A De Novo Missense MYLK Variant Leading to Nonsyndromic Thoracic Aortic Aneurysm and Dissection Identified by Segregation Analysis
Abstract
Nonsyndromic hereditary thoracic aortic aneurysm and dissection (TAAD) is an autosomal dominant disease; however, it is frequently difficult to identify the causative genes. We report in this study a 33-year-old Japanese male with TAAD (Stanford type A) that is complicated with severe aortic regurgitation. There was no family history of aortic diseases in the patient nor any specific clinical features suggestive of connective tissue diseases, such as Marfan syndrome. Genetic testing identified candidate causative variants in two different genes: MYLK (c.4819G > A, p.[Gly1607Ser]) and FBN1 (c.365G > A, p.[Arg122His]). Familial cosegregation analysis revealed that the novel de novo MYLK variant was present only in the proband, and the FBN1 variant was also found in his nonaffected mother, and thus the MYLK variant was classified as likely pathogenic. MYLK is a causative gene for nonsyndromic TAAD that requires careful management; however, the number of reports is limited. Accumulating data on the pathogenicity of rare variants by performing a comprehensive pedigree analysis would help establish better treatment strategies for life-threatening hereditary TAAD cases.
1. Introduction
Nonsyndromic hereditary thoracic aortic aneurysm and dissection (TAAD) is an autosomal dominant disorder with a wide range of clinical variabilities and incomplete penetrance [1]; however, its natural history of progress is still unknown. Most nonsyndromic TAAD cases are diagnosed due to early-onset age or family history, and the identification of pathogenic variants may provide prognostic and risk stratification information to the proband and their family members. Prophylactic aortic surgery is advised for the most severe type, which can be life-threatening, based on the specific genetic variant and aortic diameter [2–4].
Recently, the discovery of multiple genes associated with nonsyndromic TAAD was accelerated by next-generation sequencing technology [5–7], and this trend has made genetic testing indispensable for diagnosing genetic diseases and in their subsequent therapeutic management. Currently, rare variants and polymorphisms in FBN1 (OMIM∗134797), which cause Marfan syndrome (MFS), have also been reported to be involved in nonsyndromic TAAD [8, 9]; however, when assessing the pathogenicity of unclassified variants, it is important to ensure that preventive measures are taken.
MYLK (OMIM∗600922) encodes myosin light chain kinase (MLCK), which is expressed in smooth muscle cells and phosphorylates the regulatory light chain, resulting in muscle contraction. Few research has been reported on missense variants, even though MYLK pathogenic variants have been found to account for 1% of nonsyndromic hereditary TAAD, with the majority likely being explained by frameshift and nonsense variants [10, 11]. In this study, we report a young nonsyndromic male with aortic dissection carrying two missense variants of unknown significance in MYLK and FBN1, whose segregation analysis helped evaluate their pathogenicity and was useful for genetic counseling in the proband and his families.
2. Materials and Methods
2.1. Ethical Compliance
The University of Tokyo Hospital’s Institutional Ethics Committee approved the genetic testing for hereditary TAAD (Reference No. G-1538), and the patient gave written informed consent.
2.2. Genetic Analyses of TAAD
The Kazusa DNA Research Institute (Chiba, Japan) provided hybridization capture-based gene panel testing for hereditary TAAD under the coverage of Japanese health insurance. This panel testing contained the following genes: FBN1, TGFBR1 (OMIM∗190181), TGFBR2 (OMIM∗190182), TGFB2 (OMIM∗190220), TGFB3 (OMIM∗190230), SMAD3 (OMIM∗603109), ACTA2 (OMIM∗102620), MYH11 (OMIM∗160745), MYLK, and COL3A1 (OMIM∗120180) [12, 13]. The GenBank reference sequences and version numbers were as follows: FBN1 (NM_000138.4), TGFBR1 (NM_004612.3), TGFBR2 (NM_003242.5), TGFB2 (NM_003238.3), TGFB3 (NM_003239.4), SMAD3 (NM_005902.3), ACTA2 (NM_01613.4), MYH11 (NM_002474.2), MYLK (NM_053025.3), and COL3A1 (NM_00090.3).
2.3. Segregation Analysis
Segregation analysis of the candidate variant using Sanger sequencing was carried out in additional family members. For genetic testing for MYLK and FBN1 variants, genomic DNA isolated from the white blood cells of the proband and his parents was used. In order to amplify exon 28 of MYLK and exon 5 of FBN1, intron-specific primers flanking exons were designed for polymerase chain reaction (PCR). PCR was carried out using the following primers located in exon 28 of MYLK (forward, 5′-GGGCTTTCCTGCCAATTTCTCCTACCCCC-3′, and reverse, 5′-GGGGCAGGATTCAAACCTGGGAAGTCTTC-3′) and exon 5 of FBN1 (forward, 5′-GGAGTCAAGCAAGATGGAGC-3′, and reverse, 5′-AGCTGACACTACTTTTCCATTCT-3′). All sequencing procedures were performed using forward and reverse primers, as mentioned previously [14].
2.4. Histological Analysis of Human Aortic Tissue
The Institutional Ethics Committee of the University of Tokyo Hospital approved the examination of human aortic tissue (Reference No. 2233-7). Aortic root tissue samples were obtained from the patient (MT65) (III-1) who had elective aortic root surgery. After written informed consent was obtained, control aortic tissue samples were taken from a 43-year-old heart transplant recipient (MT21) who had dilated cardiomyopathy but no aortic aneurysm annulus ectasia (Valsalva 27 mm). The aortic tissue samples were fixed in 10% formalin, embedded in paraffin, and sectioned at a thickness of 5 μm. Three serial sections were stained with Elastica van Gieson and Masson’s trichrome, anti-phospho-Smad2 (Ser465/Ser467) antibody (Cell Signaling Technology, #3108, 1 : 200), and anti-phosphorylated ERK1/2 (Thr202/Tyr 204) antibody (Cell Signaling Technology, #9101, 1 : 200), using a VECTASTAIN ABC Kit (Vector Laboratories, PK-4001) and 3, 3′-diaminobenzidine tetrahydrochloride (Vector Laboratories, SK-4100).
3. Results
3.1. Case Presentation
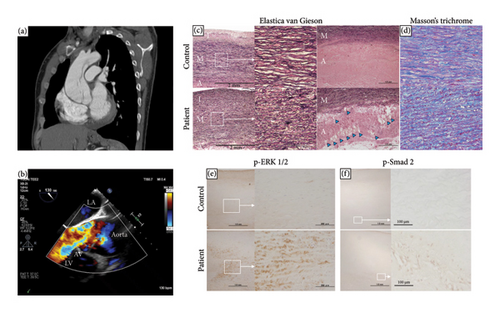
The patient was a 33-year-old Japanese male without apparent medical history. He suddenly developed chest pain and was transferred to a nearby hospital in May 2021. Computed tomography (CT) showed a 45 mm dilatation of the ascending aorta, but aortic dissection was not widely suspected. He kept experiencing symptoms of shortness of breath and chest pain on exertion afterward, and 3 months later, he returned to the outpatient clinic. Cardiac enlargement was observed on chest X-ray, and severe aortic insufficiency with ascending aorta enlargement was shown on echocardiogram. Contrast-enhanced CT revealed a Stanford type A false lumen aortic dissection that extended from the aortic root to the aortic arch without organ malperfusion. The maximum diameter of the ascending aorta increased to 55 mm compared to 3 months ago (Figure 1(a)). He was transferred to our university hospital, where he was immediately admitted for further evaluation, and transesophageal echocardiogram showed a false lumen at the aortic root and severe aortic insufficiency due to the enlargement of aortic valve ring (Figure 1(b)). Symptoms of heart failure were seen, and the diameter of the ascending aorta increased over the past few months; therefore, elective surgery was performed. Valve-sparing aortic root replacement (David procedure) and aortic valvuloplasty were performed. The thickness of the ascending aortic wall was increased with elastic fiber degeneration and collagen deposition, and the number of vasa vasorum in the adventitia was increased compared to that in the control specimen from a heart transplant recipient (Figures 1(c) and 1(d)). Moreover, compared with the control, the phosphorylation levels of Smad2 in the adventitia and ERK1/2 in the media were significantly upregulated (Figures 1(e) and 1(f)), which suggests the activation of the transforming growth factor-β (TGF-β) signaling pathways in the patient.
3.2. Segregation Analysis
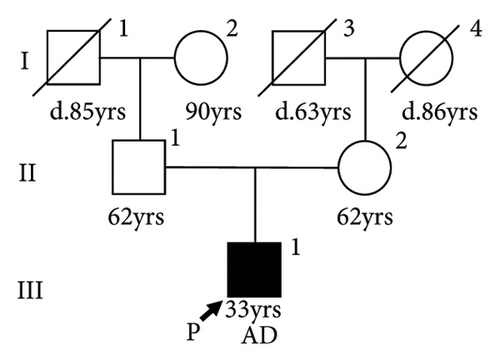
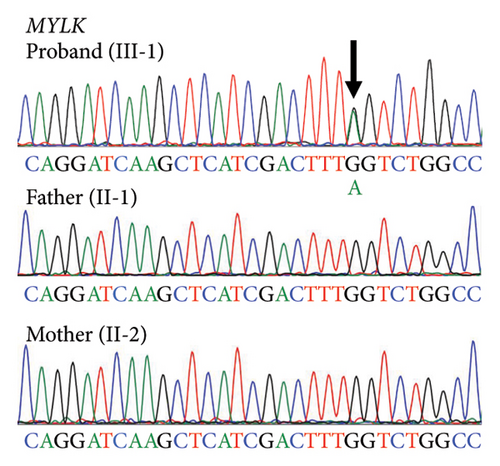
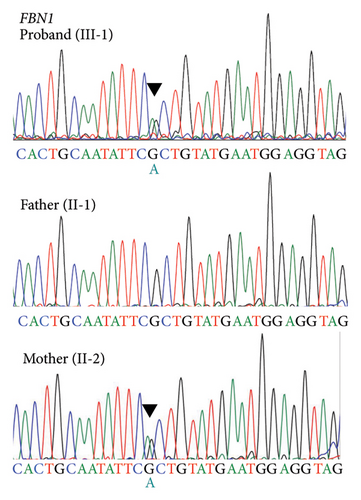
There was no apparent family history of connective tissue diseases (CTDs) or cardiovascular diseases observed in the patient (Figure 2(a)), as well as no physical examination findings suggestive of CTDs, such as MFS. Due to the aortic dissection at an early age, genetic testing for hereditary TAAD was carried out, and two missense variants of undetermined significance were detected in exon 5 of FBN1 (c.365G > A, p.[Arg122His]; NM_000138.4) and exon 28 of MYLK (c.4819G > A, p.[Gly1607Ser]; NM_053025.3). The novel missense variant of MYLK is located in the MLCK kinase domain, which is conserved across species, while the FBN1 variant has been reported as a variant of uncertain significance (VUS) in the ClinVar database (RCV001189618.2). In silico Combined Annotation Dependent Depletion scores for predicting the pathogenicity of both variants are high (28.6 and 26.3, respectively), as well as their Polymorphism Phenotyping v2 scores (0.977 and 0.991, respectively). We conducted the familial cosegregation analysis using Sanger sequencing to investigate the impact of these variants on arteriopathy in this family. Consequently, the missense variant of MYLK was detected only in the proband and not in their parents, and therefore this was considered to be a de novo variant (Figure 2(b)). The patient and his mother carried the missense variant of FBN1, whereas the patient’s father had neither (Figure 2(c)). The patient’s mother was 62 years old, and no enlargement of the sinus of Valsalva was seen on echocardiography (31 mm; aortic root Z-score [15], 0.68). According to the American College of Medical Genetics and Genomics (ACMG) guidelines [16] and the Clinical Genome Resource (ClinGen) recommendations [17], the MYLK and FBN1 variants were classified as likely pathogenic (PM1, PM2_Supporting, PM6_Supporting, PP3, and PP4) and VUS, respectively, in the present case.
4. Discussion
A young patient with nonsyndromic TAAD was reported in this study. Gene panel sequencing for TAAD-associated genes identified two candidate causal missense variants in MYLK and FBN1. FBN1 is the causative gene for MFS, the most common inherited form of syndromic TAAD, but FBN1 rare variants and polymorphisms have been also reported to contribute to nonsyndromic TAAD [8, 9]. Conversely, pathogenic variants of MYLK cause nonsyndromic TAAD, and the natural history and appropriate treatment strategy remain elusive [10].
Personalized aortic surveillance and clinical management would be supported by validation of pathogenicity of gene variants. In the present case, c.4819G > A in MYLK was a novel de novo heterozygous variant, and based on the ACMG guidelines and the ClinGen recommendations, it is classified as likely pathogenic. Although most MYLK pathogenic variants are truncating variants (e.g., frameshift and nonsense variants) which resulted in premature truncation and nonsense-mediated decay and are thought to cause disease as a consequence of haploinsufficiency, only a few pathogenic missense variants of MYLK have been previously reported, two of which remarkably reduce MLCK kinase activity in vitro [10, 11]: c.5275T > C (p.Ser1759Pro) in the calmodulin binding site and c.4471G > T (p.Ala1491Ser) in the kinase domain (ClinVar database accession numbers are RCV000023044.4 and RCV000855690.3, respectively). Although c.4819G > A located in the MLCK kinase domain may also have a loss-of-function effect in vitro, further research is required in order to fully understand the variant functional effect, since missense variants were associated with a higher risk of first aortic event (elective aortic aneurysm surgery or acute aortic dissection) than truncating variants [18].
The in vivo mechanisms through which MYLK pathogenic variants cause aortopathy are completely unknown. Activation of ERK1/2 and Smad2 and enhanced vasa vasorum formation were observed in proband’s aortic wall; however, these changes might be influenced by aortic dissection, while the increased vascularity in the aortic media has been reported from patients who had nondissecting thoracic aneurysm and MYLK pathogenic variants [10, 19]. It is interesting to note that MYLK pathogenic variants can cause aortic dissection with little to no enlargement of the aorta [10]. The ascending aorta was mildly dilated as seen on CT at the first examination (diameter 45 mm) in the present case, and 3 months later, it expanded to 55 mm. Although the exact onset time of aortic dissection was uncertain, his aortic size before dissection might have been much smaller than at dissection, since aortic dilatation occurs rapidly in aortic dissection [20].
c.365G > A in FBN1 might serve as a modifier for the disease. The penetrance of FBN1 pathogenic variants is reported to be 100% [21, 22], and c.365G > A has been observed in patient with nonsyndromic TAAD [23]; however, currently, there are not enough data to determine the impact of c.365G > A variant on FBN1 protein function and TAAD pathogenesis, which includes MFS. In the present family, the proband did not have pear-shaped aortic root enlargement typical for MFS, and his mother who has c.365G > A did not show any characteristic features of MFS which includes aortic aneurysm. These results lead us to the conclusion that c.4819G > A in MYLK, as opposed to c.365G > A in FBN1, had a stronger effect on the development of aortic dissection in the proband.
Because there are few reports on MYLK pathogenic variants, the best course of treatment and indications for prophylactic aortic surgery have not, to date, been convincingly established. Although genetic testing has grown more accessible, faster, and affordable, uncertainty in variant interpretation can result in incorrect diagnosis and prevent appropriate therapy and genetic counseling, especially when multiple candidate variants are identified as it happened this time. Better treatment options for rare diseases like nonsyndromic hereditary TAAD could be established by accumulating data on the pathogenicity of rare variants by performing a thorough pedigree analysis.
In conclusion, we reported a Japanese case of nonsyndromic TAAD with a novel likely pathogenic variant of MYLK (c.4819G > A), simultaneously carrying FBN1 c.365G > A VUS. When genetic testing identifies multiple candidate disease-causal variants, segregation analysis may help detect high-risk patients in which careful treatment strategies are needed.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Daigo Nishijo, Hiroki Yagi, Nana Akiyama, Norifumi Takeda, Masahiko Ando, and Haruo Yamauchi directly contributed to the management of the patient. Norifumi Takeda, Norihiko Takeda, and Issei Komuro revised the manuscript critically for important intellectual content. All authors approved the content of the manuscript and confirmed the accuracy or integrity of any part of the work.
Acknowledgments
We thank Ms. Asami Ogawa for her excellent technical assistance.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.




