Tailoring the Electrophilicity of Co Surface for Favorable toward Alkaline Oxygen Evolution Reaction by Metal Cation Doping
Abstract
The chemical coupling of molybdenum carbide (Mo2C) to cobalt (Co) promotes oxygen evolution reaction (OER) kinetics on the Co surface by making the surface more electrophilic. Here, to gain a deeper understanding of the effects of the surface electrophilic properties on the OER kinetics of Co and to obtain high OER activity, Fe and Ni are additionally incorporated into Co nanoparticles that are coupled with Mo2C nanoparticles (Co-Mo2C). Considering the oxidation states of Fe (Fe3+), Co (Co2+/Co3+), and Ni (Ni2+) ions, Fe and Ni are expected to affect the electronic structure of Co in the opposite direction. Lewis acidic Fe3+ doping makes the Co surface oxide more electrophilic, promoting the formation of OER-active CoOOH by strongly attracting hydroxide ions (OH−). Thus, the OER kinetics is facilitated on the Co surface of Fe-doped Co-Mo2C, resulting in a significantly lower overpotential for the OER. On the other hand, the Ni2+ doping makes the Co surface oxide less electrophilic, leading to an increase in the overpotential for the OER. Tailoring the electrophilic properties of the Co surface is presented as a key parameter in the design of a Co-based OER catalyst for alkaline water electrolysis.
1. Introduction
The oxygen evolution reaction (OER) plays a key role in determining the performance capabilities of energy conversion devices such as water electrolysis cells and metal–oxygen (e.g., lithium–O2 and zinc–O2) batteries. In general, the OER is more sluggish and has a higher overpotential compared to a counterpart reaction [1]. For the widespread dissemination of these devices, the OER kinetics should be improved [2], possibly by exploring new active catalyst materials. As OER catalysts, iridium oxide (IrO2) or ruthenium oxide (RuO2) are known to have desirable activity and stability under highly anodic potentials (Eo O2/OH− = 1.23 VRHE) [3, 4]. Recently, cobalt (Co)-based catalysts have been reported to have high OER activity in alkaline electrolytes [5] in which the Co surface is progressively oxidized through reactions with hydroxide anions (OH−) upon an increase in the anodic potential. During this process, the Co surface is restructured to form thin hydrous oxides consisting of metal cations, oxygen anions (O2−), OH−, and water [6]. Because the OER takes place at the electrode–electrolyte interface, the outermost hydrous oxides serve as actual active sites for the OER. In this case, the structural and chemical properties of the surface hydrous oxides are closely related to the OER kinetics. According to previous studies, a layered (oxy)hydroxide phase of Co is thermodynamically stable under alkaline OER conditions and can lead to rapid OER kinetics as a catalyst [7, 8, 9, 10]. However, a thermodynamically favored (oxy)hydroxide phase scarcely forms on the Co surface without an additional surface modification due to the absence of kinetically accessible routes. In order to achieve high OER activity, the surface properties of Co must be modified to form OER-active hydrous oxides which resemble a layered (oxy)hydroxide in terms of structure and composition.
The electronic structure is known to be a key surface property determining the catalytic activity of metal catalysts [11]. As an effective means of modulating the surface electronic structure, metal catalysts have been chemically coupled with heteroatoms in the form of alloy compounds or phase–phase interfaces [11, 12, 13, 14, 15, 16, 17]. The differences in the physical and chemical properties between the coupled atoms create ligand and strain effects that cause the modulation of the electronic structure [18, 19]. Our group proposed that the OER activity of Co nanoparticles can be promoted significantly through chemical coupling with molybdenum carbide (Mo2C) nanoparticles at their interfaces [20]. We demonstrated that Mo2C coupling causes a valence electron transfer from Co to Mo2C and makes the surface oxide layer (CoO and Co3O4) of the Co nanoparticles more electrophilic, giving it high OH− affinity and leading to the formation of OER-active hydrous oxides. This surface reconstruction into OER-active hydrous oxides is regarded as an essential process to achieve fast OER kinetics on Co [21, 22]. However, while acknowledging this positive aspect, we also noted that the absolute activity values slightly lagged behind those of other highly active catalysts, indicating the need for further enhancement. This study sought a deeper understanding of the effects of the electrophilic properties of the surface oxide on the OER kinetics on Co in an effort to design an active catalyst for the OER. We considered the doping of metal ions focusing on the oxidation states of the metal ions. To control the electrophilic property of the surface oxide, we incorporated iron (Fe) and nickel (Ni) into Co nanoparticles that are coupled with Mo2C nanoparticles (Co-Mo2C). Considering the structural compatibility with the host Co ions crucial, we initially considered transition metals surrounding Co as candidates. Among these, Fe, Ni, and Mn were chosen initially, excluding Cu, Zn, and Cr, due to their instability in the OER environment. However, Mn’s characteristics to possess various oxidation states made it unsuitable for assessing electrophilic characteristics based on oxidation states. Consequently, we selected for Fe and Ni, which exhibit oxidation states of +3 and +2, respectively [23, 24, 25, 26, 27]. Thus, doped Fe3+ and Ni2+ ions can affect the valence electrons of Co ions (Co2+ and Co3+) on the surface of Co oxides in a different way, leading to a different electrophilic property. Fe and Ni doping in Co-Mo2C would be effective to demonstrate a correlation between the surface electrophilic property and the OER kinetics as a design strategy for OER catalysts. In addition, we present Fe-doped Co-Mo2C with further enhanced OER activity owing to a synergetic effect of Mo2C coupling and doping with heteroatoms (Fe and Ni).
2. Experimental
2.1. Chemicals and Materials
All chemicals were purchased and directly utilized without any further purification; cobalt (II) acetate tetrahydrate ((CH3COO)2Co·4H2O, ≥98%), iron (III) nitrate nonahydrate (Fe (NO3)3·9H2O, ≥98%), nickel (II) acetate tetrahydrate ((CH3COO)2Ni·4H2O, 98%), ammonium molybdate ((NH4)2MoO4, 99.98%), and dicyandiamide (C2H4N4, 99%) were purchased from Sigma–Aldrich. Potassium hydroxide (KOH, 85%) was purchased from Junsei Chemicals. Deionized (DI) water with a specific resistance of 18.2 MΩ cm was obtained using a Milipore-DQ5 purification system. The commercial iridium oxide (IrO2) was purchased from Tanaka Kikinzoku (TKK).
2.2. Material Synthesis and Characterization
Graphitic carbon nitride (g-C3N4) was used as a carbon source for the Mo2C synthesis. To prepare g-C3N4, dicyandiamide was heat treated at 550°C (heating rate: 5°C min−1) for 4 hr under an argon (Ar) gas atmosphere. Fe and Ni-doped Co-Mo2C nanoparticles were synthesized by metal precursors and g-C3N4 mixing following heat-treatment. Cobalt (II) acetate tetrahydrate and ammonium molybdate were dissolved in DI water, and iron (III) nitrate nonahydrate or nickel (II) acetate tetrahydrate was additionally dissolved. The synthesized g-C3N4 was added in the metal precursors-dissolved water and then dried after mechanical stirring of 30 min. The dried powders were heat-treated at 800°C (heating rate: 5°C min−1) for 1 hr under an Ar gas atmosphere. The heat-treated samples were used as Fe- and Ni-doped Co-Mo2C catalysts.
The morphologies of the prepared samples were examined by transmission electron microscopy (TEM, FEI company/Tecnai G2 F30 S-Twin). X-ray diffraction (XRD, RIGAKU/SmartLab) was used to analyze phase information of the prepared samples. The content of the metallic elements (Fe and Ni) doped into Co nanoparticles was measured using inductively coupled plasma-optical emission spectroscopy (ICP-OES, Agilent/ICP-OES 720). The chemical states of the prepared samples were analyzed using X-ray photoelectron spectroscopy (XPS, Thermo VG Scientific/Sigma Probe), and 284.8 eV of C–C bond was used as a reference for calibrating the XPS results.
2.3. Electrochemical Analysis
All the electrochemical measurements were conducted in a standard three-compartment electrochemical cell using PGSTAT 302N (Metrohm AUTOLAB) with a glassy carbon electrode (GCE) in the form of a rotating disc electrode (RDE), a Pt mesh electrode and a mercury/mercury oxide (Hg/HgO) electrode filled with 0.1-M sodium hydroxide (NaOH) solution as the working, counter and reference electrodes, respectively. It was confirmed that the concentration of iron (Fe) in the DI water, which could affect the OER characteristics, was below 0.05 ppb. All potentials were measured at 20°C in a 0.1-M potassium hydroxide (KOH) solution and referenced against the reversible hydrogen electrode (RHE). The potential difference between the Hg/HgO electrode and the RHE was obtained from a cyclic voltammetry (CV) curve measured in the hydrogen-saturated 0.1 M KOH solution using a Pt disc as the working electrode. The sweep rate was 0.1 mV s−1. A catalyst ink slurry was prepared by mixing the 10 mg catalysts with 2 mL of DI water and 100 μL of a 5 wt% Nafion solution as a binding material. After mixing and ultrasonication, a drop of the 10 μL of ink slurry was loaded onto the GCE substrate. The dried electrode was then transferred to the electrochemical cell. Catalyst loading on the GCE was 0.24 mg cm−2 for all samples. The RDE rotation rate was 1,600 rpm, and the sweep rate was 5 mV s−1 during the OER measurements. The results obtained using a graphite electrode as the counter electrode confirmed that there were no changes in the electrochemical performance due to the dissolution of the Pt counter electrode (Figure S1).
3. Results and Discussion
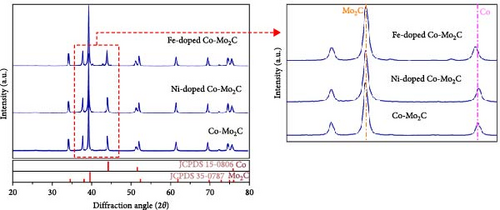
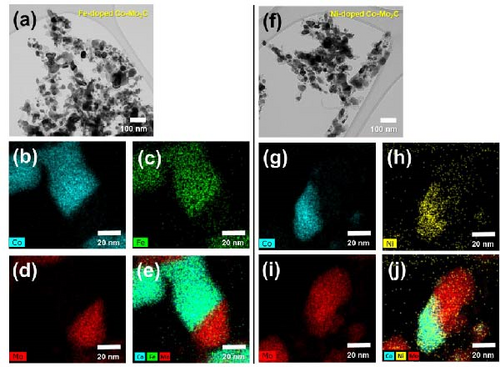
Fe- and Ni-doped Co-Mo2C samples were simply prepared by mixing all of the precursors (Fe or Ni, Co, Mo, and g-C3N4), followed by a heat-treatment at 800°C for 1 hr under an Ar gas atmosphere. Here, g-C3N4 was used as a carbon source for Mo2C. The atomic ratio of Co and the doped metals (Fe and Ni) in the precursor mixture was 9 : 1. X-ray diffraction (XRD) patterns (Figure 1) collected after the heat-treatment of the precursor mixture exhibited only Co (JCPDS 15-0806) and β-Mo2C (JCPDS 35-0787) phases without any Fe- or Ni-derived impurity phases. As shown in Figure 1 (magnified), when Fe and Ni were doped, the peaks of the Co phases shifted toward the negative and positive directions, respectively, reflecting that the d-spacing of the Co lattice changed due to a difference in the atomic radius between the host Co and the doped Fe and Ni. However, there were negligible differences in the peak positions of the Mo2C phases. The XRD patterns suggested that Fe and Ni were mostly doped into the Co nanoparticles. TEM and scanning TEM (STEM)–energy dispersive spectroscopy (EDS) images (Figures 2(a) and 2(f); Figure S2) showed that both the Fe- and Ni-doped Co-Mo2C were composed of nanoparticles 30–50 nm in size as well as some carbon residue stemming from the decomposition of g-C3N4. The similarly sized Co and Mo2C nanoparticles were uniformly dispersed in direct contact with each other. High-resolution STEM–EDS mapping images of the Fe- and Ni-doped Co-Mo2C samples (Figures 2(b), 2(c), 2(d), 2(e), 2(g), 2(h), 2(i), and 2(j)) more clearly showed that Co and Mo2C nanoparticles were coupled at their interfaces and that Fe and Ni atoms were mostly doped into the Co nanoparticles rather than into the Mo2C nanoparticles, which was coincident with the XRD patterns. In our previous report [20], only when a sufficient amount of cobalt precursor was added to the precursor mixture, Mo precursors were completely converted to Mo2C, and Co was inevitably present in the product separately (Co-Mo2C). Another previous study also suggested that a transition metal (Fe) facilitated carbide formation of a refractory metal (W) by anchoring the carbon, but it was not doped into the carbide structure [28]. We speculated that transition metals (Co, Fe, and Ni) served as anchors in the carburization of Mo and C, thereby suggesting that they were not doped into the carbide structure. The doped Fe and Ni content into the Co nanoparticles were measured using ICP-OES and found to be 8.8 and 9.4 wt%, respectively, as summarized in Table S1.
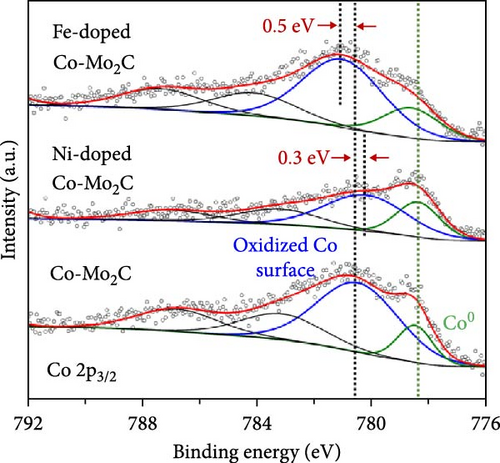
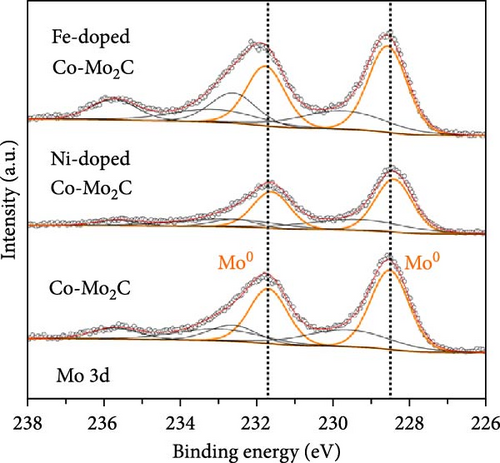
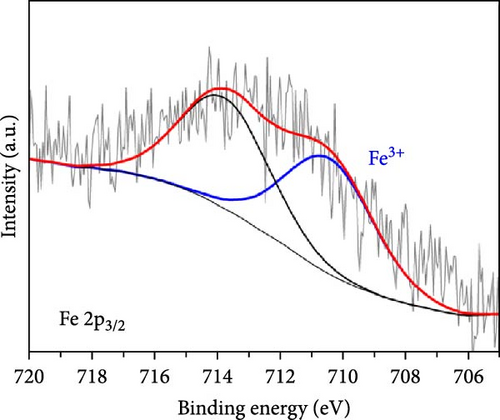
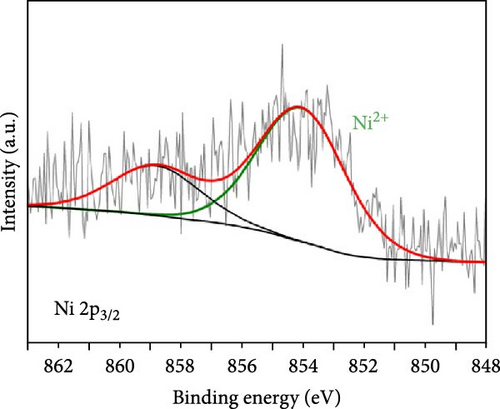
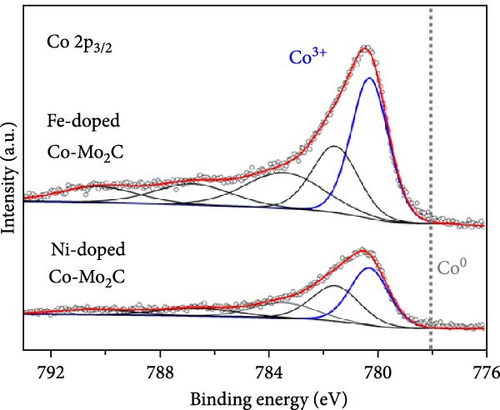
As electrochemical OER takes place on the catalyst surface, investigating surface chemical states by X-ray photoelectron spectroscopy (XPS) is important to further understand the correlation between surface electrophilic properties and OER activity. As shown in Figures 3(a) and 3(b), it is noted in the measured XPS spectra that the Fe and Ni doping dominantly influenced the chemical states of the Co surface, as those alloying elements were mainly doped into the Co nanoparticles, whereas the Mo 3d spectra were insignificantly affected by the Fe and Ni doping. For all as-prepared samples, the Co 2p spectra were composed of metallic Co (Co0) and air-oxidized Co species (CoO and Co3O4) with an oxidized Co composition of ~80%. The Mo 3d spectra were composed of Mo0 peaks for Mo2C phase and Mo4+ and Mo6+ peaks for oxidized Mo surface. The binding energies of Co 2p and Mo 3d peaks well matched the previously reported values [20, 29, 30, 31]. The binding energies of the Co 2p peaks were shifted by the Fe and Ni doping, more significantly in the oxidized Co peaks than in the metallic Co (Co0) peaks. In a comparison with Co-Mo2C, Fe doping increased the binding energy of the oxidized Co peak by ~0.5 eV, while Ni doping decreased the binding energy of the oxidized Co peak by ~0.3 eV, as summarized in Table S2. These core-level shifts in the Co 2p peaks can be ascribed to the different ionic statuses of the doped Fe and Ni in the surface oxide layer of the Co nanoparticles. As shown in Figures 3(c) and 3(d), doped Fe and Ni were present as Fe3+ and Ni2+ ions in the surface Co oxide layers. The binding energy of Fe (~710.5 eV) and Ni (~854.0 eV) closely match those reported in the literature for Fe3+ and Ni2+ [32, 33, 34]. Fe3+ ions have strong Lewis acidic properties due to the higher oxidation state compared to that of Co ions (Co2+ and Co3+) in surface cobalt oxides [35] such that the Fe3+ ions can draw valence electrons from adjacent Co ions, leading to a further increase in the binding energy of Co 2p electrons. On the other hand, doped Ni has a lower oxidation state (Ni2+) than the adjacent Co ions, leading to a Co 2p spectra peak shift in a direction opposite to that of Fe doping. In Co-Mo2C, coupling Mo2C to Co makes the Co surface oxide more electrophilic, as confirmed by a positive shift of the binding energy of Co 2p electrons [20]. Hence, we can infer that Fe doping makes Co surface oxide further electrophilic, whereas Ni doping makes the Co surface oxide less electrophilic. Therefore, these XPS results indicate that the ligand effects induced by the interactions between the Co ions and the doped Fe and Ni ions change the electrophilic property of the Co surface oxide by modifying its electronic structure. Considering that XRD peaks related to cobalt oxides were not observed, the surface oxide layer was very thin and/or amorphous. These XPS results indicate that the dominant characteristic change occurs in the oxidized surface of Co nanoparticles, suggesting the potential of these oxidized Co surfaces to catalyze OER due to the surface-oriented nature of electrochemical reactions.
The electrochemical OER performance outcomes of the Fe- and Ni-doped Co-Mo2C samples were investigated in an Ar-saturated 0.1 M KOH solution using a standard three-electrode cell. Co-Mo2C was used as a reference to assess the effects of Fe and Ni doping on the electrochemical performance, while IrO2 served as a commercial reference. The catalyst loading for all samples on GCE was 0.24 mg cm−2. As shown in the IR-corrected OER polarization curves (Figure 4(a)), the Fe-doped Co-Mo2C NPs exhibited even a higher OER current density than Co-Mo2C over the entire potential region. However, the Ni-doped Co-Mo2C exhibited a somewhat lower OER current density level than the Co-Mo2C at a higher potential region. The overpotential at 10 mA cm−2 has been generally used to evaluate the OER activity of catalysts. The Fe-doped Co-Mo2C had a much lower overpotential (318 mV) compared to those of the Co-Mo2C (347 mV) and Ni-doped Co-Mo2C (353 mV) at 10 mA cm−2. The Tafel slopes (Figure 4(b)) and Nyquist plots (Figure 4(c)) measured at 1.6 VRHE also showed a tendency identical to that in the OER polarization plots. The Fe-doped Co-Mo2C exhibited a lower Tafel slope and smaller semicircle than the Ni-doped Co-Mo2C and Co-Mo2C, indicating the lowest charge-transfer resistance. To further determine the OER capability of the surface Co sites, the turnover frequency (TOF) for OER at η = 300 mV was calculated based on the total Co mass on the GCE. As summarized in Table S3, the TOF of Fe-doped Co-Mo2C was almost three times higher than those of the other samples. Those results clearly showed that Fe doping into Co-Mo2C further promoted the OER kinetics of Co, whereas Ni doping rather lowered the OER kinetics of Co. Moreover, many studies that involved alloying of Co with Ni demonstrated a beneficial effect in increasing OER activity, and this may indeed be the case for the ordinary Co or Co oxide particles [36, 37]. However, in this study, Ni did not exhibit a beneficial effect through doping on Co-Mo2C nanoparticles. This was probably because Co could already be in a higher electrophilic state due to the formation of an interface with Mo2C, and the introduction of Ni2+ did not lead to an enhancement of OER activity.
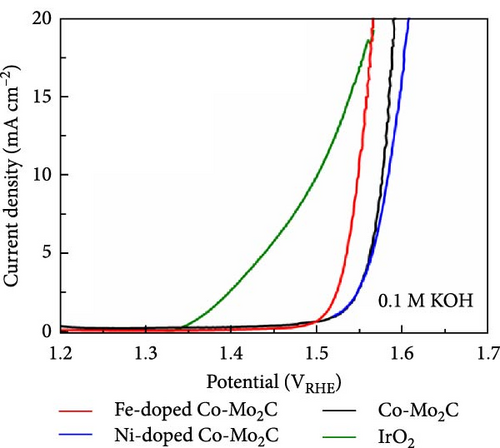
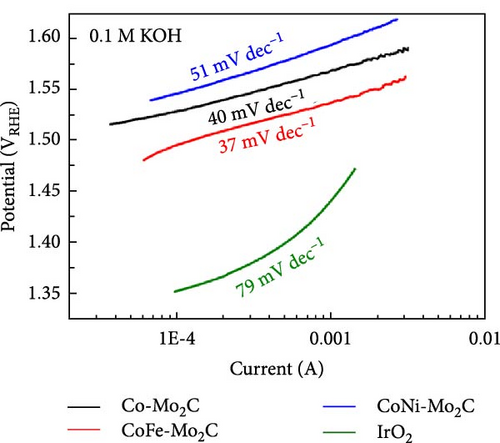
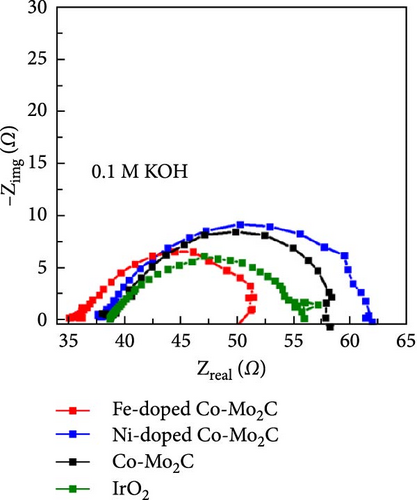
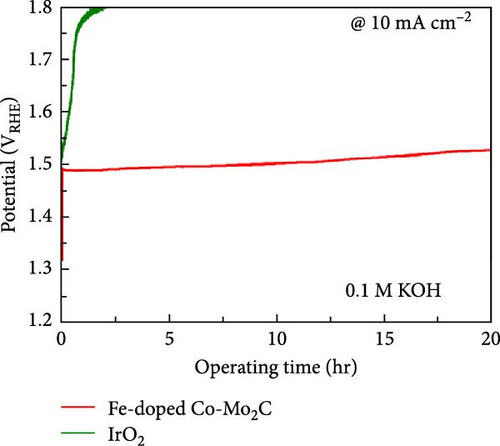
When the same amount of catalyst was used, the overpotential of the noble metal IrO2 at 10 mA cm−2 was lower than the Fe-doped Co-Mo2C, and its Tafel slope was very steep (Figure 4(b)), resulting in an increase in overpotential at high current densities. Furthermore, as shown in Figure 4(d), IrO2 underwent drastic degradation within the initial 3 hr during the stability test at 10 mA cm−2 operation. However, the Fe-doped Co-Mo2C exhibited a relatively gentle voltage increase within 40 mV for 20 hr of operation. Therefore, we can deduce that the Fe-doped Co-Mo2C catalyst would be considered valuable in various aspects regarding material cost and electrochemical performances.
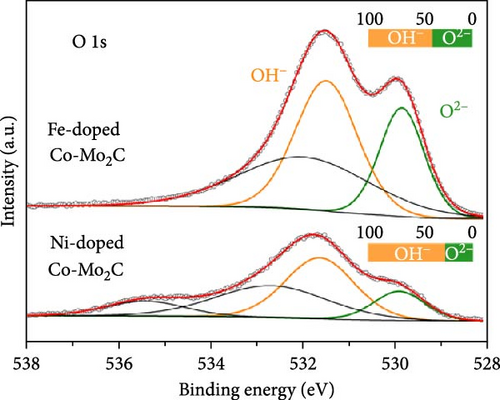
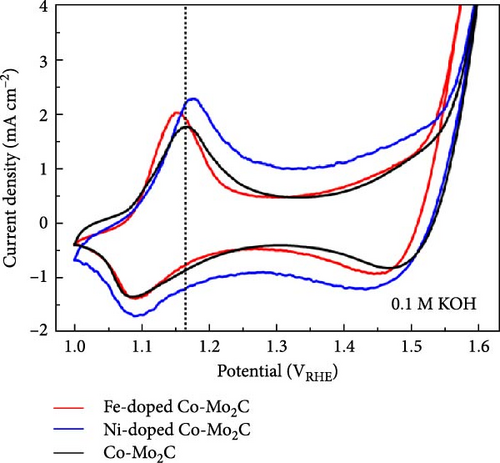
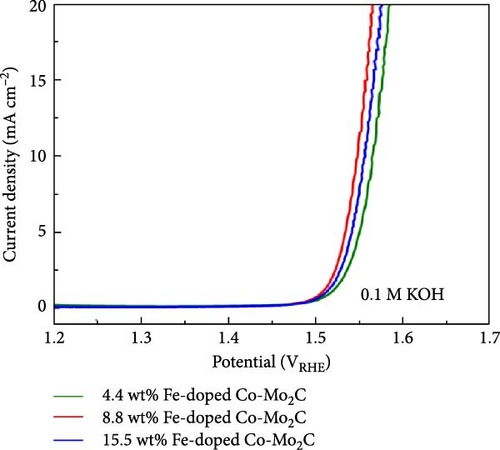
On a transition-metal-based catalyst surface, the OER takes place on the hydrous oxide that forms under the highly anodic OER potential [38]. Thus, to understand the Fe and Ni doping effects on the OER performance, the chemical states of the surface hydrous oxides were analyzed by XPS after measuring the consecutive OER polarization curves three times. As shown in the Co 2p3/2 spectra (Figure 5(a)), for both the Fe- and Ni-doped Co-Mo2C, the Co ions in the surface hydrous oxides were further oxidized to Co3+ after the three OER cycles. Fe and Ni ions were uniformly distributed in the Co nanoparticles even after the OER cycles, as shown in TEM–EDS mapping images (Figure S3). The elemental mapping images revealed that Mo2C nanoparticles were partially dissolved during the OER cycles. According to our previous study [20], the Mo dissolution negligibly affected the hydrous oxide formation process. In contrast to the Co 2p3/2 spectra, the O 1s spectra of the Fe- and Ni-doped Co-Mo2C (Figure 5(b)), which were composed of O2− and OH− peaks, demonstrated a remarkable difference; the Fe-doped Co-Mo2C exhibited significantly higher O2− peak intensity than the Ni-doped Co-Mo2C. The O2− to OH− ratio was 0.60 for the Fe-doped Co-Mo2C and 0.35 for the Ni-doped Co-Mo2C. These results indicate that while the oxidation states of Co are similar in the surface hydrous oxides layers of Fe- and Ni-doped Co-Mo2C (Co3+), the anion coordination of the Co cations can be considerably different. Because hydrous oxides form due to the oxidation reactions of the surface oxide with OH−, the anion coordination of the Co cations in the hydrous oxides is strongly affected by the surface electrophilic property as determined by the Fe and Ni doping. As discussed above from the XPS spectra (Figure 3), Fe doping causes the Co surface oxide to become more electrophilic and strongly attracting OH−. Thus, the Co surface oxide is readily oxidized further through a reaction with OH−, forming hydrous oxides with greater O2− coordination. On the other hand, Ni doping causes the Co surface oxide to be less electrophilic, weakly attracting OH−. Thus, the hydrous oxides of the Ni-doped Co-Mo2C exhibited less O2− coordination. The more O2−-coordinated hydrous oxide resembles CoOOH, which is favorable for promoting fast OER kinetics [6]. These results suggest that catalyst design considering the electrophilic characteristics of metal surfaces is necessary for activity enhancement, rather than simply relying on synergy effects between two metals. In addition, as shown in Figure 5(c), Fe doping lowers the potential for the oxidation reaction of an OER intermediate, enabling the OER to commence at lower potentials relative to that of Co-Mo2C. The OER polarization curves of Fe-doped Co-Mo2C catalysts with different Fe content in Co nanoparticles (Figure 5(d)) support the Fe doping effects. Here, 4.4 wt% Fe-doped Co-Mo2C delivers lower OER activity (336 mV @ 10 mA cm−2) than 8.8 wt% Fe-doped Co-Mo2C (318 mV @ 10 mAcm−2) but higher activity than Co-Mo2C (347 mV @ 10 mA cm−2). On the other hand, 15.5 wt.% Fe-doped Co-Mo2C shows slightly reduced OER activity (328 mV @ 10 mA cm−2) than 8.8 wt% Fe-doped Co-Mo2C, which is likely due to the formation of an impurity phase such as CoFe (Figure S4). The OER performance of Fe-doped Co-Mo2C with optimum Fe content was compared with previously reported results of transition metal oxides or hydroxides, as summarized in Table S4 [39, 40, 41, 42, 43, 44, 45, 46]. Combining the experimental observations discussed thus far, we concluded that Fe doping into Co-Mo2C significantly lowered the OER overpotential by making Co surface oxide more electrophilic, thereby promoting the formation of OER-active CoOOH. On the other hand, Ni doping decreased the OER activity by making the Co surface oxide less electrophilic. In addition to this OER catalyst design strategy, the facile synthesis of Fe-doped Co-Mo2C can be utilized as an efficient and practical means of preparing highly active OER catalysts for alkaline electrolysis.
4. Conclusions
In summary, we synthesized Fe- and Ni-doped Co-Mo2C samples with a simple precursor mixing step followed by heat-treatment. The TEM–EDS and XRD results clearly showed that Fe and Ni atoms were doped into the Co nanoparticles. Both the Fe- and Ni-doped Co-Mo2C were composed of nanoparticles 20–30 nm in size in which the Fe- and Ni-doped Co nanoparticles were coupled with Mo2C nanoparticles at their interfaces. From XPS studies, we found that Fe and Ni were present as Fe3+ and Ni2+ ions in the surface oxide layer of the Co nanoparticles. Fe3+ doping made the Co surface oxide more electrophilic, whereas Ni2+ doping made Co surface oxide less electrophilic. The more electrophilic Co surface oxide of the Fe-doped Co-Mo2C created a higher OH− affinity, enabling the formation of OER-active hydrous oxides with a higher degree of O2− coordination, similar to CoOOH, and lowering the onset potential of the OER. As a result, the Fe-doped Co-Mo2C exhibited a very low overpotential of 318 mV at 10 mA cm−2 in a 0.1 M KOH solution. This study provides a novel catalyst design strategy as well as a facile and practical method by which to prepare a new class of efficient OER catalysts for use in alkaline electrolysis.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Research Foundation (NRF) of Korea grant and funded by the Korean Government (Ministry of Science and ICT) (no.2021M3H4A3A02086516). This work was also supported by the Technology Innovation Program (20022449, Development of Large-Area Diaphragm Membrane and Electrode Production Technology for Alkaline Water Electrolysis) and funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Open Research
Data Availability
Data are available on request.




