One Dose Risankizumab Effectiveness in Psoriasis: A Real-Life Multicentre Study
Abstract
Background. Rapid efficacy is an important item to psoriasis patients. Risankizumab, a humanised immunoglobulin G1 monoclonal antibody that inhibits IL-23, has demonstrated early and sustained efficacy in patients with moderated-to-severe psoriasis. Effectiveness data in real world, particularly regarding short-term response, however, are scarce. Objective. To explore the short-term effectiveness of risankizumab in patients with moderate-severe psoriasis in normal clinical practice. Methods. This was an observational, retrospective, multicentre study carried out at thirteen hospitals in Valencia, Spain. It was conducted on a sample of adult outpatients over 18 years of age, diagnosed with moderate-to-severe psoriasis who received at least one subcutaneous injection of 150 mg of risankizumab. Psoriasis Area and Severity Index (PASI) was used to assess the short-term (4 weeks) effectiveness of risankizumab. Results. One hundred and sixteen patients (63.8% men) with a mean age (standard deviation (SD)) of 50 (16) years were included in the study. 90.6% were overweight or obese, and 22.7% were biologic-naïve. The mean (SD) PASI score decreased from 11.9 (7.2) at the baseline to 3.3 (2.7) at week 4, with a median (SD) PASI score reduction of 8.6 (2.3) (p < 0.05). The absolute PASI score of <2 was reached by 52.6% of patients. Overall, PASI scores of 75, 90, and 100 were achieved in 56%, 37.1%, and 25.9% of patients, respectively, at week 4. PASI 90 was achieved by a significantly higher proportion of naïve patients than biologic-experience failure patients (59.3% vs. 30.3%; p = 0.01). Conclusion. This study, which reflects our initial risankizumab experience in a real-life setting, seems to show quick effectiveness in psoriasis treatment after one single dose. This trial is registered with NCT04862286.
1. Introduction
Psoriasis is a chronic immune-mediated inflammatory disease which has been estimated to affect 2-3% of the population worldwide [1, 2]. It is characterized by the appearance of erythematous, itchy plaques which are most commonly found on the knees, elbows, trunk, and scalp. Psoriasis can also occur on the joints, which is the case in about 30% of those affected [3]. In addition, numerous concomitant diseases, such as depression and cardiovascular and metabolic diseases, are associated with psoriasis [4–6]. This leads to a severe reduction in the quality of life for sufferers [3, 4, 7].
Psoriasis is caused by a complex interplay between the innate and adaptive immune system [1]. Currently, the interleukin (IL)-23/IL- 17 axis is considered to play a major role in the pathogenesis of psoriasis [1]. Interleukin (IL)-23, a regulatory cytokine that is overexpressed in psoriatic skin, composed of the specific p19 subunit and the p40 subunit also shared by IL-12, is considered to be a key cytokine in the development and maintenance of chronic plaque psoriasis, as it induces the differentiation and amplification of T-helper-17 and innate immune cells, which are major sources of proinflammatory cytokines, such as IL-17 [8, 9].
In recent years, a number of biologics targeting IL-17 or IL-23 receptors have been approved, and several new compounds are currently under development [1]. Compared with older biologics, drugs targeting IL-17 or IL-23 have demonstrated significantly greater reductions in the Psoriasis Area Severity Index (PASI) in clinical trials [1, 10]. In addition, these results are supported by real-world evidence, which allow the evaluation of efficacy and safety in patient profiles more similar to those seen in routine clinical practice, thus aiding in decision-making [11–13]. Based on the evidence, biologic drugs are a promising option also in special populations such as elderly patients [14] or children (NCT04862286).
Risankizumab is a humanised immunoglobulin G1 monoclonal antibody that binds with affinity and specificity to the p19 subunit and selectivity inhibits IL-23 [4, 15]. In five phase 3 randomized and controlled clinical trials, risankizumab demonstrated early and sustained efficacy in patients with moderated-to-severe psoriasis [4, 16–18].
Rapid efficacy is important to patients and clinicians [7]. Fast improvements in the skin and the ability to feel better quickly are important treatment attributes of a psoriasis therapy [7]. These are important patient preferences for treatments and are ranked among the highest desired priorities in multiple reports [7]. Limited evidence is available on the effectiveness of risankizumab in real-life patients [2, 8, 19–23], particularly regarding short-term response [20, 24, 25]. The purpose of our study was to explore the short-term effectiveness of risankizumab in patients with moderate-to-severe psoriasis in normal clinical practice.
2. Patients and Methods
This was an observational and retrospective multicentre study carried out at thirteen hospitals in the Autonomous Region of Valencia in Spain. The study was carried out on outpatients of both genders over 18 years of age, diagnosed with moderate-severe psoriasis who received at least one subcutaneous injection of risankizumab on-label use. Patients suffering exclusively from psoriasis at special body locations (scalp and palmoplantar) were excluded.
All patients provided their informed written consent. In accordance with Spanish recommendations, the study received a favourable approval from the Ethics Committee of Manises Hospital and was conducted in accordance with the principles of the Declaration of Helsinki for studies involving humans. Data were collected from January to June 2021.
Patients’ demographic data, psoriasis characteristics, and use of previous biologic treatment were collected from clinical records. In order to assess the short-term effectiveness of risankizumab, the Psoriasis Area and Severity Index (PASI) score, the percentage of patients achieving 75% and 90% improvement in PASI (PASI 75, PASI 90) and the Dermatology Life Quality Index (DLQI) score were also registered at the baseline and at week 4.
2.1. Statistical Methodology
Descriptive statistics were performed for each variable, using frequencies and percentages for categorical variables and mean and SD for quantitative/continuous variables. Student’s t-test, Mann–Whitney-U test, or Kruskal–Wallis H test were used to compare quantitative variables and Pearson’s chi-square or Fisher exact tests for qualitative variables, when appropriate. Statistical analyses were carried out with SPSS v21.0, and a p value <0.05 was considered significant.
3. Results
A total of 116 patients were included in the study. The majority of patients were men (63.8%), and the mean (SD) age was 50 (16) years. Mean (SD) body mass index (BMI) was 30.1 (7.0), and nearly all patients (90.6%) were overweight or obese. At the baseline, the mean (SD) DLQI score was 12.2 (3.4). 41 patients (33%) had scalp, and 12 (9.8%) had palmoplantar involvement, while 13.8% of patients were affected by psoriatic arthritis. Risankizumab was the first-line biologic therapy for psoriasis in 27 patients (22.7%), and the remaining 89 patients (76.7%) were previously treated with at least one biologic. Fifty-three (45.7%) patients had received TNF-α, 42 (36.2%) anti-interleukin (IL) -12/23, 44 (37.9%) anti-IL-17, and 11 (9.5%) anti-IL-23 (Table 1).
| Characteristics | Patients N = 116 |
|---|---|
| Female, n (%) | 42 (37.8) |
| Age, years, mean ± SD | 50 ± 16 |
| BMI, kg/m2, mean ± SD | 30.1 ± 7 |
| BMI >25, n (%) | 70 (60.8) |
| BMI >30, n (%) | 45 (29.2) |
| LTBI without prophylaxis, n (%) | 6 (4.9) |
| LTBI with prophylaxis, n (%) | 11 (10) |
| Disease duration years, mean ± SD | 12 ± 7.8 |
| PASI score, mean ± SD | 11.9 ± 7.2 |
| DLQI score, mean ± SD (n = 70) | 12.2 ± 3,4 |
| Plaque psoriasis, n (%) | 116 (100) |
| Special location, n (%) | |
| Scalp | 41 (33) |
| Palmoplantar | 12 (9.8) |
| Psoriatic arthritis, n (%) | 16 (13.8) |
| Naïve, n (%) | 27 (22.7) |
| Biologic-experience patients, n (%) | 89 (76.7) |
| 1 previous biologic | 46 (39.5) |
| 2 or more previous biologics | 44 (37.8) |
| Previous biologics, n (%) | |
| Anti-TNF | 53 (45.7) |
| Anti-IL-12/IL-23 | 42 (36.2) |
| Anti-IL-17 | 44 (37.9) |
| Anti-IL-23 | 11 (9.5) |
- BMI: body mass index; DLQI: Dermatology Life Quality Index; IL: interleukin; LTBI: latent tuberculosis infection; PASI: Psoriasis Area and Severity Index; SD: standard deviation; TNF: tumour necrosis factor.
3.1. Effectiveness of Risankizumab at Week 4
The mean (SD) PASI score decreased from 11.9 (7.2) at the baseline to 3.3 (2.7) at week 4, with a median (SD) PASI score reduction of 8.6 (2.3) (p < 0.05). The absolute PASI score <2 was reached by 52.6% of patients. Overall, PASI scores of 75, 90, and 100 responses were achieved in 56%, 37.1%, and 25.9% of patients, respectively, after the first risankizumab dose. At week 4, the mean (SD) DLQI score (n = 63) was 4.6 (1.5).
No significant differences were observed regarding effectiveness in relation to sex, BMI, or presence of arthropathies (Table 2).
| Characteristics | p value | |
|---|---|---|
| PASI <2, % | ||
| Men | 46.6% | |
| Women | 59.1% | 0.06 |
| BMI >30 | 53.8% | |
| BMI ≤30 | 45.9% | 0.4 |
| With psoriatic arthritis | 33.3% | |
| Without psoriatic arthritis | 51.3% | 0.1 |
| Naïve | 66.7% | |
| Biologic-experience patients | 48.3% | 0.09 |
| PASI 90, % | ||
| Men | 36.1% | |
| Women | 38.6% | 0.7 |
| BMI >30 | 43.6% | |
| BMI ≤30 | 31.1% | 0.2 |
| With psoriatic arthritis | 26.7% | |
| Without psoriatic arthritis | 39.8% | 0.3 |
| Naïve | 59.3% | |
| Biologic-experience patients | 30.3% | 0.01 |
- BMI: body mass index; PASI: Psoriasis Area and Severity Index.
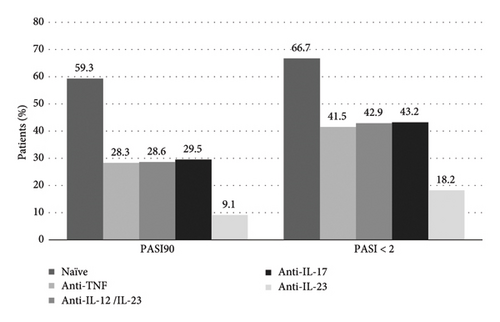
Figure 1 shows the proportion of patients who achieved PASI 90 and an absolute PASI <2 according to previous treatment. More than a half of naïve patients (59.3%) and 30.3% of patients who had previously failed biologic therapy achieved PASI 90 (p = 0.01). Among the latter, the worst response group corresponded to patients who had previously failed anti-IL-23 treatment. Absolute PASI <2 was also achieved by a higher proportion of naïve patients (66.7%) than by those who had previously failed biologic therapy (48.3%) (p = 0.09). Similarly, the worst response group corresponded to patients who had previously failed anti-IL-23 treatment (Figure 1).
Significant safety findings were not observed. After the first dose, two patients reported an eczematous reaction and two other patients discomfort at the injection site.
4. Discussion
Risankizumab is positioned as an alternative biologic therapy for moderate-severe plaque psoriasis, not only offering good long-term results, but also obtaining significant short-term improvement, as shown in a recent meta-analysis comparing several biologic therapies [26].
This study shows the short-term effectiveness of risankizumab in a real world and wide population composed of a high proportion of biologic-failure and obese patients with moderate-severe plaque psoriasis in some cases with difficult-to-treat areas such as the scalp (33%) and palmoplantar (9.8%).
Herein at week 4, after one dose, a PASI score reduction of 8.6 (2.3) (p < 0.05) was observed and an absolute PASI score <2 was reached by 52.6% of patients. Overall, PASI 75, 90, and 100 responses were achieved in 56%, 37.1%, and 25.9% of patients, respectively.
Even if several real-life data have been reported in long-term use of risankizumab [27, 28], few data are available in short term [20, 24, 25]. Hansel et al. conducted a real-life multicentre study on 57 patients (28.1% naïve, 57.9% BMI ≥25, 52.6% PASI baseline <20). At week 4, 38.6% of patients reached PASI 75, 15.8% PASI 90, and 5.3% PASI 100 [20]. Similar to our results, a single-centre, prospective study carried out on 14 patients showed a mean PASI score which decreased from 12.3 ± 5.2 at the baseline to 4.4 ± 2.7 at week 4 (p < 0.01) [24]. Finally, Rivera-Díaz et al. conducted a retrospective multicentre study on 44 patients, that also showed a decrease on the mean PASI score from 12.3 ± 2.8 at the baseline to 5.3 ± 0.9 after one month of treatment with risankizumab [25]. Previous real-world evidence with other biologics suggests that efficacy may differ when compared to phase 3 trials [22]. It is noteworthy that herein, the mean baseline PASI (11.9 ± 7.1) was lower and the proportion of patients who were overweight/obese (90.6%) or with failure to previous biologic treatment (76.7%) was higher than those referred to in the randomized clinical trials. Egeberg et al. in recent review on phase III clinical trials examined the rapidity of onset of action of novel biologic treatments. For risankizumab, 150 mg every 12 weeks over a period of 6.1 weeks (SD: 0.4 weeks) was required to achieve PASI 90 in 25% of patients, and 9.4 weeks (SD: 0.5 weeks) were needed for 50% of patients to achieve PASI 90 [1]. An analysis using integrated data from UltIMMa-1 and UltIMMa-2 and data from the open-label extension study LIMMitless showed 16.6% of patients achieving absolute PASI scores of ≤3 at week 4 [29, 30].
Several real-life studies have evaluated possible differences in risankizumab response according to different patient characteristics [8, 19–21]. A better response has been found in females, patients with baseline PASI≥ 20, or among those with a lower BMI [8, 20, 21]. Published data showed that biologic experience was irrelevant for therapeutic outcomes [19, 20]. Herein, no significant differences were observed regarding effectiveness in relation to age, sex, BMI, or presence of arthropathies. However, it was noticed that patients who had experienced previous failure with biologics had a significantly lower PASI 90 but not PASI<2 response. The group of patients with the lowest response rates corresponds to those who had previously failed anti-IL-23 treatment. In this regard, Borromi et al. found an association between patients who had failed more than 2 biologic treatments and lower probabilities of reaching PASI 75 and 90 at week 16 and PASI 100 at week 40 compared with those who had failed one biologic or naïve patients [8].
Although no significant safety findings were observed, two patients reported an eczematous reaction after the first dose of risankizumab. There are few reports of the occurrence of eczematous reactions associated with to biologics targeting IL-17 or IL-12/23. The pathogenesis of eczema involves both Th1 and Th2 responses, and inhibition of Th17 cytokines may disrupt the Th2/Th17 immune balance, potentially resulting in eczematous eruptions [31, 32].
4.1. Strengths and Limitations
The study shows the rapid treatment response of risankizumab in routine clinical practice, which has been questioned in the case of IL-23 inhibitor drugs. In addition, sample size is large enough to allow robust conclusions. Some limitations should be borne in mind, the main one derived from the observational and retrospective nature which does not allow missing data to be retrieved, such as the treatment response assessment in patients with psoriasis in special areas (scalp and palmoplantar). A further limitation is the short follow-up period.
5. Conclusion
This study, which reflects our initial experience with risankizumab in a real-life setting, seems to show a quick effectiveness in psoriasis patients after receiving one single dose of treatment.
Ethical Approval
The study was approved in March, 2021, by the Clinical Research Ethics Committee of Manises Hospital, Valencia (Spain), who acted as reference EB and was conducted in accordance with the principles contained in the Declaration of Helsinki for studies involving humans.
Consent
All patients provided written informed consent prior to their participation.
Disclosure
The authors maintained complete control over the manuscript content, and it reflects their opinions.
Conflicts of Interest
Jorge Magdaleno-Tapial received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Sanofi, and UCB. Isabel Belinchón-Romero received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Janssen, Lilly, Novartis, Sanofi, and UCB. Antonio Sahuquillo-Torralba received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Novartis, Janssen, Leo Pharma, and UCB. Sergio Santos-Alarcón received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Gebro, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB. José María Ortiz-Salvador received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, and Novartis. Víctor González-Delgado received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, LEO Pharma, Lilly, and Sanofi. Antonio Martorell received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Gebro, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB. Rafael Carmena-Ramón received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Sanofi, and UCB. Almudena Mateu received consultancy/speaker’s honoraria and/or travel grants and/or participated in clinical trials sponsored by AbbVie, Almirall, Janssen, Lilly, Novartis, and Sanofi. The rest of authors declare that they do not have any conflicts of interest.
Authors’ Contributions
JMT, JSC, and AM conceived the research. JMT, JMOS, SSA, IBR, AST, VGD, JSA, MAC, JIMR, AMP, JMD, LSP, RCR, and AN contributed to patient identification and data acquisition. JMT and AM contributed in the data analysis and results’ interpretation. JMT participated in the manuscript conceptualization and drafting. All authors have read and approved the final manuscript.
Acknowledgments
Editorial assistance and medical writing support were provided by Esther Tapia, PhD, and Adelphi Targis. The current risankizumab study is independent. The medical writer for this manuscript was supported by Abbvie with no input to the preparation, review, approval, and writing of the manuscript.
Open Research
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.