Stream Quality and Benthic Macroinvertebrate Diversity in the Badeye Area, Logone Occidental Province, Southwestern Chad
Abstract
The aim of this study of benthic macroinvertebrates in the southwestern canton of Badeye in Chad was to analyze the community structure in relation to the physicochemical quality of water in the Man baptem tributary. A total of 4,012 benthic macroinvertebrates were identified and counted in 38 families. Arthropods were the most diverse, with 35 families and the most abundant (92.32%) of the total, followed by molluscs (6.65%) and annelids (1.02%). Insects predominated at all sampling stations (89.40%), followed by Gastropoda (6.75%), Malacostracans (2.85%), Hirudinea (0.57%), and Oligochaetes (0.45%). The preponderance of the Chironomidae, Psychodidae, and Physidae families in station A3 implies that the water at this station is of poor quality compared with the other 3 stations. The Shannon–Weaver and Pielou indices are higher at station A3, confirming some of the values of the physicochemical parameters at this station. In general, the four sampling stations are diverse. The abundance of certain benthic groups such as Diptera (Chironomidae) in the Man baptem tributary shows that this watercourse is subject to anthropogenic disturbance.
1. Introduction
Water is the most important element in life, and people are used to living in riparian areas [1]. Most of the human population is located close to waterways, particularly along large rivers because they are important sources of fresh water. In fact, they are the most susceptible to pollution [1]. Pollution represents a serious problem for the environment due to discharges into watercourses [2]. However, sewage systems, septic tanks, factory wastewater, and solid waste are the main sources of groundwater pollution in the urban sector. In periurban and rural areas, agriculture, through untreated domestic wastewater, is the main source of organic pollution. It is also one of the causes of surface water quality degradation [3].
The aquatic compartment, which is the ultimate receptacle for residues, is the most affected environment. These hazardous products deteriorate populations, cause intoxication, induce biocenotic disturbances, and sometimes even lead to the extinction of certain populations [4–6]. Preserving water quality is not only a necessary means of environmental management but also of conserving biodiversity. Furthermore, good water quality is essential, as it plays an important role in sustaining life, food production, energy production, and environmental support [7, 8]. In fact, aquatic ecosystems are of vital importance. However, they are subject to strong anthropogenic pressures and a growing population [9, 10]. Consequently, its monitoring must be carried out by evaluating reliable high-performance indicators such as bioindicators [11, 12]. Among the bioindicators, macroinvertebrates constitute organisms used the most frequently for the monitoring and evaluation of the global state of health of aquatic systems [13–16]. Due to their sedentary nature, their great diversity, and their tolerance of variable pollution and degradation of humid environments, bioindicators (macroinvertebrates) are good tools for evaluating the quality of the water of aquatic ecosystems [17]. Several studies on benthic macroinvertebrates have shown their importance in the food chain and in aquatic environments [18]. The main advantage of using these benthic macroinvertebrates lies in the fact that they are sensitive to physicochemical variables and environmental disturbances [10, 13].
The Republic of Chad is endowed with an immense hydrographic network: Chari, Logone, Lake Chad, Lake Fitri, Barh, etc. This hydrographic network is quite diversified and has numerous natural resources [19]. Unfortunately, these rivers, like other African cities, are experiencing major problems: the steady decline in their volume, the anthropic pressure exerted on them, and the degradation of their quality. [11, 20, 21].
Occidental Logone Province is considered the country’s leading economic zone. However, the Logone River and its tributaries are subject to various and considerable anthropogenic pressures as a result of discharges of industrial and domestic effluent waste from agricultural activities, bathing, detergent-based washing powder, etc. Nomadic pastoralists who water their animals and defecate in streams at the same time have degraded water quality and altered the diversity and settlement of benthic macroinvertebrates. Ultimately, the consequences of these disturbances could be reduced biodiversity and the risk of waterborne diseases within this [10, 22–24]. These anthropogenic disturbances observed at the sampling sites help to differentiate the richness and abundance of aquatic macroinvertebrates [21].
Currently, in Chad, there is no information on the benthic macrofauna of the Logone River and its tributaries. With a view to preserving aquatic biodiversity and contributing to knowledge of benthic macroinvertebrate fauna, an inventory of these organisms was carried out in the rural area of Badeye. The aim of this study was to analyze the diversity and structure of the benthic macroinvertebrate assemblages and to estimate the quality of the waters in this area.
2. Material and Methods
2.1. Study Area
The present study was carried out in the province of Logone Occidental, the Lac Wey Department, and the Ngandong subprefecture, and precisely in the canton of Badeye, located 41 km from Moundou at the southern end of Chad. Its geographical location lies between latitude 8° 31′60″ north and longitude 15° 43′60″ east. Located in the eastern part of the Ngandong subprefecture, Badeye straddles the Departments of Dodjé and Lac Wey. Its relief is generally flat, with numerous floods from the various surrounding valleys.
The soil is ferralitic, hydromorphic, and sandy clay. The area is subject to a Sudan-type climate characterized by two distinct seasons: the first dry season from October to April and the second rainy season from April to September. Rainfall varies between 900 and 1,200 mm/year [19]. Vegetation consists of herbaceous savannahs, trees, and semiwetlands. The area is endowed with surface water and groundwater from traditional wells and human-powered wells. The area has considerable agropastoral potential, which constitutes the main economic activity [19]. To carry out this work, four (4) stations from upstream to downstream were selected. These stations were selected on the basis of criteria such as accessibility, anthropogenic activities, microhabitat diversity, and stream depth.
- (1)
Station A1 (08°50′85.3″N, 015°74′52.9″ E) coming towards Doiti is located upstream and serves as a control station, unaffected by human activity. It is surrounded by kapok, shea, and small shrubs and grasses.
- (2)
Station A2 (08°50′98.7″ N, 015° 74′64.2″ E) is located far from station A1, on the Moundou-Banamar axis via Tapol. The area around the station is used for agriculture, with fields of manioc, sesame, and red millet. Fishing is also practiced here.
- (3)
Station A3 (08°51′47.7″ N, 015°74′99.1″ E) is located further downstream than the first station. It was chosen to estimate the impact of human activities on this environment. This station receives a large number of people who take baths in it, do their laundry, wash their dishes, and water the animals that defecate in it. Both banks are used for agricultural purposes, such as growing peanuts, sesame, and sorghum. The vegetation consists of mango and fig trees and small shrubs.
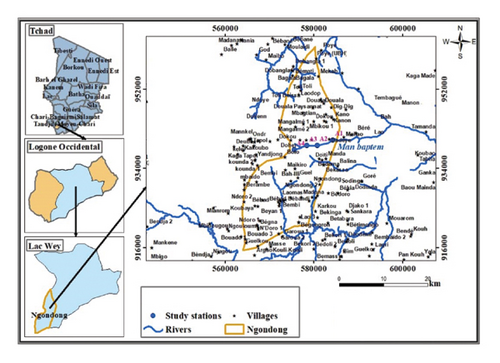
Station A4 (08°51′84.8″ N, 015° 76′62.2″ E) is located further downstream than the first three stations. It is also on the Douala Paysannat road, not far from the cemetery. The surrounding area is used for agriculture, with fields of sesame, groundnuts, sorghum, and beans. The vegetation consists in part of mango trees and a few grasses. This station is also home to a number of local residents, who wash their clothes and dishes in it, as well as some ruminants that drink and defecate in it.
2.2. Benthic Macroinvertebrate Sampling
Benthic macroinvertebrates were collected according to the standardized protocol. Sampling was carried out by taking into account the three components of the aquatic habitat: substrate, current speed, and station depth.
The sampling was carried out using a dip net consisting of a conical net with a mesh opening of 400 μm and depth of 50 cm, attached to a 30-cm square metal frame mounted on a 150-cm-long steel handle. It was carried out following the multihabitat approach proposed by [17, 25], which consists in carrying out at each station, in each campaign, a total of 20 net shots in different microhabitats characterized by the substrate/velocity pair.
Organisms caught in the mesh were collected using entomological forceps and a hand-held magnifying glass, then placed in pillboxes containing a 95% ethanol solution, and labeled with the date, station number, and time of collection. The samples obtained were returned to the Animal Physiology and Health Laboratory of the Faculty of Agriculture and Agricultural Sciences (FASA) for identification.
2.3. Identification of Benthic Macroinvertebrates
In the laboratory, the samples were washed under running water on a mesh sieve of 400 μm and then preserved in a 95% ethanol solution. Identification and counting were carried out station by station. For each station, collected organisms were poured into Petri dishes and then grouped according to size and morphological characteristics. They were identified using identification keys. These keys are those of [26, 27], which deal with invertebrates in general. Observations were made with the naked eye and under a Wild M38 stereoscopic binocular with episcopic lighting.
2.4. Measurements of the Physicochemical Parameters of Water
For each station, the physicochemical parameters were measured to gain a better understanding of the influence of environmental factors on the distribution of macroinvertebrate benthic structures.
In situ measurements of the water’s physicochemical parameters, namely, temperature, conductivity, and TDS, were determined using an OAKLON portable conductivity meter. The pH was measured using a portable HQdl pH meter.
Water samples were taken for nutrient analysis. Water samples were taken at each station in plastic bottles of 750 ml and stored in a cooler with ice for transport to the laboratory. In the laboratory, TSS, turbidity, salinity, dissolved oxygen, chlorine, phosphate, nitrite, nitrate, and ammonia nitrogen were measured using a HACH DR/890 spectrophotometer, and the values were expressed in mg/l.
Biochemical oxygen demand (BOD5 in mg/l of O2) was measured by respirometer using a LIEBHERR BOD incubator for 5 days at 20°C, according to the AFNOR method NFT 90-103. The rise in mercury in the tube was read every day for 5 days. COD was carried out using a powerful oxidizing agent, and organic compounds present in water measure the total organic matter content, including those that are not degradable by bacteria. Values are expressed in mg/l.
2.5. Data Analysis
Pearson’s, Shapiro–Wilk correlation tests, and the Kolmogorov–Smirnov normality test were performed using SPSS 26 software. They allowed the physicochemical variables to be compared between the different study stations at the significance level α = 5%.
The principal component analysis (PCA) made with R software made it possible to establish the physicochemical characteristics (temperature, pH, conductivity, etc.) that better define each sampling station.
The frequency of occurrence of taxa provides information on the level of constancy of a species or taxon in each habitat, without any indication of its quantitative importance. Expressed as a percentage (F), it is the quotient of the number of samples in which species i was found (Pi) by the total number of samples during the study period (Pt).
F is given by F (i) = Pi x100/Pt.
Using this formula, the following three groups of species can be distinguished according to the F values: constant species, present in at least 50% of surveys (F ≥ 50%); accessory species, present in at least 25% of the records (25% ≤ F < 50%); and rare species, present in at least 25% of the surveys (F < 25%).
The aim of principal component analysis (PCA) is to graphically present the maximum amount of information in a large table of quantitative data. PCA was applied to environmental data and metrics describing the structure of the benthic macroinvertebrate population to establish the abiotic and biotic typology of the various sampling stations.
3. Results
3.1. Environmental Variables
The spatial values of the physicochemical parameters recorded throughout the study are shown in Table 1. Analysis of water temperatures measured at the various stations reveals that station A4 (32°C) has the highest temperature and station A1 (23.80°C) has the lowest, i.e., a thermal amplitude of 8.2°C. pH ranged from 5.47 UC (UC is the unit of pH) at station A3 to 8.27 UC at station A1. Conductivity values ranged from a low value of 36 µS/cm to a high value of 96.40 µS/cm at station A4. TDS levels ranged from 19.10 mg/L at station A2 to 49.50 mg/L at station A4. Turbidity fluctuated around 2 NTU at stations A1, A3, and A4 and 36.60 NTU at station A2.
| Variables | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| Temperature (°C) | 24.97 ± 0.84 (23.80–25.80) | 25.50 ± 0.76 (24.50–26.30) | 25.50 ± 0.89 (24.90–26.50) | 27.95 ± 3.0 (25.30–32.00) |
| pH (U.C) | 7.07 ± 1.10 (5.96–8.27) | 6.33 ± 0.35 (6.10–6.86) | 5.85 ± 0.38 (5.47–6.31) | 6.96 ± 0.49 (6.41–7.50) |
| Conductivity (µS/cm) | 47.42 ± 11.71 (39.60–64.60) | 47.05 ± 11.60 (37.80–63.80) | 60.80 ± 17.60 (42.00–76.30) | 57.15 ± 28.43 (36.00–96.40) |
| TDS (mg/L) | 24.45 ± 5.87 (20.50–33.10) | 23.40 ± 6.22 (19.10–32.30) | 30.95 ± 9.05 (22.10–39.20) | 29.45 ± 14.03 (19.50–49.50) |
| Turbidity (NTU) | 13.65 ± 11.03 (2.00–28.60) | 22.60 ± 10.29 (11.90–36.60) | 14.77 ± 11.90 (2.00–26.80) | 11.85 ± 8.20 (2.0–20.80) |
| Salinity (mg/L) | 0.02 ± 0.005 (0.02–0.03) | 0.02 ± 0.05 (0.2–0.3) | 0.03 ± 0.005 (0.03–0.04) | 0.03 ± 0.02 (0.02–0.06) |
| Suspended solids (mg/L) | 68.92 ± 109.78 (7.60–233.30) | 72.55 ± 71.03 (16.60–176.60) | 72.85 ± 93.44 (9.50–210.00) | 84.40 ± 90.91 (8.80–216.60) |
| Color (Pt. Co) | 266.82 ± 250.10 (121.00–641.00) | 308.40 ± 140.35 (98.70–389.92) | 212.67 ± 217.01 (48.00–51.50) | 230.32 ± 231.53 (67.00–566.70) |
| Dissolved oxygen (mg/L) | 5.33 ± 1.45 (3.67–7.18) | 4.53 ± 0.87 (3.46–5.46) | 5.03 ± 1.59 (2.97–6.83) | 4.73 ± 1.78 (2.12–5.87) |
| Chloride (mg/L) | 6.37 ± 2.56 (4.00–10.00) | 9.75 ± 8.84 5.00–23.00 | 8.20 ± 5.16 (3.00–14.00) | 7.00 ± 3.16 (4.00–11.00) |
| NH4+ (mg/L) | 1.17 ± 0.71 (0.30–1.90) | 7.40 ± 8.61 (1.60–20.20) | 5.85 ± 6.32 (2.10–15.30) | 2.20 ± 0.57 (1.50–2.80) |
| Nitrate (mg/L) | 1.26 ± 2.16 (0.001–4.50) | 4.90 ± 4.76 (0.50–10.30) | 6.37 ± 4.57 (0.40–10.30) | 2.39 ± 2.39 (0.09–5.50) |
| Nitrite (mg/L) | 0.004 ± 0.003 (0.001–0.009) | 0.03 ± 0.04 (0.001–0.090) | 1.51 ± 2.99 (0.002–6.00) | 1.39 ± 2.41 (0.01–5.00) |
| Orthophosphates (mg/L) | 0.47 ± 0.35 (0.10–0.90) | 1.30 ± 0.82 (0.4–2.40) | 1.22 ± 0.60 (0.50–1.90) | 9.75 ± 15.56 (0.90–33.00) |
| BOD5 (mg/L) | 8.87 ± 0.64 (5.50–11.60) | 26.05 ± 30.85 (9.80–72.30) | 10.90 ± 4.01 (7.50–16.90) | 15.50 ± 5.37 (9.90–22.00) |
| COD (mg/L) | 17.25 ± 5.23 (10.80–23.20) | 52.55 ± 64.13 (18.10–148.70) | 21.70 ± 8.11 (15.70–33.10) | 28.50 ± 12.9 (17.60–45.80) |
The TSS values ranged from 7.60 mg/L to 216.60 mg/L at stations A1 and A4, respectively. For salinity, the minimum value was 0.03 (A3), and the maximum value was 0.06 mg/L (A4). The color ranged from 48.00 PtCo (A3) to 641.00 PtCo (A1). Suspended solids, pH, and electrical conductivity showed a significant difference at P = 0.05 between stations A1, A2, A3, and A4. However, there is a strong correlation between stations A2 and A4. The lowest chloride value was 3.00 mg/L (A3), and the highest chloride value was 23 mg/L (A2). The dissolved oxygen levels ranged from 2.12 mg/L to 7.18 mg/L at stations A4 and A1, respectively. For ammonium, the lowest concentration is 0.30 mg/L at station A1, and the highest concentration is 20.20 mg/L at station A2. Nitrate concentrations in the Man baptem stream ranged from 0.001 mg/L (A1) to 10.30 mg/L in A2 and A3 each. Minimum nitrite values were 0.001 mg/L at stations A1, A2, and A4, and maximum value was 6.00 mg/L at station A3. Orthophosphate values ranged from 0.10 mg/L to 33.00 mg/L at stations A1 and A4, respectively.
BOD5 levels ranged from 5.50 mg/L (A1) to 72.30 mg/L (A2). For COD, the lowest value was 10.80 mg/l at station A1, and the highest value was 64.13 at station A2. There is a correlation between dissolved oxygen, nitrate, and phosphate between stations A1, A2, A3, and A4. However, there were no significant differences between these variables and the stations.
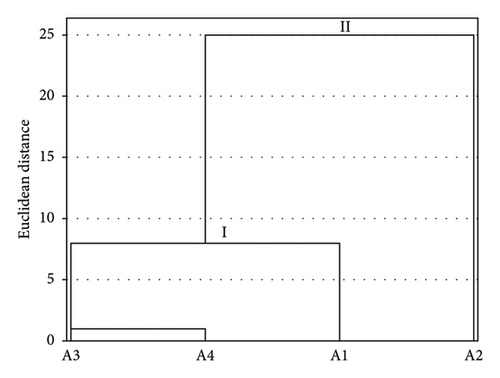
The hierarchical ascending classification (HAC) of stations based on the similarity of physicochemical parameters shows that sampling stations in the study area are grouped into two classes (Figure 2). Class I is that of stations A3, A4, and A1. However, station A1 located upstream is considered a reference; it is distinguished from stations A3 and A4 which are considered too anthropized with similar abiotic characteristics (they directly receive landfills from agricultural inputs and domestic waste). Class II is made up only of the little anthropized station A2, which differs by its physicochemical characteristics compared to the stations A3 and A4.
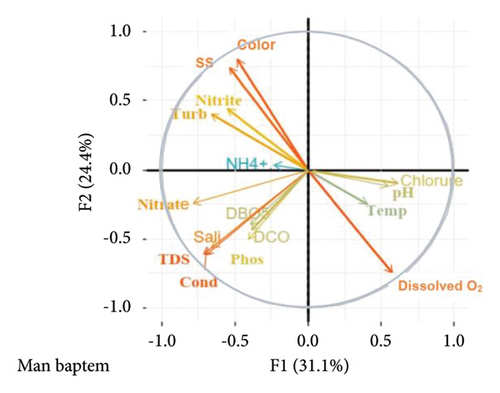
The principal component analysis (PCA) carried out from the physicochemical variables (Figure 3) shows on the correlation circle that conductivity (−0.313), TDS (−0.317), nitrate (−0.35), and dissolved oxygen are negatively correlated to the axes F1F2 < 0, and suspended solids (SS) (0.37) and color (0.40) are positively correlated with the F2 axis, while dissolved oxygen (−0.37) is also negatively correlated on the F2 axis.
3.2. Benthic Macroinvertebrate Assemblages
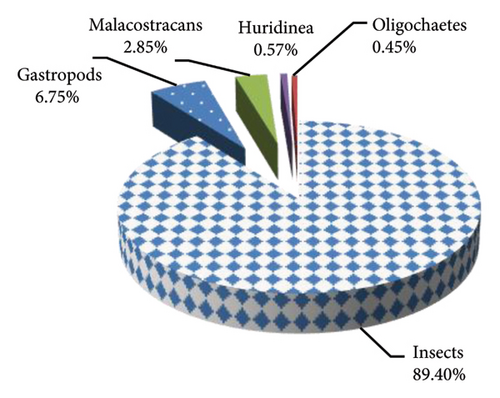
A total of 4,012 individuals including Arthropods (92.32%), 267 Gastropods (6.65%), and 41 Annelids (1.02%) were identified and counted during this study (Figure 4). Insects are the most represented, in 7 orders and 28 families, followed by Gastropods (1.2), Malacostracans (1.1), Oligochaetes (1, 1), and Hirudinea (1.1). In terms of quantity, insects predominate with 89.40% of total abundance, dominated by the Chironomidae family (36.54%), followed by Gasteropoda (6.75%), Malacostraca (2.85%), Hirudinea (0.57%), and Oligochaetes (0.45%).
3.3. Structure of the Benthic Macroinvertebrate Assemblages
Analyses of the numbers of different families show that in station A1, Baetidae and Caenidae are dominant, with 19.04% and 13.96%, respectively. They are followed by Perlidae (8.46%) and Ephemerythidae (8.18 M) of the total abundance. At station A2, the Dytiscidae and Hydraenidae families dominate with 10.44% and 9.20%, respectively, followed by Libellulidae (9.02%), Nepidae (7.78%), and Gyrinidae (7.43%). At station A3, Chironomidae predominate (65.71%), followed by Psychodidae (10.33%) and Physidae (8.86%) in relative abundance. Station A4 is dominated by Dytiscidae families (14.18%), followed by Helodidae (13.83%) and Nepidae (10.50%) of total abundance.
Generally, station A3 has the highest abundance, with 54.01% of specimens collected in this river, that is, 2,167 individuals. This station is followed by A1 with 709 individuals (17.61%), then A4 with 571 individuals (14.4 233%), and A2 with 565 individuals (14.08%). In terms of taxonomic diversity, station A4 is the richest. Then, 26 families of benthic macroinvertebrates were counted at stations A1 and A2, with 21 families each, with 22 families at station A3.
Figure 5 shows the spatial evolution of macroinvertebrates according to Shannon’s diversity indices and Pielou’s equitability. The Shannon index shows that station A2 (H = 3.33 bits/ind) is the most diverse, while station A3 (H = 2.54 bits/ind) is the least diverse. There is a significant difference between station A3 and stations A1, A2, and A4. The highest value of the equilibrium index is observed at station A2 (0.92) and the lowest at station A3. Equitability at all stations in the Man baptem stream is close to 1, indicating that benthic macroinvertebrate assemblages are balanced. According to the Kolmogorov–Smirnov normality test, there is no significant difference between the 4 stations.

Of a total of 38 families collected in the Man batem stream, 4 macroinvertebrate families are very frequent in station A1 with a frequency of occurrence of 50%, including the Baetidae, Caenidae, Ephemerythidae, and Perlidae families. At station A2, only the Dytiscidae family is very frequent. At station A3, the most anthropized, 4 families, Chironomidae, Psychodidae, Grapsidae, and Physidae, are very frequent. At station A4, two families, Nepidae and Dytiscidae, are very frequent. Table 2 shows the different signs of frequency of occurrence of the families collected in the Man baptem stream.
| Families | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| Baetidae | +++ | + | + | + |
| Caenidae | +++ | + | + | + |
| Machadorythidae | − | − | − | + |
| Ephemerythidae | ++ | − | − | − |
| Ephemeridae | + | + | − | − |
| Nepidae | ++ | ++ | ++ | +++ |
| Notonectidae | − | − | − | + |
| Gerridae | ++ | − | + | − |
| Pleidae | + | − | − | + |
| Veliidae | − | + | + | − |
| Belostomatidae | − | + | + | ++ |
| Mesoveliidae | − | − | + | − |
| Perlidae | +++ | − | − | − |
| Aeshnidae | ++ | − | + | + |
| Coenagrionidae | − | + | + | − |
| Gomphidae | − | − | − | + |
| Libellulidae | + | ++ | + | + |
| Lestidae | ++ | − | − | + |
| Corduliidae | + | − | − | + |
| Dytiscidae | +++ | +++ | ++ | +++ |
| Helodidae | +++ | + | + | ++ |
| Hydrophilidae | − | − | + | + |
| Hydraenidae | ++ | ++ | ++ | + |
| Géorissidae | + | − | − | − |
| Spercheidae | + | − | − | − |
| Gyrinidae | + | + | − | − |
| Elmidae | − | − | + | − |
| Philopotamidae | + | − | − | + |
| Ecnomidae | − | − | − | + |
| Hydropsychidae | − | + | − | − |
| Tabanidae | − | ++ | − | − |
| Dolichopodidae | − | ++ | − | − |
| Chironomidae | − | − | +++ | + |
| Psychodidae | − | − | +++ | + |
| Grapsidae | ++ | ++ | +++ | ++ |
| Physidae | − | ++ | +++ | ++ |
| Erpobdellidae | − | − | + | ++ |
| Tubificidae | − | − | ++ | + |
- −: F = 0% (absent taxa), +: F 0% and <25% (rare taxa), ++: 50% > to F 25% (frequent taxa), +++: 50% F (very frequent taxa).
4. Discussion
4.1. Physicochemical Characteristics
The high-temperature values would be due to the ambient temperature of the environment on public supply water in the city of N’Djamena in Chad. Man baptem waters are practically neutral, which can be attributed to the nature of the substrate. This result confirms those of [18] in the Nguitto stream in the Central African Republic. The low values of electrical conductivity, TDS, turbidity, and salinity in the Man baptem stream reflect low mineralization of the water in place of the geological substrate. This agrees with those of [29] in periurban streams in the city of Dschang (Cameroon). The low concentrations of TSS at station A1, located upstream, are very little anthropized.
This result is similar to that of [30] in the Nsapé stream in Douala. The low levels of dissolved oxygen in the stations would be related to the low anthropogenic activities in these stations. This confirms those of [31] in streams in the western region (Cameroon). The high values of chloride, ammonium, nitrate, nitrite, orthophosphate, BOD5, and COD in stations A2, A3, and A4 located downstream of the discharge are due to the discharges of crop-related fertilizers, domestic waste, and detergents into this watercourse. This result is similar to that of [32] on the tropical urban river of Douala (Cameroon). It also corroborates that of [6] on the stream waters of the middle Alibori River (Benin). The principal component analysis carried out from the physicochemical data highlights the physicochemical variables on which we can base ourselves to define the waters of the Man baptem watershed. These include TDS, conductivity, nitrate, suspended solids (SS), color, and dissolved oxygen. On the other hand, the other parameters are ignored by the physicochemical characterization of this watercourse.
4.2. Biological Characteristics
The taxonomic richness of macroinvertebrates in Man baptem (38) families is similar to that found in rivers under very low anthropogenic pressure. Our results also corroborate those obtained by [14] who identified 38 macroinvertebrate families in Mefou. The results obtained in this study show that insects are the most abundant. They corroborate several previous studies that have shown the predominance of the insect class in aquatic environments, as well as the scientific comments obtained from the preprint of the said manuscript [10, 33]. The other classes, namely, Malacostracans, Molluscs, Oligochaetes, and Hirudinea, are poorly represented. The Diptera are the most abundant order in terms of individuals, specifically Chironomidae, followed by Psychodidae at station A3, considered the most anthropized. Ephemeroptera, Odonata, Coleoptera, and Hemiptera are poorly represented. The total taxonomic richness is 89 taxa, including 20, 21, 22, and 26 at stations A1, A2, A3, and A4, respectively. However, the authors of [32] have shown that the preponderance of Chironomidae and Psychodidae in station A3 is indicative of a high organic matter load in the water. However, station A1, which is considered a control station, offers favorable conditions for the proliferation of macoinvertebrates such as Baetidae and Caenidae, which are highly sensitive to the action of pollution. Equitability calculations reveal the balanced nature of the watercourse studied.
This result corroborates that of [10] in the streams of Moukalaba-Doudou National Park (Gabon). However, station A3 shows low diversity compared to the other 3 stations, which could be explained by the fact that this station is considered the most accessible. The frequency of occurrence of taxa exceeding 50% allows us to identify macroinvertebrate taxa that can be qualified as polluosensitive and polluoresistant. At station A1, 3 constant taxa, Caenidae, Baetidae, and Perlidae (Table 2), were recorded, which are classified as polluosensitive. This station would provide a favorable environment for these species. These results are similar to those of [29] in the Dschang streams (Cameroon). Station A2, with one constant taxon (Dytiscidae), and A4, with two constant taxa (Nepidae and Dytiscidae), which are also considered sensitive to pollution. In station A3, Chironomidae, Psychodidae, and Grapsidae families are omnipresent at this station, where the water is highly mineralized. These results corroborate those of [32] in Douala streams, which showed that Chironomidae and Psychodidae are characteristic of aquatic environments disturbed by human activities. They can be considered to be pollutant-resistant and could serve as bioindicators.
5. Conclusions
This study has enabled us to characterize the Man baptem stream in terms of environmental and biological parameters. The physicochemical analysis carried out in this tributary shows that the water quality is satisfactory at station A1. However, at stations A2, A3, and A4, certain parameters such as orthophosphate, BOD5, and COD have very high levels. This study allowed us to identify benthic macroinvertebrates in the Ngandong subprefecture (Chad), which is very similar to the structure of these communities in tropical African rivers, characterized by a preponderance of arthropods, and insects in particular. The results accurately contrast upstream reference stations with downstream polluted ones. Station A1 is characterized by a wealth of species (Ephemeroptera, Plecoptera) indicative of good-quality water. Station A3, on the other hand, is characterized by a high abundance of species (Diptera, Chironomidae, and Psychodidae) indicative of an environment impacted by human activities. This study confirms the value of the bioindication of benthic macroinvertebrates in the Ngandong subprefecture and opens up a significant field of research for this region, as well as for all the rivers in Chad.
Ethical Approval
All experiments were reviewed and approved by the COESR (Committee of Ethics of Scientific Research) of the Faculty of Sciences of the University of Dschang (no. 1806/21/UDS/FS/DBA). All authors hereby declare that the principles relating to animal care have been respected.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
All authors contributed to the study. Design, material preparation, data collection, and analysis were performed by Djikoloum Beosso Theophile, Seino Richard Akwanjoh, and Norbert Tchamadeu Ngameni. All authors participated in the writing and the final approval of the manuscript.
Acknowledgments
We would like to thank the local authority and its team for their agreement to data collection. We would also like to thank Mr. Aseaku Frederik Nkakah and Mr. Mbaitelssem Bienvenu for their participation in the statistical analyses. We used R, SPSS, Excel, Word, and DeepL which enabled us to process and write the manuscript.
Open Research
Data Availability
Datasets are available from the corresponding author on reasonable request.




