Gender Differences in Adverse Events of Ketamine Drugs: A Real-World Study Based on FAERS
Abstract
Objective. To identify gender differences in the adverse events (AEs) of ketamine, reduce the AEs among patients, and contribute to the advancement of personalized medicine. Methods. A normalized dataset from 2004 Q1 to the 2022 Q4 in the US Food and Drug Administration Adverse Event Reporting System (FAERS) was analysed. The reporting odds ratio (ROR), proportional reporting ratio (PRR), and P value were used to detect the risk signals from the data in the FAERS database and quantify the presence and extent of gender differences in ketamine AEs. Results. Totally, 5,477 ketamine (female/male (2507/1795)) AE reports were analysed, and sedation (ROR 1.30 (1.07, 1.58)), suicidal ideation (ROR 1.30 (1.03, 1.64)), nausea (ROR 1.37 (1.05, 1.78)), depression (ROR 1.22 (1.13, 1.61)), dizziness (ROR 2.25 (1.78, 2.90)), anxiety (ROR 1.48 (1.09, 1.99)), and other adverse events were found to be significantly more frequent in male patients than in female patients. Conclusion. Using FAERS, we identified gender as factors associated with ketamine-related AEs. With the limitations inherent to this open data source, our data need prospective validation but elucidate potential factors for a personalized side effect profiling.
1. Introduction
Depression ranks among the most prevalent mental health conditions, manifested by a persistent low mood and associated with symptoms such as fatigue, impaired concentration, and insomnia [1, 2]. Ketamine, an N-methyl-D-aspartic acid (NMDA) receptor antagonist, was originally used in clinical practice as a vital anesthetic in the 1960s, but its application in the treatment of major depressive disorders (MDDs) is a relatively more recent addition to the arsenal of treatments for depression [3]. Early research on ketamine proved that a single intravenous subanesthetic dose of ketamine could quickly and significantly reduce depressed symptoms [4–8], and intravenous administration can significantly reduce depressive symptoms within 2–4 hours, with an effective rate of 70%–80% [9–12]. Studies have revealed that there are differences in drug metabolism, absorption, and distribution between men and women [13, 14], which contribute to the differences in individual responses to drugs. Epidemiological studies have identified notable gender differences in MDD incidence rates [15–17]. Studies have consistently demonstrated that women are more likely to have MDD than men [18] (roughly 21% for women and 12% for men) [19, 20]. Over 12 months, 3.5% of men and 5.8% of women worldwide had MDD [21].
There were differences in the efficacy of ketamine in the treatment of depression between genders [22, 23], and there may also be gender differences in the occurrence of drug adverse events (AEs). A randomized controlled study showed that the most common adverse events in patients treated with ketamine were nausea, separation, and dizziness, and the incidence of adverse events was higher in women than in men [24]. In addition, studies have implied that adverse events in the nervous system mainly affect women [25]. However, these studies were limited by their inclusion of small cohorts of patients, which limited power to detect statistically significant differences in AEs between male and female patients. The lack of a clear understanding of the contribution of gender to AE risk and the potential clinical impact of this knowledge merit a more comprehensive and multisource characterization of AE differences between genders.
Spontaneous reporting offers a potentially superior method to study AEs in the real world. This research was a retrospective pharmacovigilance analysis using the FDA Adverse Event Reporting System (FAERS) database, and signal analysis evaluation of AE gender differences in ketamine drugs was conducted to provide decision support for treatment plans of patients with different genders and help clinically rational drug use.
2. Materials and Methods
2.1. Data Acquisition and Preprocessing
More than 19 million global case reports on possible medication side effects are available in the FAERS database. From the FAERS website (https://fis.fda.gov) on April 1, 2023, patient information reported between Q1 2004 and Q4 2022 was retrieved, because more than 20% of FAERS records have been duplicated [26]. Moreover, in contrast to the AE terms, which are standardized and coded by the MedDRA [27] (http://www.meddra.org), the drug names in FAERS are not normalized. Instead, they may be full names, trade names, and abbreviations, and spelling mistakes are not uncommon, which further complicate downstream analysis. Previously, we have standardized the FAERS data into 3 steps [28]. The first step is data deduplication, where redundant reports are deleted in compliance with the suggested method of the FDA. In the second step for drug name normalization, RxNorm [29], a standard nomenclature that provides a normalized naming system for clinical drugs, was used. Drug names, together with administration route and dose information, were mapped to concept-unique identifiers in RxNorm through a medication information extraction system named MedEx [30]. The AE terms were matched to MedDRA’s preferred term code and classified into MedDRA System Organ Class (SOC). In the third step, drugs were aggregated into classes by NDF-RT 24, a drug terminology dictionary belonging to RxNorm. Drug search names are listed in Supplement 1. Data with the names “DEMO,” “DRUG,” “REAC,” “OUTC,” “RPSR,” “THER,” and “INDI” are among those found in the FAERS database. We mostly use the following three data: (1) “DEMO” gives the case ID, gender, age, year of the event, country of the event, and type of reporter’s occupation; (2) “REAC” lists all adverse events that may have been brought on by the DRUG each patient used; and (3) “Drug” gives the name, dosage, indication, dosing, and discontinuation date of each drug that may be linked to the AE. The reporter labels the causal determination of the association between each drug and its reported AE in the “DRUG” table as “primary suspected (PS),” “secondary suspected,” “concomitant,” or “interaction.” Only “PS” was included in our analysis to lower the possibility of drawing conclusions that were falsely positive. Only the most current report acquired from the same case ID was kept after duplicate reports were removed.
To more clearly define the signals of ketamine drug gender differences, signals were mined and analysed from the level of Preferred Term (PT), and classified into various SOC, and High-Level Group Term(HLGT).
2.2. Statistical Analysis
All data categorizations and statistics were performed using SAS version 9.4 and Microsoft Excel version 2023.
3. Results
The basic data of AEs reporting were extracted to obtain 5,477 ketamine AE reports, with female/male of 2,507/1,795. The clinical outcomes of AE patients, reporting crowd, age distribution, and country are shown in Table 1. It was observed that men reported a higher incidence of severe clinical outcomes resulting from AEs, including death, compared with women who reported a greater quantity of AEs overall.
| Female | Percentage (%) | Male | Percentage (%) | |
|---|---|---|---|---|
| Clinical outcome | ||||
| Death | 115 | 5 | 197 | 11 |
| Hospitalization | 641 | 26 | 454 | 25 |
| Threat to life | 124 | 5 | 82 | 5 |
| Disability | 32 | 1 | 18 | 1 |
| Other | 1096 | 4 | 835 | 47 |
| Reporting crowd | ||||
| Medical workers | 502 | 20 | 327 | 18 |
| Consumer | 830 | 33 | 511 | 28 |
| Unknown | 1174 | 46 | 956 | 53 |
| Age distribution | ||||
| ≤18 | 69 | 3 | 92 | 5 |
| 18∼64 | 1763 | 70 | 1067 | 59 |
| ≥65 | 280 | 11 | 135 | 8 |
| Unknown | 395 | 16 | 500 | 28 |
| Country | ||||
| USA | 1891 | 75 | 1257 | 70 |
| Others | 610 | 24 | 526 | 29 |
| Unknown | 6 | 1 | 11 | 1 |
Signal detection results at the PT level showed that the high-risk signals of ketamine in women included dissociation (ROR 1.32 (1.11, 1.56)), sedation (ROR 1.30 (1.07, 1.58)), suicidal ideation (ROR 1.30 (1.03, 1.64)), nausea (ROR 1.37 (1.05, 1.78)), depression (ROR 1.22 (1.13,1.61)), vomiting (1.26 (0.95, 1.67)), anxiety (ROR 1.48 (1.09,1.99)), and dizziness (ROR 2.25 (1.78,2.90)). In men, high-risk signs included dissociation, sedation, drug ineffectiveness, suicidal thoughts, drug abuse, and nausea (Table 2).
| SOC | HLGT | PT | Male | Female | ROR (95% CI) | PRR (95% CI) |
|---|---|---|---|---|---|---|
| Psychiatric disorders | Various dissociative disorders | Dissociation | 398 | 221 | 1.32 (1.11, 1.56) | 1.32 (1.11, 1.56) |
| Various acts of suicide and self-harm (not classified) | Suicidal ideation | 202 | 113 | 1.30 (1.03, 1.64) | 1.30 (1.03, 1.64) | |
| Depressive mood disorders and confusion | Depression | 142 | 84 | 1.22 (1.13, 1.61) | 1.22 (1.13, 1.61) | |
| Anxiety disorders and symptoms | Anxiety | 130 | 64 | 1.48 (1.09, 1.99) | 1.48 (1.09, 1.99) | |
| Various acts of suicide and self-harm (not classified) | Suicide attempt | 72 | 41 | 1.27 (0.86, 1.87) | 1.27 (0.86, 1.87) | |
| Nervous system disorders | Neurological diseases (not otherwise classified) | Sedation | 294 | 165 | 1.30 (1.07, 1.58) | 1.30 (1.07, 1.58) |
| Neurological diseases (not otherwise classified) | Dizziness | 113 | 78 | 2.25 (1.78, 2.90) | 2.25 (1.78, 2.90) | |
| Headache of all kinds | Headache | 75 | 24 | 2.27 (1.43, 3.60) | 2.27 (1.43, 3.60) | |
| Neurological diseases (not otherwise classified) | Somnolence | 43 | 26 | 1.19 (0.73, 1.95) | 1.19 (0.73, 1.95) | |
| Vomiting | Signs and symptoms of gastrointestinal system | Nausea | 162 | 86 | 1.37 (1.05, 1.78) | 1.37 (1.05, 1.78) |
| Signs and symptoms of gastrointestinal system | Vomiting | 136 | 78 | 1.26 (0.95, 1.67) | 1.26 (0.95, 1.67) | |
| Signs and symptoms of gastrointestinal system | Abdominal pain upper | 19 | 4 | 3.43 (1.17, 10.10) | 3.43 (1.17, 10.10) | |
| Oral soft tissue diseases | Hypoaesthesia oral | 12 | 5 | 1.73 (0.61, 4.92) | 1.73 (0.61, 4.92) | |
| Inappropriate schedule of product administration | Off-specification use and intentional misuse/use of products | Off-label use | 86 | 85 | 0.73 (0.54, 0.98) | 0.73 (0.54, 0.98) |
| Medication errors and other product use errors and problems | Product dose omission issue | 83 | 29 | 2.08 (1.36, 3.18) | 2.08 (1.36, 3.18) | |
| Various injuries (not otherwise classified) | Fall | 23 | 10 | 1.66 (0.79, 3.50) | 1.66 (0.79, 3.50) | |
| Medication errors and other product use errors and problems | Product dose omission issue | 23 | 18 | 0.92 (0.50, 1.71) | 0.92 (0.50, 1.71) | |
| Overdose and underdose (not classified separately) | Underdose | 23 | 11 | 1.51 (0.74, 3.10) | 1.51 (0.74, 3.10) | |
| Hypotension | Vascular hypertensive disease | Hypotension | 92 | 55 | 1.21 (0.86, 1.69) | 1.21 (0.86, 1.69) |
| Decreased blood pressure and abnormal blood pressure of unknown nature and shock | Hypotension | 22 | 22 | 0.72 (0.40, 1.30) | 0.72 (0.40, 1.30) | |
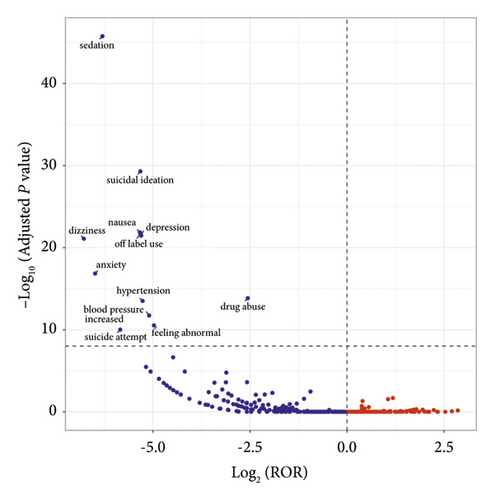
The visualization of signal detection outcomes was accomplished through the creation of a “volcano plot.” This analytical technique facilitated the visualization and interpretation of the gender-specific AEs signals associated with ketamine. The values of −Log10 P for the vertical axis and Log2 ROR for the horizontal axis of the volcano diagram were taken as scales, and the results are shown in Figure 1. According to the figure, sedation, suicidal ideation, nausea, depression, dizziness, anxiety, and other adverse events in male patients were significantly higher than those in female patients.
4. Discussion
Research has established a connection between hippocampal glutamate concentrations and psychiatric symptoms. It has been demonstrated that ketamine can induce a selective increase in hippocampal glutamate levels in males [35], which indicated that there were gender-specific responses to the drug’s effects on the glutamatergic system. Studies on animals have revealed distinct gender differences in ketamine’s mechanism of action. Zarate Jr. et al. [36] found that female rats require only half the minimum dose of males to produce antidepressant effects, which suggested that there are gender differences in the mechanism of action of ketamine [23]. As confirmed by Carrier and Kabbaj [37], female rats are more sensitive to ketamine because they are sensitive to low doses of ketamine (2.5 mg/kg) compared with male rats. However, several meta-analyses did not find gender differences in the antidepressant effects of ketamine [36]. Coyle and Laws [38] noted a slight increase in sensitivity to ketamine in men, but this discrepancy only emerged seven days post-infusion [38]. Similarly, Freeman et al.’s investigation into ketamine as a rapid treatment for treatment-resistant depression revealed no substantial gender-based differences in tolerance [22]. Despite the varied findings of these studies, our real-world research indicated that the frequency of psychiatric AEs, such as sedation, suicidal thoughts, nausea, depression, dizziness, and anxiety, in male patients treated with ketamine is significantly greater compared with female patients. These results offer valuable insights into the study of the gender-specific effects of ketamine.
Jones et al. found that the incidences of nausea and dissociation were higher among women than among men among esketamine-treated patients, regardless of age [24, 39]. Due to the limitation of their study’s small sample size, we have achieved a real-world study to complement RCT. Lee et al. [40] found that pharmacovigilance databases contain larger numbers of adverse drug events (ADEs) that occurred in women compared to men. The cause of this disparity is frequently attributed to sex-linked biological factors. They offer an alternative Gender Hypothesis, positing that gendered social factors are central to the production of aggregate sex disparities in ADE reports. Fisher et al. [41] provided evidence that many sex-biased adverse events (SBAEs) are associated with drug targets and drug metabolism genes that are differentially expressed and regulated between males and females. These SBAE-associated drug metabolism enzymes and drug targets may be useful for future studies seeking to explain or predict SBAEs.
Ketamine is a racemic mixture of two enantiomers (S-ketamine and R-ketamine). Currently, there is a lack of clinical studies on gender differences between the two enantiomers. Studies have revealed that when S-ketamine, which is more clinically relevant, was used as an indication for analgesia, women eliminate it faster than men [42], which may explain the results of this study.
The study proved that the United States accounted for 70% of the total reports. This disparity may stem from the fact that ketamine is not approved for use as an antidepressant in China, and its application is restricted to short-term and low-dose administration for inducing anesthesia and analgesia during surgical procedures. After analysing the distribution of reporters, a significantly greater proportion consists of consumers rather than health workers, which may be explained by ketamine’s primary indications for treating depression and its consequent use in home-based care in the United States.
Despite the obvious physical and physiological gender differences, gender differences in AEs are rarely considered in clinical treatment [43]. Lack of awareness among physicians may be one of the main reasons. A survey has illustrated that information regarding gender aspects of medicine was not fully embedded in the existing curriculum of US medical schools [12]. Yu et al. randomly picked 20 drug-event combinations for diabetes mellitus and 20 drug-event combinations for hypertension and asked 2 primary care physicians to identify the AEs with gender differences; both physicians were not aware of any gender difference in these drug-event combinations, even though their data suggested that half of the drug-event combinations had gender differences [44].
In conclusion, this study harnessed the power of signal detection in the FAERS database to analyse the gender-related differences in AEs associated with ketamine. This research furnished valuable insights that can assist healthcare providers in tailoring treatment plans that account for gender differences, ultimately enhancing medication safety. Nonetheless, the study did not take potential confounders such as polypharmacy and comorbid conditions that might influence the AEs signals into account. Consequently, additional comprehensive assessments, confirmatory studies, and longitudinal follow-ups are warranted to corroborate and expand upon these findings.
5. Conclusions
Our study substantiated the existence of gender differences in AEs associated with ketamine use, which suggested that these distinctions should be integrated into clinical practice to optimize therapeutic outcomes.
5.1. Limitations
FAERS cannot establish a causal relationship between males and females. The reporting habits may be impacted by recent publications of AEs in the literature and media attention. Comorbidities and concurrent medications confused the link between a drug and an AE. FDA claims that the submitted information has not been examined by a medical expert. Manufacturers, consumers, and healthcare professionals may all submit FAERS data. A submission’s source must be taken into account. Incomplete or missing data can be found in FAERS. In other instances, the age was not provided or the medicine names were spelled incorrectly. Due to the inability to obtain the patient’s medication dose, it is impossible to rule out the bias in delirium caused by different medication doses. Not every AE or medication mistake involving a product was reported to the FDA. Additionally, ROR only investigated a risk of AE reporting that was elevated rather than a risk of AE incidence in general. The FAERS database has the advantage of having a huge sample size. Although there are some flaws, it is very important to discover new and rare AEs.
Ethical Approval
Ethical approval was not necessary because there are no data to be approved by the Ethics Committee in this paper.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
All authors made a significant contribution to the work reported in the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the Shanxi University of Chinese Medicine’s New Ability Cultivation Plan (no. 2021PY-X-01).
Open Research
Data Availability
This study analysed publicly available datasets. These data can be found in the following: https://research.cchmc.org/aers/explore.jsp.




