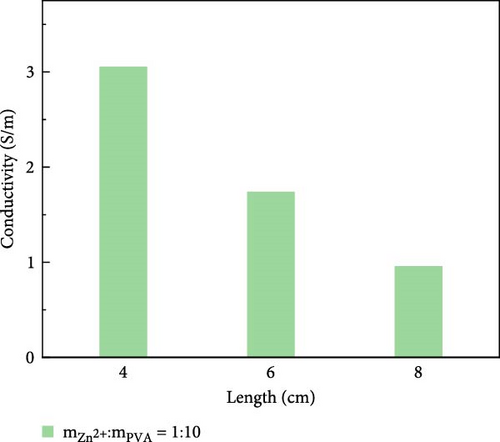
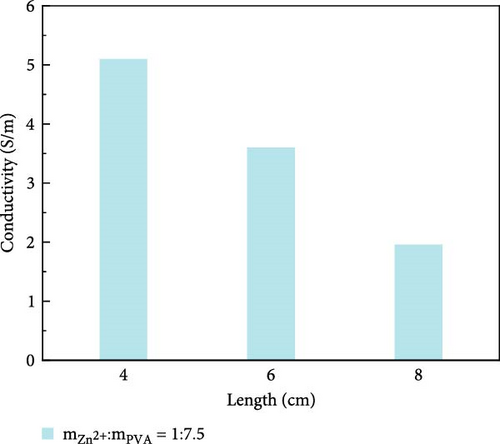
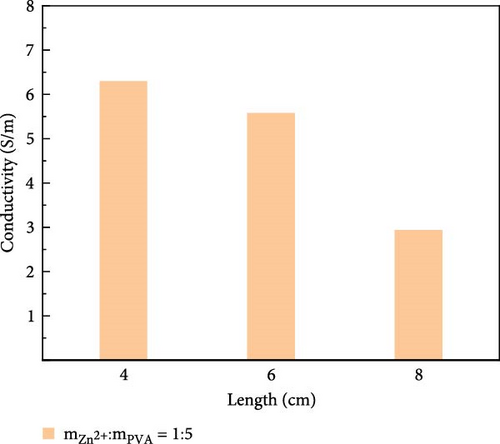
The variation in conductivity of different concentration hydrogels at different elongation levels (Figure 3). The sensitivity of hydrogels is expressed by the change of conductivity; the experimental results reveal an inverse relationship between the conductivity of hydrogels and length [31, 32]; the longer the length, the lower the conductivity. The conductivity of 10 wt% PVA hydrogel was 0.032 S/m without stretching and decreased to 0.0165 S/m after stretching for 2 cm (Figure 3a). The conductivity of PVA hydrogels doped with varying concentrations of ionic liquid based on zinc chloride exhibits maximum values without mechanical stretching, gradually diminishing with the progressive increment of the stretching length. (Figure 3b–f). After stretching, 10 wt% changed from 0.0325 to 0.017 S/m and then to 0.005 S/m, and the conductivity changed by 0.0275 S/m; the conductivity of PVA-Zn-1 changed from 0.35 to 0.22 S/m and then to 0.125 S/m, and the conductivity changed by 0.225 S/m; the conductivity of PVA-Zn-2 changed from 0.46 to 0.275 S/m and then to 0.16 S/m, and the conductivity changed by 0.3 S/m; the conductivity of PVA-Zn-3 changed from 3.1 to 1.85 S/m and then to 1.15 S/m, and the conductivity changed by 1.95 S/m; the conductivity of PVA-Zn-4 changed from 5.1 to 3.85 S/m and then to 2.15 S/m, and the conductivity changed by 2.95 S/m; and the conductivity of PVA-Zn-5 changed from 6.3 to 5.75 S/m and then to 3.25 S/m, and the conductivity changed by 3.05 S/m. It can be observed that the higher concentrations of ionic liquid based on zinc chloride are associated with increased sensitivity in composite hydrogels, resulting in large changes following stretching.
3.2. Analysis of Mechanical Performance
ΔL represents the total deformation of the gauge length segment after the tensile fracture of the specimen. L represents the initial gauge length.
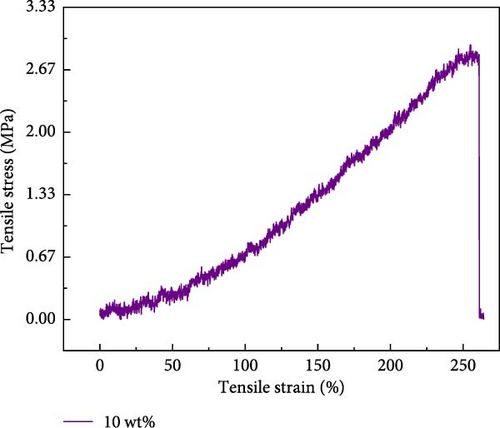
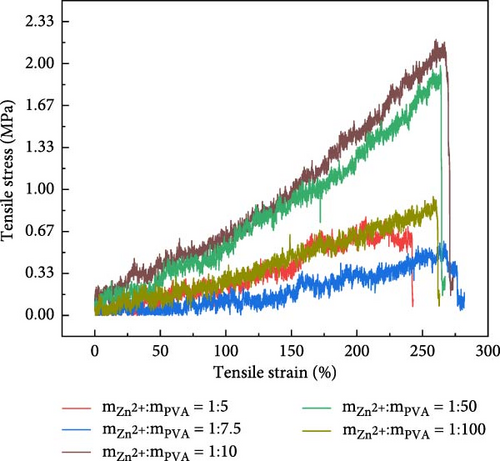
In the context of hydrogel applications, mechanical properties are significant influencing factors, particularly in flexible electronics [33] and biomimetic electronics [34–36], which exert a paramount influence. The variation of tensile length and its impact on the applied pressure were investigated for a 10 wt% PVA hydrogel (Figure 4a,b). The tensile strength of Zn ionic liquid/PVA composite hydrogels reaches 3 MPa with the elongation at a break of 275%, evaluating the tensile performance of Zn ionic liquid/PVA composite hydrogels at different concentrations. The experimental results reveal that the optimal tensile performance of the hydrogel can be achieved by incorporating a suitable amount of zinc ion-containing ionic liquid [37–39]. The PVA-Zn-3 gel demonstrates superior tensile performance. The tensile strength of Zn ionic liquid/PVA composite hydrogels reaches 2.25 MPa with the elongation at a break of 270%. The tensile properties of the PVA-Zn-2 gel are relatively inferior; the tensile strength of PVA-Zn-2 gel reaches 2.0 MPa. The tensile performance of PVA-Zn-1 gel, PVA-Zn-4 gel, and PVA-Zn-5 gel exhibits a significant decrease. Hence, it can be inferred that PVA-Zn-3 gel uniquely combines favorable electrical conductivity with excellent tensile properties.
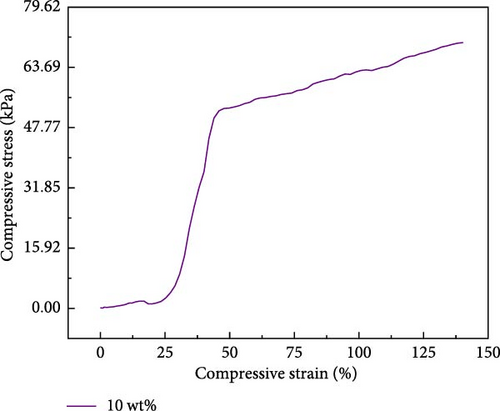
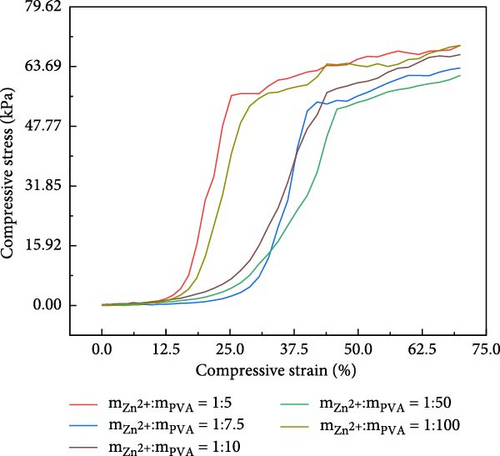
The changes in the compressive performance of PVA upon adding zinc ion-based ionic liquids are depicted (Figure 4c,d). The PVA is placed into a tensile testing machine with a certain gap during the compression process. It is only after a compressive strain of 12.5% that the sample exhibits noticeable force transmission. The experimental results reveal that the introduction of metal ion-based ionic liquids has a negligible impact on the compressive properties of the hydrogel. The compressive strength of 10 wt% PVA hydrogel measures 49 kPa. The compressive strength of the PVA-Zn-3 gel measures 50.6 kPa.
The PVA-Zn-3 gel displays superior electrical conductivity and commendable mechanical properties (Figures 2 and 4b), coupled with notable sensitivity (Figure 3e). The addition of a zinc ion-containing liquid induces a diminishment in the tensile properties of the hydrogels. Optimal incorporation of zinc ion-containing liquid is essential to simultaneously enhance the conductivity of the hydrogel while maintaining its favorable mechanical properties. In conclusion, PVA-Zn-3 gel demonstrates the optimal concentration for achieving a harmonious combination of mechanical performance and conductivity in the hydrogels.
3.3. Sensor Application Analysis
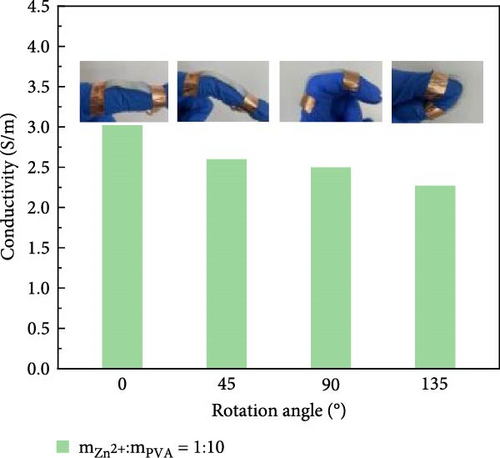
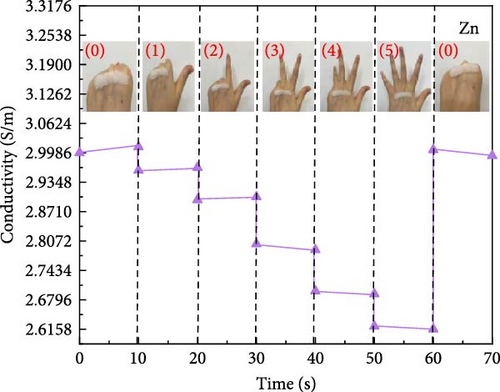
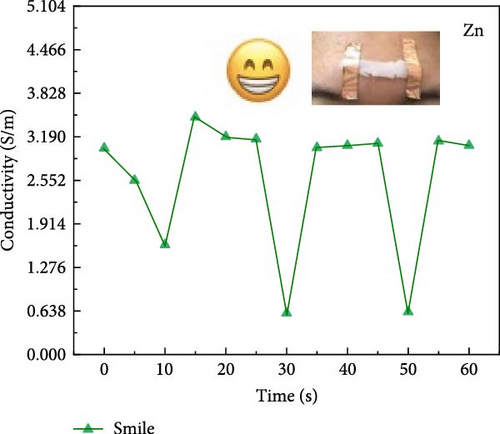
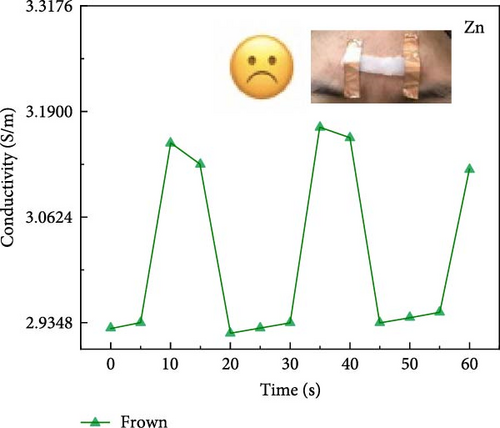
Attaching composite hydrogels onto a rubber glove can be used to simulate monitoring the movement of human joints (Figure 5a). The experimental results reveal that the electrical resistance of the hydrogel exhibits proportional changes with the various angles of finger flexion and extension. With increasing degrees of finger joint flexion at 0°, 45°, 90°, and 135°, the electrical conductivity of the hydrogel decreases proportionally. The electrical conductivity of hydrogel exhibits variations across different hand gestures (Figure 5b). The experimental results reveal that the more extended fingers, the lower the electrical conductivity. The electrical conductivity of hydrogel exhibits variations across different facial expressions. Smiling was performed at 10, 30, and 50 s (Figure 5c), and frowning occurred at 10, 40, and 60 s (Figure 5d). The experimental results reveal that during a smiling facial expression, the hydrogel experiences elongation, leading to a decline in electrical conductivity. During brow furrowing, the hydrogel experiences endpoint convergence, leading to an abrupt increase in electrical conductivity. Therefore, the hydrogel can monitor various human behavioral states [40].
PVA hydrogels demonstrate high sensitivity and stability toward subtle variations [41–44]. Thus, its incorporation as a sensor enables the detection of minute fluctuations. A simple writing board is created by sandwiching the hydrogel between insulating cling film (Figure 6a). The conductivity variation curve of the hydrogel while writing “OK” three times on the simple writing board (Figure 6b). When writing “OK” at 0–20 s, 40–60 s, and 80–100 s, the conductivity has obvious fluctuation during writing. Schematic diagram of sound sensing principle (Figure 6c). Performing LCR measurements with an insulated conductive tape applied to the throat and the utterance of “中国计量大学 (China Jilaing University)” by the tester elicits subtle muscular changes in the throat region. There is a change in the conductivity curve of the hydrogel after the tester vocalizes (Figure 6d). The experimental results reveal that the composite hydrogel demonstrates enhanced conductivity with an ascending trend in the curve when vocalization is performed in the second tone. The conductivity of the composite hydrogel decreases along a descending curve when vocalization is in the fourth tone. Thus, the conductive hydrogel converts subtle motions into measurable electrochemical signals. The diverse amplitude of muscle contractions results in variable rates of resistance change, demonstrating the high sensitivity of the hydrogel-based monitoring sensor.
3.4. Antibacterial Property
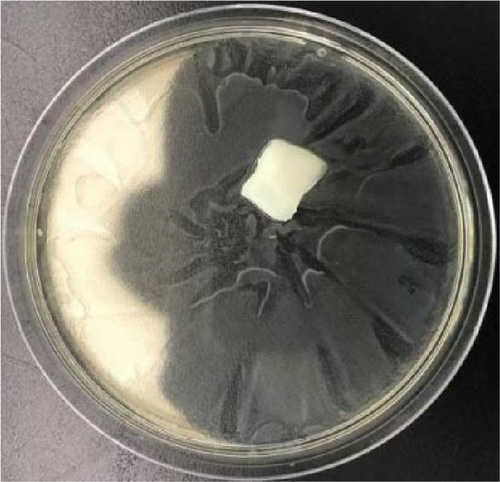
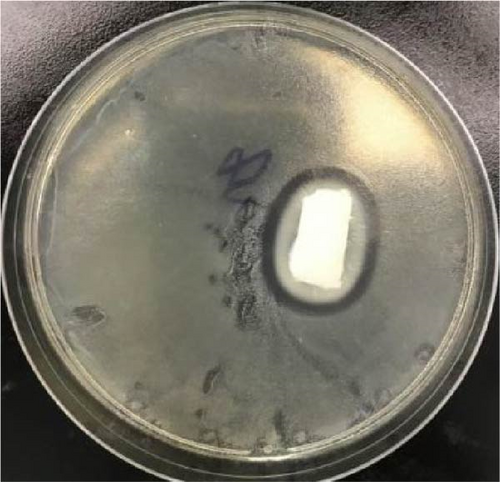
Polymeric hydrogels can also be used for electronic skin, requiring excellent antibacterial properties [45–48]. No inhibition circle was observed around the 10 wt% PVA (Figure 7a,b). Thus, the 10 wt% PVA demonstrates no antibacterial activity against E. coli. An inhibition circle was observed around PVA-Zn-3. The PVA-Zn-3 exhibits inhibitory effects against E. coli. Therefore, the Zn ionic liquid/PVA composite hydrogels exhibit excellent antibacterial properties.
4. Conclusions
In conclusion, adding ionic liquid based on zinc chloride has significantly enhanced the conductivity performance of the hydrogels. Furthermore, excellent mechanical properties are maintained even at a mass ratio 1 : 10 between ionic liquids based on zinc chloride and PVA hydrogels. The synthesized PVA-Zn-3 hydrogel can be used to detect subtle variations in human body movements. This includes bending fingers at different angles, hand gestures, facial expressions, and vocalizations. It can also detect subtle variations during handwriting on a simple writing board. Additionally, the prepared PVA-Zn-3 hydrogel exhibits antibacterial properties, making it suitable for electronic skin applications. In summary, the prepared Zn ionic liquid/PVA composite hydrogels exhibit high conductivity, good tensile strength, and toughness. The hydrogel exhibits high sensitivity in detecting subtle movements, demonstrating significant potential for applications in the field of human sensory sensors.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Liuqing Zheng, Junjie Wang, Ruochen Lei and Jia Wang. The first draft of the manuscript was written by Liuqing Zheng, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The research was funded by Hangzhou Science and Technology Plan Guiding Project (Agriculture and Social Development) (no.20201231Y055).
Acknowledgments
The research was funded by Hangzhou Science and Technology Plan Guiding Project (Agriculture and Social Development) (no.20201231Y055).
Open Research
Data Availability Statement
The corresponding author will make the datasets created and/or analyzed during the current study available upon reasonable request.




