Analysis of Predator–Prey Model With Inclusion of Temperature Variability in Prey Refugees
Abstract
One of the central concerns in ecology is assessing the impact of environmental changes, such as global temperature shifts, on the dynamic interactions between predators and prey across diverse ecosystems. This study explores the relationship between prey and predator populations in the context of climate-induced temperature changes. Utilizing linearization and the Lyapunov approach, we analyze the local and global stability of the system’s equilibrium points. Applying Sotomayor’s theorem, we further investigate the local bifurcation of the fixed points. Additionally, the persistence of the model system is studied. The ecological models developed in this research aim to explore the stability of these predator–prey systems and provide insights to prevent the decline of endangered species. Numerical simulations are conducted to study the impact of environmental changes on the proposed models. The simulation results indicate that the temperature dependence of the predator–prey population dynamics is directly proportional to the size of the reserved habitat area for the prey when the temperature is high. These findings suggest that the preservation of sufficient habitat reserves plays a crucial role in promoting the long-term survival of species and stabilizing their ecological interactions.
1. Introduction
One of the most essential components of biological activity is the biosphere, which is responsible for driving changes in the environment and ecology. Refuges play a crucial role in conservation management, as they have the potential to protect species from difficult-to-manage threats such as changing climate, extreme events (e.g., drought, fire), and biotic threats (e.g., disease, invasive species) [1]. According to Oxford University Press (2019), a “refugium” is a place where a population of organisms can endure challenging conditions without fear of hardship, danger, or being sought after [2]. Since prey escape has a major impact on the system’s dynamic behavior, it is an essential component of the prey–predator system. It is known that in many dynamic systems, prey react to a predator attack by seeking refuge, which allows them to escape from the predator.
The relationship between climate change and the distribution and abundance of various organisms has been the subject of much research; as a result of their incapacity to adapt to their changing environment, some species have declined or even gone extinct [3, 4]. One of the most popular methods for predicting future range boundaries is the climate envelope method, which estimates a species’ fundamental niche space by utilizing correlations between observed environmental variables and the species’ current range boundaries [5]. Nevertheless, recent studies have revealed that organism distribution predictions based on body temperature differ significantly from those based only on environmental factors [6]. Understanding how weather and climate affect body temperature is especially crucial when studying predator–prey interactions [7].
Through a variety of mechanisms, such as changing the quantity and quality of vegetation that primary consumers depend on for foraging, climate variation and climate change may affect predator–prey interactions. This could result in differentiating foraging strategies and patterns of space use [8]. Temperature variations in the climate may also have an effect on prey’s nutritional status, which could alter how they perceive the risk of predators and react to attacks [9]. Climate change may affect the interactions between all species in a community, but predators are more vulnerable to environmental disturbance than plant herbivores, which could have important ramifications for the structure and functioning of the community [10].
Many species have varying climates, which are brought on by variables including humidity, temperature, rainfall, food availability, shifts in daylight, and elements that impact population survival in ecological communities [11]. Temperature affects ecological communities through its effects on the physiological performance of individuals. Temperature variability in the biosphere is having an increasingly noticeable impact on biological systems. Because of this, an organism’s survival and reproduction directly decline when its equilibrium with its surroundings is disturbed, which has an impact on population distribution across different geographic areas. Indeed, problems with temperature, population dynamics, species stability forecasting, and understanding population dynamics when behavior shifts due to climate change all have an impact on prey–predator systems.
The dynamics of prey–predator systems with prey refuge have been extensively studied using mathematical models. Refuges can have stabilizing or destabilizing effects, impacting prey regulation and population outbreaks [12]. Mismanagement, overexploitation, overpredation, and climate change contribute to prey refuges, necessitating steps like designating reserved regions to protect populations [13, 14]. As mentioned in [15], climate variability (through drought) has the potential to throw organisms out of balance with their surroundings.
In this context, we address a gap in the existing literature by incorporating temperature variability and prey refugees. Our study is aimed at examining the local and global stability associated with equilibrium, providing insights into the dynamics of prey–predator systems under changing environmental conditions. Additionally, we note the ecological significance and look into predator–prey dynamics as well as how temperature variability affects the stability of the system. The dynamic result of the system is examined through numerical simulations carried out with MATLAB programming.
2. Mathematical Model Formulation
2.1. Model Assumptions
The effect of climate alteration on the prey–predator is intuitive. Considering this into account made the following assumptions. In this research, the food web consists of three species: P (the prey species in the unreserved area), N (the prey species in the reserved area), and R (the predator species at a specific location) and T (temperature due to climate change) at time t. Predator feeding behavior is governed by the Holling Type-II functional response, which is contingent upon favorable weather conditions and seasonal variations in the predator’s attack rate. Predation rates are temperature dependent. The maximum temperature where prey disturbance is high is Tmax and the balanced temperature for prey is T0, r1(T) is the intrinsic growth rate (temperature dependent) of the prey species in the unreserved area, r2(T) is the intrinsic growth rate (temperature dependent) of the prey species in the reserved area, μ(T) is the predation rate (temperature dependent), and prey refugees and natural death rate of predator populations are temperature independent (T). The conservation management (especially in light of temperature variability) identifies potential refugees’ location. When there is significant temperature fluctuation, the populations move around. Both the prey and the predator coexist in a free region. Prey populations can migrate from unreserved to reserved areas, but predator populations are not allowed to enter the reserved area, and vice versa is not permissible. The movement can be attributed to various factors such as drought, deforestation (potentially for the production of charcoal), and poor resource management. These issues have collectively contributed to the current situation.
Because of the refuge’s limited resources or size, there would be a maximum amount of prey that the area could sustain. Table 1 discusses the parameters in the model system and their biological significance.
| Variables and parameters | Biological meaning |
|---|---|
| P(t) | The population size of the prey in the unreserved area at time t. |
| N(t) | The population size of the prey in the reserved area at time t. |
| R(t) | The population size of the predator in the free region at time t. |
| T(t) | Temperature due to climate change at time t. |
| r1(T) | Intrinsic growth rate (temperature dependent) of the prey species in the unreserved area |
| r2(T) | Intrinsic growth rate (temperature dependent) of the prey species in the reserved area. |
| r3 | Increment rate of temperature. |
| μ(T) | Predation rate (temperature dependent). |
| α | Half-saturation constant. |
| c | Conversion efficiency at specific location. |
| β | Natural death rate of predator. |
| δ | The migration rate of the prey population from the unreserved area to the reserved area. |
| k1, k2 | The carrying capacity of the prey in the unreserved and protected region, respectively. |
The predator’s deadly maximum temperature T ≡ 300 or higher is the point at which the life process is irreversibly disrupted.
The rate at which prey migrates from unreserved to reserved areas, contingent upon predator density, is presumed to be positive and to escalate with the number of prey species, N. In principle, this implies that as the number of predators in the unreserved region increases, more prey either departs or collaborative hunting enhances the predators’ ability to capture prey populations and procure food.
3. Model Analysis
3.1. Positivity, Uniqueness, and Boundedness of Solution
The positivity, uniqueness, and boundedness of the solutions of the model system (2) are all examined in this section.
3.1.1. Positivity of the Solution
For a given initial condition (n(0), p(0), r(0), q(0)), we observe that the system (2) is well-posed. We show positivity of the solutions in the following proposition:
Proposition 1. The solution (p(τ), n(τ), r(τ), q(τ)) of the model system with a positive initial condition (p0, n0, r0, q0) is positive.
Proof 1. Here, it is possible to write the first equation of the model system (2) dp/dτ = p(1 − p) − ηp − σ(pr/(1 + mp)) as
Hence,
Since p is positive, the second equation can be written in the form
This implies
Then
For the third equation of system (2)
Integration gives r(τ) = r0e((ξp/1 + mp) − ϕ)τ which is positive.
Finally, the fourth equation
That is,
Solving this equation, we obtain
Hence, for positive initial conditions, all solutions of model system (2) are positive for all t ≥ 0.
3.1.2. Boundedness of Solutions of the Model System
The following proposition serves to establish the boundedness of the solution model system (2).
Proposition 2. All solutions (p(τ), n(τ), r(τ), q(τ)) of the model system (2) with nonnegative initial condition (p0, n0, r0, q0) is uniformly bounded in the region
Proof 2. We present the following sum relation function in order to demonstrate model system (2)’s boundedness, defined as the total population (2).
Differentiating Z(τ) with respect to τ
Since dp/dτ = p(1 − p) − ηp − σ(q)(pr/(1 + mp)), dn/dτ = φ(q)n(1 − n) + γp, dr/dτ = ξ(q)(pr/(1 + mp)) − ϕr.
Adding ϕZ on both sides for positive ϕ, we have
Therefore, Z(τ) = (ϕ + φ(ϕ + 2)/ϕφ) + ce−ϕτ, where c is constant.
As τ⟶∞, Z⟶(ϕ + φ(ϕ + 2)/ϕφ), that is 0 < Z(τ) ≤ (ϕ + φ(ϕ + 2)/ϕφ) which is bounded. It follows each of the state variables p(τ), n(τ), r(τ) that are also bound.
To show the boundedness of q(τ), we consider the last equation of the model system.
After simplification, we obtain
Hence, as τ⟶∞, q⟶β1 + α1, that is 0 < q(τ) ≤ (β1 + α1) which is bounded.
Here, one can conclude that all the solutions of the system (2) that are initiated in are attracted to the region
Therefore, the solutions p(τ), n(τ), r(τ) and q(τ) are bounded and positive invariant under the given condition.
3.1.3. Uniqueness
The solution of a system starting in the nonnegative octant is bounded, and the right side of the model system (2) is continuous and has continuous partial derivatives of all orders in a domain Ω of the positive octant . Therefore, the solution of the model system (2) with nonnegative initial condition exists and is unique.
3.2. Existence of Equilibrium Points
We demonstrate the existence of a positive solution of model system (2) with omitting equation of temperature in this section. Because it can be difficult to comprehend how temperature and threshold vital rates relate to one another.
- i.
The equilibrium points E0 = (0, 0, 0) and E1 = (0, 1, 0) exist with no condition.
- ii.
There are also additional equilibrium points. The third equilibrium point is , and it exists if 0 < η < 1.
The interior equilibrium points exist if ξ > mϕ, and 0 < (ϕ/(ξ − mϕ)) + η < 1.
3.3. Local Stability of the Equilibrium Point
Utilizing the eigenvalue method, we examine the local dynamic behavior of the model system (2) around each of the equilibrium points shown above.
We examine local stability and the equilibria of system (2) in the following propositions.
Proposition 3. The trivial equilibrium point E0(0, 0, 0) is unstable.
Proof 3. The Jacobian matrix of the model (2) at E0 is
Then, clearly, the eigenvalues of J(0, 0, 0) are λ1 = 1 − η, λ2 = φ, λ3 = −ϕ.
Hence, the trivial equilibrium point E0 is saddle point which is unstable. This biologically means that total annihilation is not possible.
Proposition 4. The equilibrium point E1(0, 1, 0) is locally asymptotically stable if η > 1.
Proof 4. The Jacobian matrix at E1 takes the form
The three eigenvalues of the Jacobian matrix are λ1 = 1 − η, λ2 = −φ, and λ3 = −ϕ.
Therefore, E1 is locally asymptotically stable, if η > 1.
This, in turn, biologically means that prey populations in the unreserved area survive.
Proposition 5. The predator-free equilibrium point E2(p∗, n∗, 0) is locally asymptotically stable if either of the following is true:
Proof 5. The Jacobian matrix of the model system (2) at E2 is
The eigenvalues of the Jacobian matrix J(p∗, n∗, 0) are and λ3 = (−(mϕ − ξ)(ϕ − (1 − η)))/(m(1 − η) + 1).
Here, we notice that since 0 < η < 1, λ1 and λ2 are negative. Moreover, the third eigenvalue λ3 < 0 if ξ − ϕm < 0 and ϕ − (1 − η) > 0, or ξ − ϕm > 0 and ϕ − (1 − η) < 0.
Proposition 6. The positive equilibrium point E3(p∗, n∗, r∗) is locally asymptotically stable in , if
Proof 6. The Jacobian matrix of the system (2) at the positive equilibrium point E3(p∗, n∗, r∗) can be written as
The eigenvalues are obtained from the characteristic equation Det(J(E3) − λI) = 0 and can be written as
Accordingly, the characteristic equation Det(J(E3) − λI) = 0 is
Here,
Hence, by Routh–Hurwitz criteria, the interior equilibrium point is locally asymptotically stable under the conditions A1 > 0, A2 > 0, A1A2 − A3 > 0, combined with ξ > mϕ, 0 < (ϕ/(ξ − mϕ)) + η < 1.
Proposition 7. The interior equilibrium point E4(p∗, n∗, r∗) is locally asymptotically stable in the positive orthant if the following conditions are met:
Proof 7. The Jacobian matrix of the model system at the equilibrium point E4(p∗, n∗, r∗) becomes
The eigenvalues are obtained from the characteristic equation Det(J(E3) − λI) = 0 and can be written as
The characteristic equation Det(J(E3) − λI) = 0 becomes
Here, the explicit expressions are
Hence, we can conclude by the Routh–Hurwitz criteria that the interior equilibrium point E4 is locally asymptotically stable if the conditions B1 > 0, B2 > 0, B1B2 − B3 > 0, along with ξ > mϕ, 0 < (ϕ/(ξ − mϕ)) + η < 1, are satisfied.
3.4. Global Stability
The Lyapunov function is a powerful tool for figuring out the global stability of an equilibrium point [21–24].
Theorem 1. The steady state E2(p∗, n∗, 0) is globally asymptotically stable in the region R+ = {(p, n): p > 0, n > 0} if it exists and is locally asymptotically stable in the interior of the region
Proof 8. Let E2(p∗, n∗, 0) be locally asymptotically stable in the region R1. The model system (2) can be reduced to the form
Consider the function
It is Dulac’s in the region R+ and .
Then, and
Thus, the system has no periodic orbit in pn-plane region, and hence, E2(p∗, n∗, 0) is globally asymptotically stable in the region R+ = {(p, n): p > 0, n > 0}.
Theorem 2. The equilibrium point E3(p∗, n∗, r∗) is globally asymptotically stable.
Proof 9. We consider a Lyapunov function of the form
Rearranging it becomes
Compactly, this can be expressed as
Choosing C1 = 1, C2 = r∗ψp(p∗)/p∗ψr(r∗), we have
Since the terms 1 + (p∗ + η − 1)/p and 1 + ((n∗ − 1)/n) are positive, then (dV/dτ) ≤ 0, and V(τ) is a Lyapunov function. Hence, the positive equilibrium point E3(p∗, n∗, r∗) of the model system (2) is globally asymptotically stable.
3.5. Persistence
- 1.
The right-hand side of the model system (2) is C1 in (p, n, r).
- 2.
All solutions of the system (2) with initial conditions are bounded in forward time according to Proposition 2.
- 3.
E1(0, 1, 0) exists and hyperbolic saddle point if 0 < η < 1.
- 4.
An equilibrium point E2(p∗, n∗, 0) in the pn-plane exists, and it is unstable in the positive direction orthogonal to the pn-plane if ξ − mϕ > 0 and ϕ − (1 − η) > 0 or ξ − mϕ < 0 and ϕ − (1 − η) < 0. Since E2(p∗, n∗, 0) is locally asymptotically stable whenever it exists, it is globally asymptotically stable with no limit cycles around this point [25]. In the pn-plane, the stable point is designated as the unique equilibrium point E2. This theorem generalized the existence of the model system (2). The proof of positivity and boundedness is also provided in Proposition 1 and Proposition 2.
The observations outlined above are formalized into the following theorem.
Theorem 3. The model system (2) meets the necessary conditions for persistence whenever the equilibrium point E2(p∗, n∗, 0) exists, provided that 0 < η < 1, ξ − mϕ < 0 and ϕ − (1 − η) < 0, or ξ − mϕ > 0 and ϕ − (1 − η) > 0.
Proof 10. Suppose Φ(Y) be the orbit through the point in the positive octant Y = (p, n, r) with p > 0, n > 0, r > 0. Let ρ(Y) be the ω-limit set of Φ(Y). Note that ρ(Y) is bounded.
We claim that E0 does not belong to ρ(Y). If E0 ∈ ρ(Y), then by Lemma A1 of [25], there exists another point u in ρ(Y) ⋂ Ws(E0) where Ws(E0) denote the stable manifold of E0. Since, Φ(u) and Ws(E0) in the r-direction, we conclude that Φ(u) is unbounded, which is a contradiction.
Similarly, E1 ∉ ρ(Y). If E1 ∈ ρ(Y), then there exists a point u in ρ(Y) ⋂ Ws(E1) where Ws(E1) denote the stable manifold of E1. Since, Φ(u) lies ρ(Y) and Ws(E1) in the nr-direction, we conclude that Φ(u) is unbounded, which is a contradiction.
In the same way, E2 ∉ ρ(Y). If E2 ∈ ρ(Y), the condition 0 < η < 1, ξ − mϕ > 0 and ϕ − (1 − η) > 0 or ξ − mϕ < 0 and ϕ − (1 − η) < 0 implies that E2 is a stable point and Ws(E2) is the pnr-direction; again, we conclude that unbounded orbit lies in ρ(Y), which is a contradiction.
As a result, the model system (2) persists and ρ(Y) lies in the positive octant. Ultimately, the model system (2) is dissipative and only the closed orbits and equilibria form the ω-limit set of the solutions in the boundary of .
3.6. Local Bifurcation Analysis
Many ecologists are exploring the applications of mathematics in species biology.
Theorem 4. The model system (2) undergoes no bifurcation around the trivial equilibrium point E0(0, 0, 0).
Proof 11. The Jacobian matrix around the equilibrium point E0(0, 0, 0) is
Computing the characteristic equation, we have λ1 = 1 − η, λ2 = φ, λ3 = −ϕ; hence, it is a saddle node. If 1 − η = 0, then there is a characteristic root with eigenvalue λ = 0 and eigenvectors corresponding to the matrix J and JT given as
Further
On the other hand
Computing the third condition of Sotomayor’s theorem wT[D2f(E0, η = η∗)(V, V)], using the following:
Thus,
Then, we investigate the condition: wTfη(E0, η = η∗) = 0, wT[Dfη(E0, η = η∗)V] = 0, wT[D2f(E0, η = η∗)(V, V)] = 0.
Thus, there is no bifurcation around the trivial fixed point E0(0, 0, 0), which is unstable.
Theorem 5. The model system (2) exhibits a transcritical bifurcation at η = 1 near the steady state E1.
Proof 12. The Jacobian matrix at E1 takes the form
The three eigenvalues of the Jacobian matrix are λ1 = 1 − η, λ2 = −φ, and λ3 = −ϕ, which is stable if η > 1. By substituting E1(0, 1, 0) with η = 1 in the Jacobian matrix J(E1), the characteristic equation has zero eigenvalues.
The eigenvectors V and w which are associated to zero eigenvalues of matrices J and JT, respectively, are
Let f be the right-hand side of the model system (2),
The derivative of the model system (2) with respect to the control parameter η is
It follows that
Hence, fη(E1, η) = (0, 0, 0)T.
The first condition of Sotomayor’s theorem is satisfied:
In addition,
The second condition of Sotomayor’s theorem is again satisfied:
Computing the third condition of Sotomayor’s theorem wT[D2f(E1, η)(V, V)], using Equation (10), we obtain
Thus,
Thus, we conclude that all three conditions of Sotomayor’s theorem are satisfied. That is
Then, transcritical bifurcation occurs in the start-up equilibrium point, and the stability of this equilibrium will be changed at the bifurcation point η.
Theorem 6. A transcritical bifurcation occurs near the equilibrium point E2(p∗, n∗, 0) when the system parameter satisfies the restriction η1 = 1 + (φ/4γ).
Proof 13. If p ≠ 0, n ≠ 0, r = 0, then we have
Let the discriminant of Equation (12) be represented by Δ and express Δ in terms of η, that is
Let η1 be the zeros of Δ(η). Upon computation, we have
The Jacobian matrix at E2 is
If l1 = 0, there is zero characteristic root. The eigenvectors V and W associated to zero eigenvalues of matrices J and JT, respectively, are
The derivative of the model system (2) with respect to the control parameter η is
The first condition of the Sotomayor’s theorem is
Using Equation (10), we obtain
Accordingly,
Then, it follows that
Therefore, WTfη(E2, η) ≠ 0, WT(Dfη(E2, η)V) ≠ 0, WTD2fη(E2, η)(V) ≠ 0.
Hence, Sotomayor’s theorem for transcritical bifurcation [26] indicates that there is a transcritical bifurcation around the predator-free fixed point E2 with threshold parameter η1 = 1 + (φ/4γ).
4. Numerical Simulation
In this study, we present simulation results obtained through an analysis of model (1) using ode45. The numerical outcomes not only reinforce our analytical conclusions but also contribute to a deeper comprehension of their dynamical characteristics. Subsequent sections will exemplify the numerical solutions for model (1), by taking one parameter at a time, based on the information provided in the system. This approach allows us to systematically investigate the influence of varying parameter values on the system’s behavior (1).
4.1. Simulation Results
This section’s primary goal is to use numerical simulation to determine the critical model system (1) that influences the suggested model’s performance. In the present study, the rate of temperature (T), growth rate r(T), refuge coefficient (δ), and predation rate (μ(T)) are the key parameters which will be taken as a control parameters.
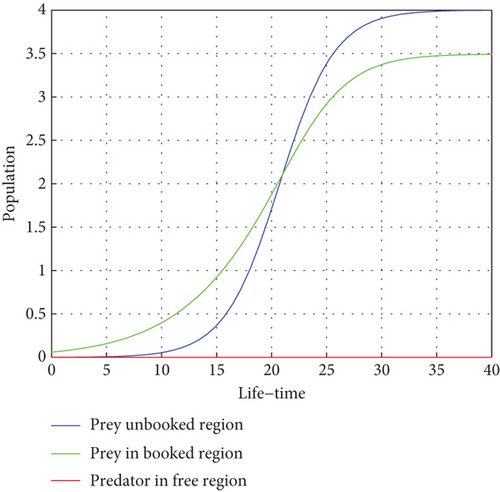
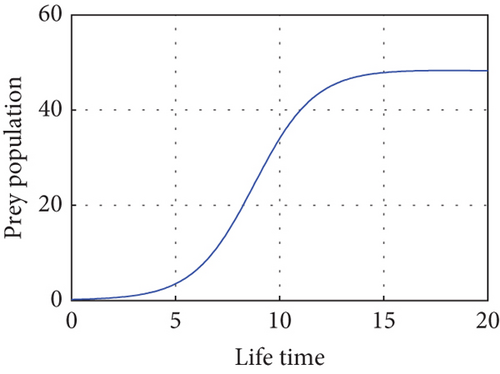
Figure 1 illustrates how there are fewer predators than prey, and the number of prey will rise. Within a population cycle of expansion and fall, these dynamics persist. Both prey populations are still bounded, and T = 27.21°C indicates that, in the absence of predators, the migration rate of both prey regularly surpasses a certain threshold. This supports Theorem 1.
Here, we verify the fulfillment of the prerequisites outlined in Theorem 2, establishing the global asymptotic stability of the system. The established local stability along the solution branch serves as a tool to characterize the overall qualitative behavior of the system. In Figure 2, we depict the dynamics of the three populations based on the data provided at a temperature of T = 26.3°C. This specific temperature condition allows the predator to coexist in the model system (1) with focal prey during periods of elevated temperatures and diminishing population growth rates.
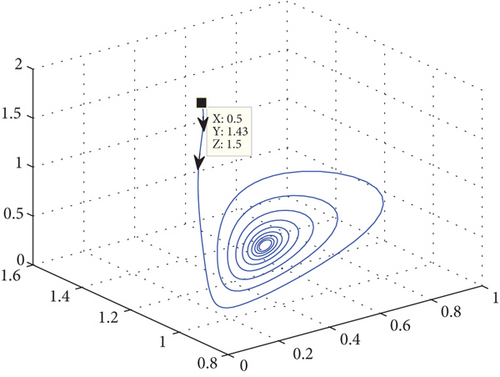
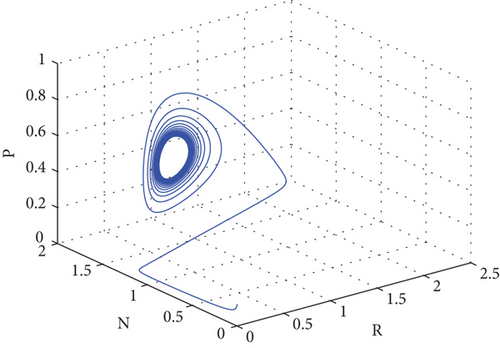
The most significant exogenous variable influencing predator populations on prey is temperature. There are two stages to the relationship between temperature and life history factors, such as development rate. The intrinsic growth rate of a population can be described as having a rise during the first stage, which takes place in the interval between some base and optimal temperature. Should this ideal temperature ever be surpassed, the population growth rate will decrease to zero at the highest point during the second stage. Using the parameters in the Table 2, we can comprehend Figure 2. Theorem 2 is verified first in this analysis, and E(P∗, N∗, R∗) = (0.86,1.64,3.52) indicates the existence of the interior equilibrium point of the model system (1) with parameter values r1(T) = 0.45, r2(T) = 1.19604, δ = 0.06, μ(T) = 5.9.
The oscillation behavior of the other interior equilibrium point is seen here in Figure 2. Figure 2 is the corresponding phase portrait which shows a stable limit cycle around the fixed point. We note that, because the initial value is taken near to E2, the system achieves the attractor soon when the oscillation amplitudes for all time series have the same size, which is globally stable in Figure 3.
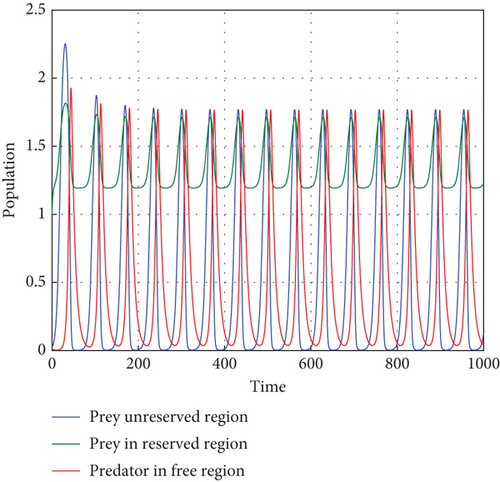
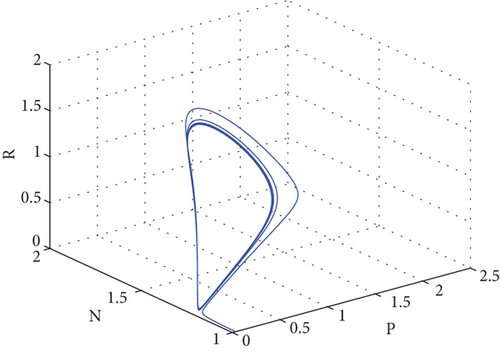
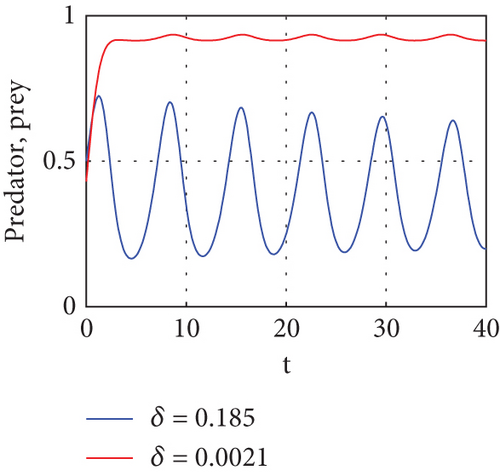
One effective strategy to safeguard the prey–predator system is to build a refuge zone; another is to give the predator more food. Predators stop attacking their prey when they have enough food, allowing the prey to live in the wild. The addition of food supplementation benefits the prey as well as the predator and is generally employed as a biological control tactic. Parameter δ is a significant metric that indicates the quantity of additional food. Thus, we carry out more research to learn how and influence system dynamics. We numerically simulate in multiple ways.
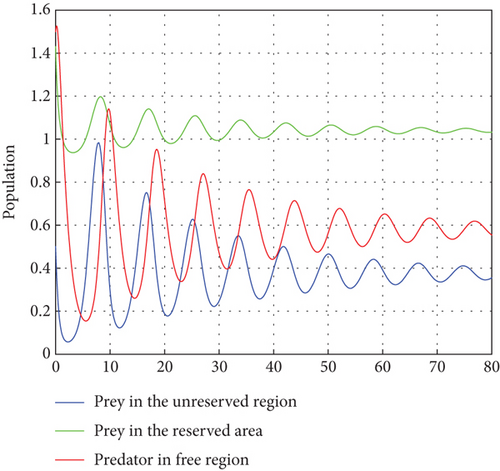
The interior equilibrium point of the system (1) is (0.72,0.012, 0.25), with parameter r1 = 0.1, r2 = 0.19, α = 0.5, μ = 0.2. We confirm that Theorem 1 is locally asymptotically stable. Look at Figure 4.
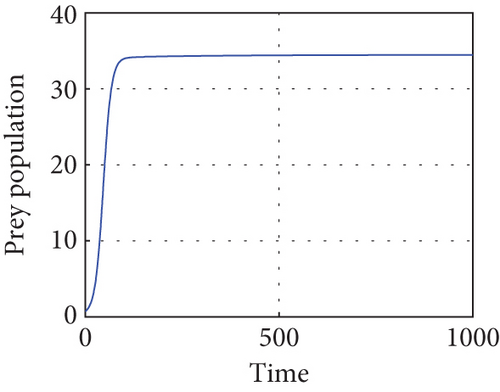
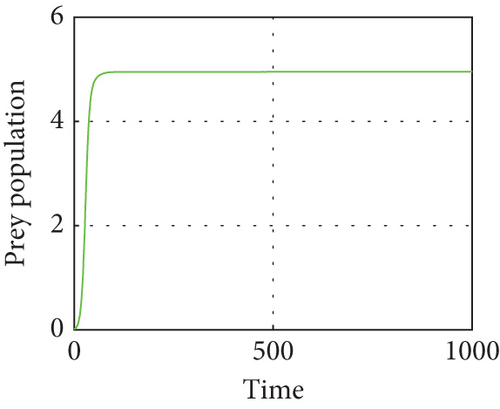
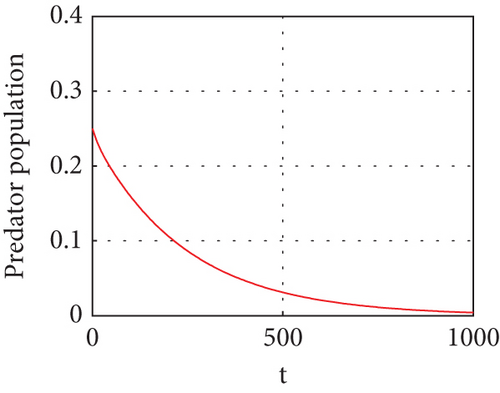
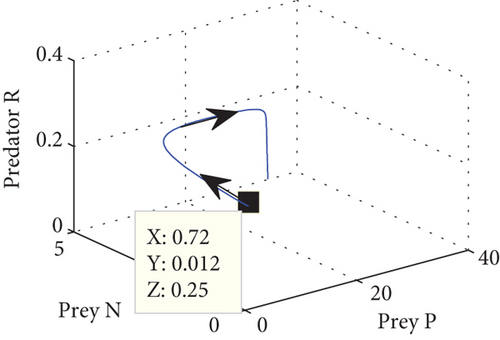
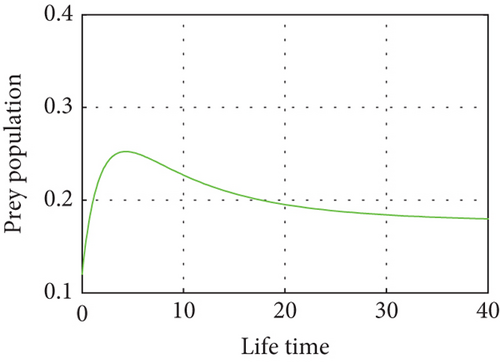
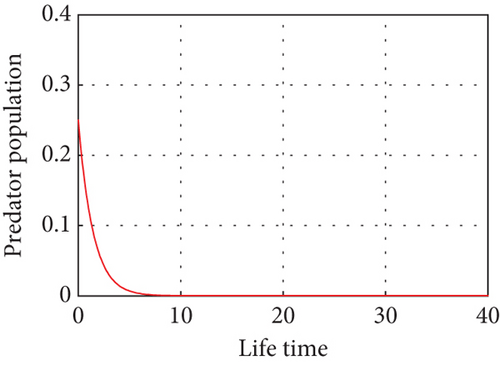
Figure 4 shows that prey populations are increasing as the decreasing of predator populations.
Figure 5 shows that if prey refugees increase, the predator population decreases. That is, prey is inversely proportional to the predator. Increases in the prey refugee can be due to drought (high temperature). Increasing temperature becomes more disturbance of the existing population. Consequently, increased prey refuge becomes a predator population that will die out.
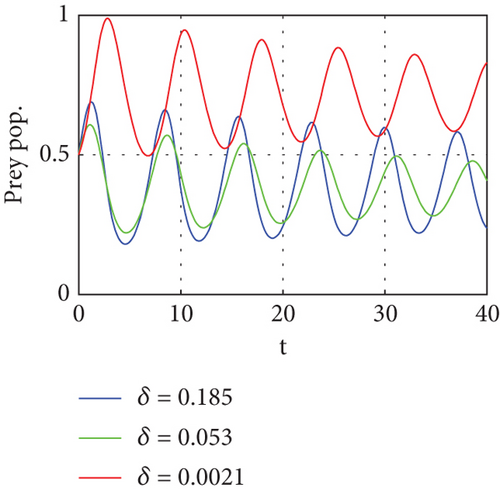
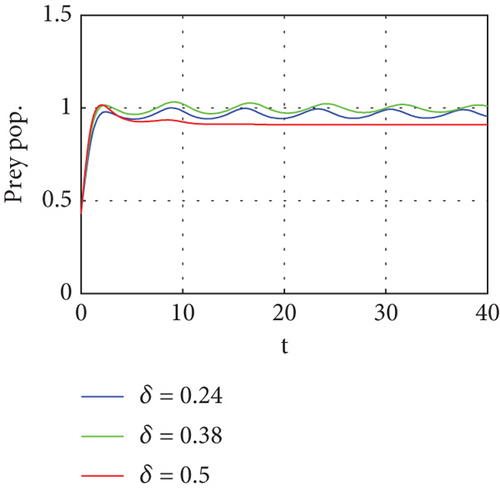

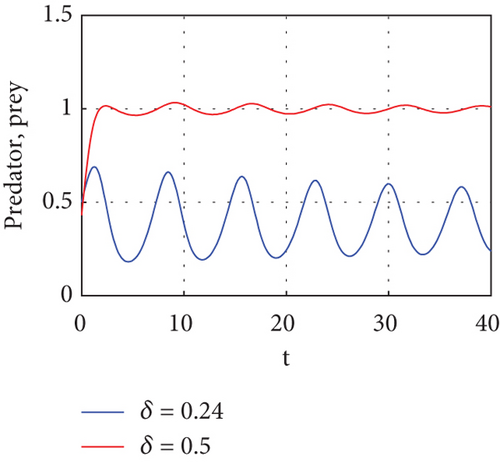
Figure 6 demonstrates that when prey refugee numbers decline, predator populations grow and have access to more food. Declines in prey refuge population size can be due to temperature balance. The stability range of predators increases gradually due to a decrease in prey refuge and an increase in prey in reserved areas.
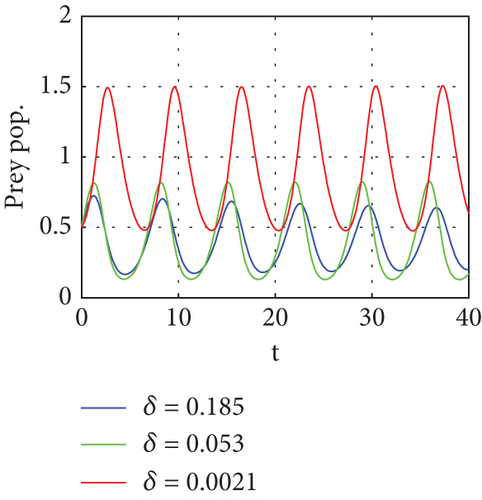
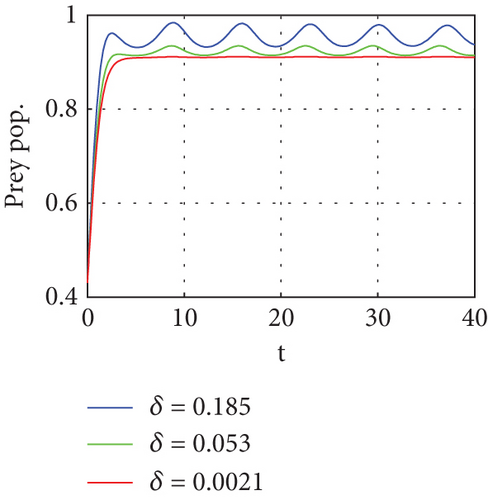
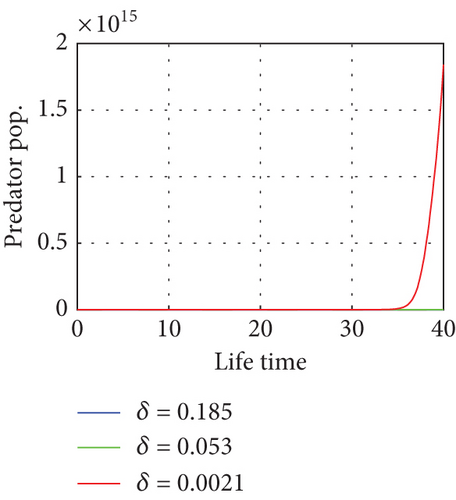
The bifurcation parameters are summarized in Table 3.
| Bifurcation | Thresholds of parameters | Numerical values | References |
|---|---|---|---|
| Transcritical bifurcation | φ | 0.1667 | |
| γ | 0.8333 | ||
| η | 1, 1.8 | [4] |
Figure 7 illustrates the bifurcation diagram around the parameter η that shows the impact of prey population in the reserved area, and it confirms Theorem 5.
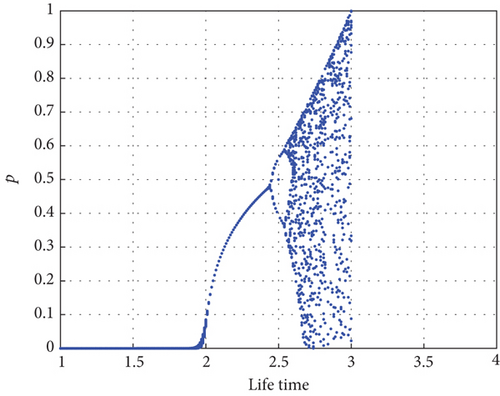
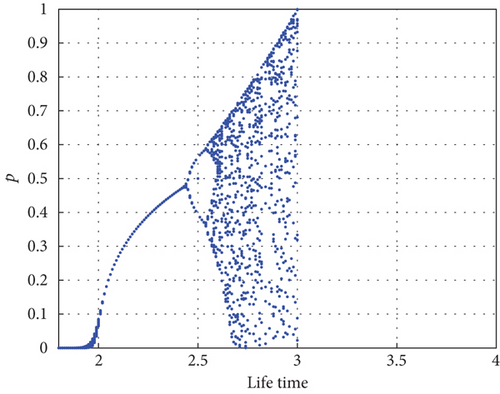
The bifurcation diagram shown in Figure 8 examines the impact of parameter η1. Predator–prey interaction only displays equilibrium dynamics if the proportion δ of prey refuge is increased to 0.45, as determined from dimensionless data. The red color dash of Figure 8 indicates that the predator conversion is disruptive when the prey refuge is high. For η1 < 0.45, there is an asymptotically stable equilibrium E2, as shown by Proposition 4, which is indicated by a solid line (blue) where the red color curve in Figure 8 serves as the saddle point.
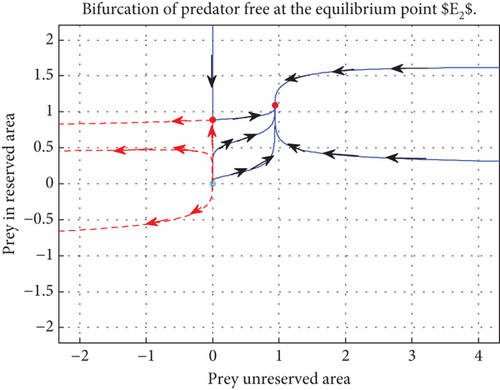
The main results indicate that refuge has a major impact on the stability of the system. Two strategies that work well to lessen the effects of prey refuge and maintain both predator and prey for their natural survival are the creation of refuge areas and the release of more food for predators. Although these methods are both positive and negative, improper application of them can lead to the extinction of a species in addition to maintaining its ongoing survival.
4.2. The Impact of Temperature on Predator–Prey Population
Climate change may impact populations in a number of ways, ranging from individual attributes to population-level impacts that may reshape predator–prey relationships. Climate-related temperature variations can affect a species’ developmental characteristics, particularly its behavior, which can influence how a predator–prey relationship plays out. Look at the following figures.
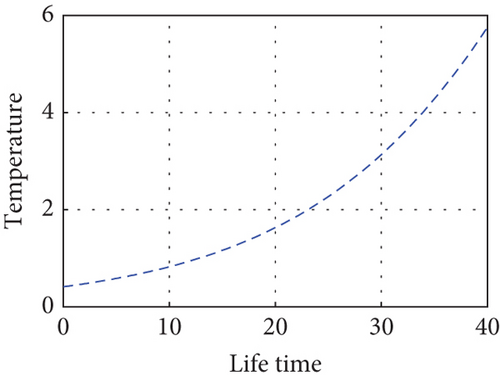
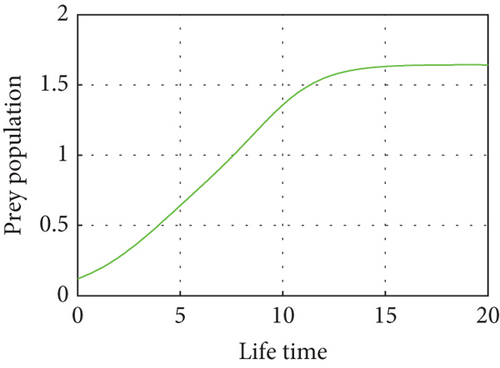
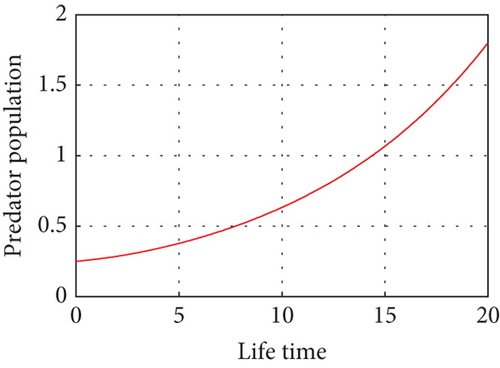
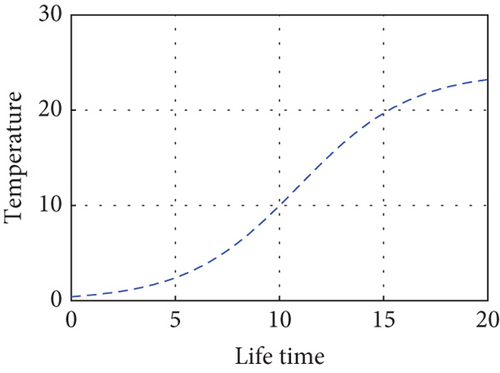
Here, we propose a conceptual model that explains how the relative performances of predators and prey affect the predator–prey populations’ temperature dependence. The predator population is declining as the temperature rises above (27.3°C) due to a lack of food sources and drought, and the prey population in the unreserved area is also declining as a result of climate variability, high temperatures (drought), and the prey reserved area gradually declining in the reserved area as shown in Figure 9. Drought-related climate change may alter predator–prey interactions by altering the spatial and temporal makeup of local communities. This demonstrates how predator–prey populations experience disruptions in their lives when temperatures are high during drought seasons. Populations may exhibit shifts in cyclic length or system stability in response to rising temperatures. The temperature balance is between 10°C and 26°C, and the predator population in the free region is growing modestly, in direct proportion to the increases in the prey population as shown in Figure 10.

5. Conclusion
In conclusion, our study highlights the crucial role of refuges in the biological regulation of predator–prey populations. Expanding refuges, however, may lead to higher prey densities and population epidemics, particularly in hotspot regions qualified as refugia where predators struggle to subdue prey effectively. The solution to our model asymptotically approaches its equilibrium level in the interior of for various values of δ, indicating the significance of migration to reserved regions. As a result of many predator–prey groups actually having access to some kind of shelter, the model becomes more realistic when refuge is added to the system (1). While prey immigration can lessen the chance of extinction due to predation, much like sanctuary does, predator immigration can help manage prey populations and preserve predator populations. From our perspective, the models are significant because they provide a theoretical framework and a way to assess the effects of adding temperature dependency to the intrinsic growth rate.
Indeed, the intrinsic growth rate of the prey population as a function of temperature is represented by the parameters r1(T) and r2(T), which have been taken to be constant. Following completion of the work described in this study, we included a temperature-dependent relationship for μ(T). This relationship was derived from relevant parameter values in a manner similar to determining the temperature-dependent values of r1(T) and r2(T). Consequently, it makes sense to conclude that the temperature-dependent regions that are important to the population dynamics of the system are the relationships shown in Figures 1 and 4. We present quantitative evidence in favor of the hypothesis that the equilibrium density of prey decreases while that of predators rises as prey refuges grow. In actuality, predator equilibrium density rises and then falls as prey refuges increase.
Furthermore, varying the parameter δ, representing the impact of the unreserved region on population behavior, influences the system’s solution near the equilibrium point. Decreasing δ, indicative of abundant prey, negatively impacts predator density. These findings contribute to our understanding of how environmental factors, refuge dynamics, and population behavior interact in complex ecological systems.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Negeri Negese Wayesa: methodology, software, formal analysis, investigation, writing—original draft, writing—review and editing. Legesse Lemecha Obsu: conceptualization, methodology, validation, formal analysis, investigation, writing—review and editing, supervision. Mohammed Yiha Dawed: conceptualization, investigation, validation, formal analysis, writing review and editing, supervision.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors thank Bule Hora University for its hospitality and support during this work.
Open Research
Data Availability Statement
The data utilized in this study are provided within the manuscript itself.




