Sodium-Decorated SiB Monolayer for Reversible Hydrogen Storage: High-Capacity Achieved by Carbon Doping
Abstract
Based on the first-principles calculation and grand canonical Monte Carlo (GCMC) simulation, the reversible hydrogen storage of Na-decorated silicon boron (SiB) and C-doped SiB monolayers have been investigated. For double side 4Na-decorated SiB monolayer, each Na atom can adsorb five H2 molecules with the average adsorption energy of −0.18 eV/H2, yielding an H2 gravimetric density of 9.09 wt% and desorption temperature TD of 227 K. To further improve the H2 gravimetric density and desorption temperature, doping effect was employed to enhance the internal electric field between Na atom and SiB substrate. When two Si atoms in the SiB monolayer were replaced by C atoms, the adsorption energy for H2 was significantly improved. The H2 gravimetric density reaches to 10.63 wt% with the average adsorption energy of −0.22 eV/H2. Meanwhile, the desorption temperature TD was raised from 227 to 282 K, reaching the ideal condition near room temperature. In addition, the GCMC simulations further confirm that the H2 gravimetric density can fully meet the latest hydrogen storage target (5.5 wt%) for both Na-decorated SiB monolayer and Na-decorated C-doped SiB monolayer. Our results indicate that both Na-modified SiB and C-doped SiB monolayers can be used as promising materials for reversible hydrogen storage.
1. Introduction
Due to the efficient, clean combustion and renewable properties, hydrogen energy is expected to be the ideal candidate to replace fossil fuels [1, 2, 3]. The latest hydrogen storage goal set by the U.S. Department of Energy (DOE) is 5.5 wt% before 2025 at the near ambient thermodynamic environment (233−333 K) and stable controlled pressures (35−100 bar) [4]. However, the low density and low efficiency of hydrogen storage seriously restrict the sustainable development of hydrogen energy [5]. Until now, researchers are still exploring efficient, safe and cheap hydrogen storage methods.
Among the various hydrogen storage technologies, solid-state storage, which chemically or physically adsorbs hydrogen onto the surface of a material, is considered to be the ideal hydrogen storage option due to its safe and reversible characteristics. In particular, physical adsorption is the binding of hydrogen molecules (H2) to the material through van der Waals (vdW) interactions rather than covalent bonds, which may lead to a rapid adsorption/desorption process. In recent years, due to the large specific surface area, excellent chemical stability, and lightweight [6], 2D materials have attracted much attention in terms of hydrogen storage performance, which are expected to promote a revolution in hydrogen energy development [7, 8, 9, 10].
- (1)
The first method is to modify the substrate by metal ions, such as alkaline metals [13], alkaline earth metals [14], and transition metals [15]. For example, Zhang et al. [16] found that lithium-modified naphthalene can reach an impressive hydrogen storage capacity of 14.9 wt% by first-principles calculation. Dou et al. [17] also explored the potential of magnesium-modified g-C2N monolayer as a reversible hydrogen storage material, which can reach a hydrogen storage weight capacity of 8.03 wt%. In addition, Trivedi et al. [18] theoretically predicted that Ti-modified BeN4 could absorb up to seven H2 molecules through Kubas interactions with high corresponding hydrogen storage weight and average hydrogen adsorption energy.
- (2)
The second method is to make suitable adjustments and modifications to the substrate, such as implementing doping, applying strain, and so on [19]. For example, we systematically studied lithium- and sodium-modified graphdiyne and ultimately obtained an expected hydrogen gravimetric density of 8.81 wt%, higher than that of the undoped case [20]. Similarly, Deng et al. [21] revealed that a hydrogen storage capacity of 12.2 wt% can be obtained for lithium-modified C-doped BN. Compared with 7 wt% in the undoped case, the hydrogen storage capacity is significantly enhanced.
- (3)
The third method is to search for new 2D materials, such as carbon-based systems [22, 23, 24], CN systems [25], and BN systems [26]. Recently, 2D SiB material with a honeycomb structure, which exhibits excellent thermodynamic stability, has been theoretically predicted [27, 28, 29]. Subsequently, Jiang et al. [30] studied the H2 gravimetric capacity of SiB monolayer with Li modification via density functional theory (DFT) calculations. It is found that the H2 storage capacity of the Li-modified original SiB substrate is 8.1 wt%. Surprisingly, this hydrogen storage density increases by about 30% under 7% biaxial tensile strain, as the tensile strain provides more space for H2 adsorption. These results reveal the excellent applications of SiB monolayer with large strain for hydrogen storage. However, applying large strain on 2D materials is extremely challenging and requires a high level of experimental techniques. Given this situation, some natural but not negligible questions will arise: In addition to the large tensile strain, is there a more direct way to further increase the H2 storage density of the SiB monolayer? How to further enrich and expand the application of SiB monolayer in the field of hydrogen storage? Is it possible to achieve both high hydrogen storage density and desorption temperature close to room temperature?
Motivated by the above-mentioned several issues, in this work, the SiB monolayer will be modified with sodium (Na) atoms to establish the internal electric field and improve the hydrogen adsorption energy. In addition, replacing Si atoms with carbon (C) atoms will further strengthen the binding energy between Na atoms and SiB monolayer. The results reveal that the hydrogen storage density and the desorption temperature TD can be significantly increased with C doping (i.e., 10.63 wt% and 282 K, respectively), further confirmed by the grand canonical Monte Carlo (GCMC) simulations. These findings expand the application of SiB as a hydrogen storage material and provide additional references for further experimental studies.
2. Computational Details
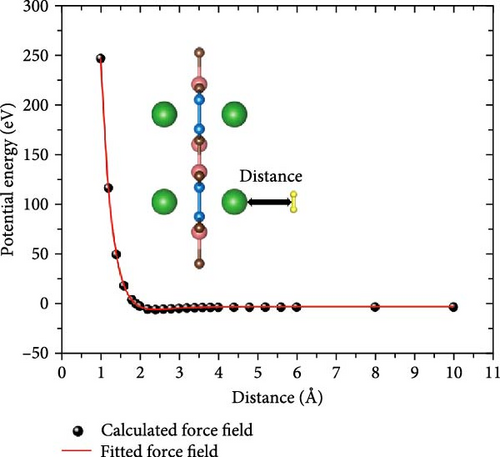
To check the structural thermal stability, the ab initio molecular dynamics (AIMD) simulations were performed. The time step and total duration were set to 1 fs and 10 ps, respectively. In addition, to calculate the H2 adsorption capacity of the whole system in more detail, GCMC simulation was employed. Since the interaction between H2 and Na-decorated substrate can be described by the Morse potential function, the corresponding force field parameters will be obtained by fitting the Morse equation. The detailed steps are as follows: First, the potential energy of interaction between H2 and Na-decorated substrate is calculated by DFT. Second, the potential energy curve is fitted based on the Morse equation. Then, the fitted force field parameters are written into the Dreiding force field file [36, 37]. Finally, MC simulation is performed using the grand canonical ensemble. To ensure the convergence of the data, an MC simulation with 5 × 106 steps for each point was employed.
3. Results and Discussion
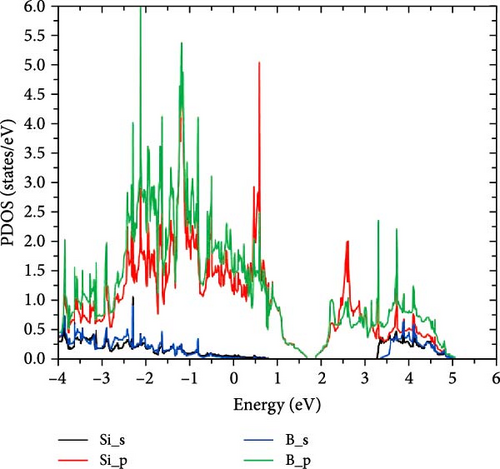
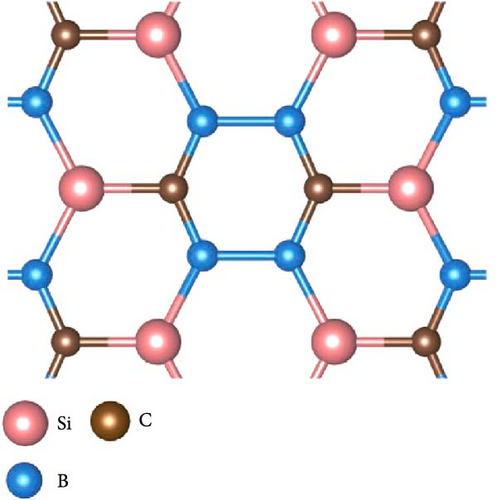
SiB monolayer is a structural analog of graphene and hexagonal boron nitride, as shown in Figure 1(a). The cohesive energy of SiB is 4.71 eV/atom, which is larger than that of β-silicene [27]. In the SiB monolayer, three nonequivalent B─B, Si-B, and Si─Si bonds construct two kinds of planar six-membered rings, i.e., Si4B2 and Si2B4, unlike the singularity of the C six-membered ring in graphene. First, the SiB primitive cell (marked by black dashed lines) was expanded into a 3 × 2 supercell and then relaxed. After lattice optimization, no obvious folds or distortions were found in the SiB monolayer structure, and the positions of Si and B atoms were still located in the same plane, indicating the structural stability of the SiB monolayer. In fact, thanks to the high symmetry of SiB monolayer and the strong hybridization between Si’s p orbitals and B’s p orbitals (see the projected density of states (PDOS) shown in Figure 1(b)), the structural stability of SiB monolayer has been repeatedly confirmed [28, 29]. In addition, the relaxed lattice constants (a = 5.96 Å and b = 3.34 Å) and corresponding bond lengths (2.28 Å for Si─Si, 1.97 Å for Si-B, and 1.61 Å for B─B, respectively) in our calculations are also in good agreement with the results of previous calculations [38]. These calculations show that the computational parameters set in this work are feasible.
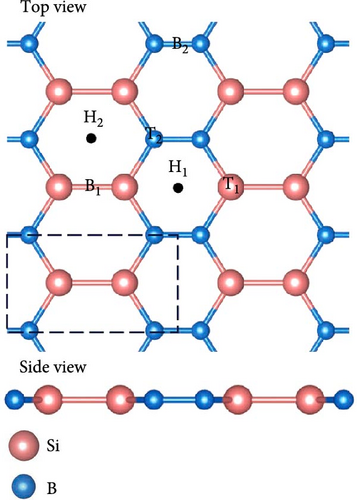
Then, the SiB monolayer modified by Na atoms has been checked. In our calculations, the six possible adsorption sites for a single Na atom on pristine SiB (labeled as H1, H2, T1, T2, B1, and B2) are tested, as shown in Figure 1(a). H1 and H2 denote the hollow sites above the centers of the Si2B4 and Si4B2 six-membered rings, respectively. T1 and T2 represent the top sites of Si and B atoms, respectively. B1 and B2 denote the bridge sites of Si─Si and B─B bonds, respectively. However, when Na atoms are initially placed on top of Si and B atoms (i.e., T1 and T2 sites), Na atoms cannot be stabilized in these locations and will spontaneously move to the H1 or B2 site. As summarized in Table 1, the hollow site H1 is preferred as the desorption position of Na atom, and the adsorption energies of all tested sites are obviously larger than the cohesive energy of Na (−1.13 eV) [39], suggesting that Na atoms will be uniformly distributed on SiB without forming clusters.
| Adsorption site | H1 | H2 | T1 | T2 | B1 | B2 |
|---|---|---|---|---|---|---|
| Adsorption energy | −3.00 | −2.81 | / | / | −2.21 | −2.65 |
- “/” Indicates that the adsorption position of Na is unstable.
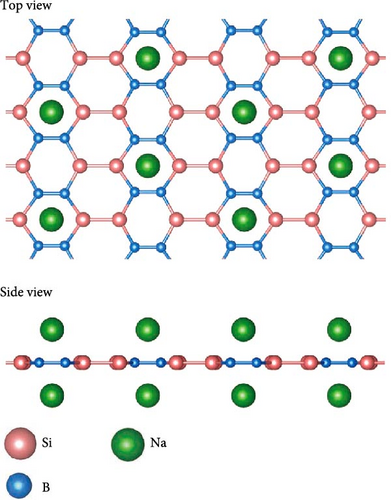
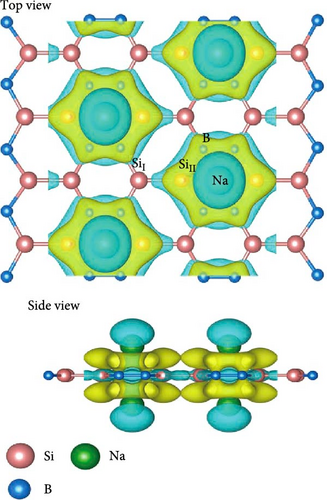
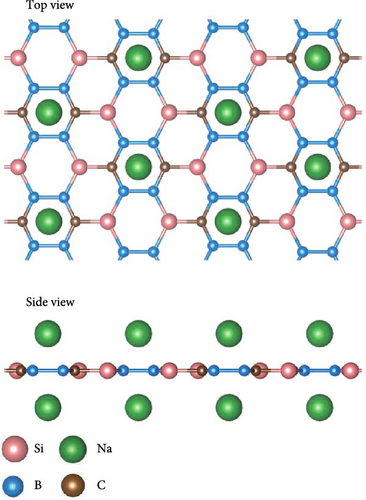
To obtain as high hydrogen storage capacity as possible, both sides of the SiB monolayer are symmetrically modified by Na atoms, as shown in Figure 2(a). Such Na-modified SiB substrate is dynamically stable according to its phonon spectrum (Figure S1). After structural optimization, the distance and average binding energy between Na and SiB monolayer are 2.06 and −2.89 eV, respectively. To understand the adsorption mechanism of Na atoms decoration in the system, the charge density difference Δρ (defined as Δρ = ρNa-SiB-ρNa-ρSiB, where ρNa-SiB, ρNa, and ρSiB are the charge densities of Na-modified SiB, Na atoms, and SiB, respectively) has been analyzed. As reflected in Figure 2(b), because the Na atom is weaker in electronegativity than Si and B atoms, the charge transfer from Na to SiB occurs, which can be quantitatively confirmed by Bader charge analysis. As summarized in Table 2, Na atom transfers −0.798 e−/atom to SiB monolayer. Compared to the original SiB monolayer, the transferred electrons have been redistributed between Si and B atoms, and hybridization occurred between Na’s s orbital and SiB’s 2p orbitals (Figure S3), indicating a significant interaction between Na atoms and SiB substrate.
| Systems | Atom | Max | Min | Average |
|---|---|---|---|---|
| 4Na-SiB | Si | −0.975 | −0.509 | −0.742 |
| B | 1.213 | 1.069 | 1.141 | |
| Na | −0.798 | −0.798 | −0.798 | |
| 4Na-SiB + 20H2 | Si | −0.974 | −0.525 | −0.758 |
| B | 1.150 | 1.049 | 1.097 | |
| Na | −0.806 | −0.806 | −0.806 | |
| H | 0.100 | −0.069 | 0.013 | |
| 4Na-C2Si2B4 | Si | −1.679 | −1.678 | −1.679 |
| B | 0.154 | 0.033 | 0.093 | |
| C | 2.276 | 2.275 | 2.276 | |
| Na | −0.783 | −0.783 | −0.783 | |
| 4Na-C2Si2B4 + 20H2 | Si | −1.632 | −1.626 | −1.629 |
| B | 0.029 | −0.004 | 0.018 | |
| C | 2.253 | 2.239 | 2.246 | |
| Na | −0.812 | −0.812 | −0.812 | |
| H | 0.107 | −0.068 | 0.016 | |
To get the exact charge transfer direction, the net atomic charges of Si, B, and Na atoms before and after forming Na-SiB have been calculated by using DDEC6 [40], as shown in Table S1. For the results given by DDEC6, we can analyze the data from Table S1 in conjunction with Figure 2(b). As shown in Figure 2(b), Si atoms can be classified into two groups in terms of electron gain and loss during Na adsorption: (1) Si atoms are not in the Na-modified Si2B4 six-membered ring, labeled as SiI; and (2) Si atoms are located in the Na-modified Si2B4 six-membered ring, labeled as SiII. When Na is modified above the six-membered ring of Si2B4, both SiII and B gain electrons because they are more electronegative than Na. In addition, since B is more electronegative than Si, it can gain electrons from both Na and SiI, thus becoming more negative. However, in the case of SiII gaining electrons, SiI must lose more electrons than before to ensure charge conservation of the system; hence, SiI becomes more positive. Such the charge accumulation and depletion behavior will induce a local internal electric field, which can effectively improve the adsorption energy of hydrogen. The underlying physical mechanism is that the positive Na ion could produce the stronger local electric field than the neutral Na atom. When H2 molecules are adsorbed, this electric field will polarize the H2 molecules (Figure S2), and the H─H bond length extends from ~0.74 Å (original H2 molecular bond length) to ~0.76 Å (Figure 3(c)) due to the polarization interaction between Na ion and H2. Therefore, the adsorption energy of H2 is significantly improved.
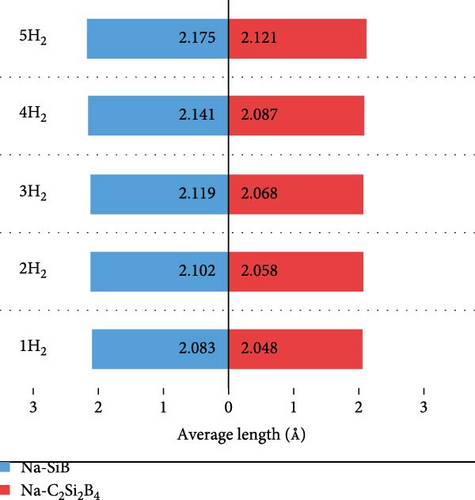
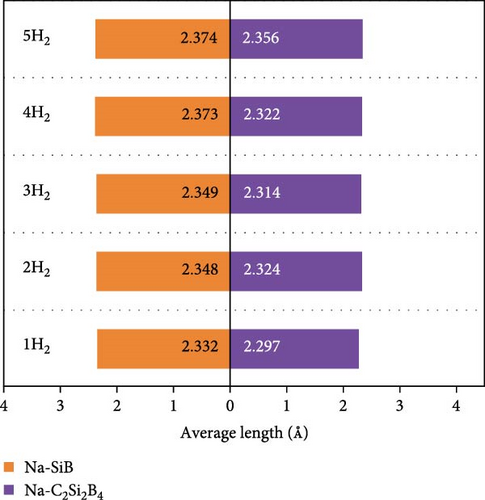
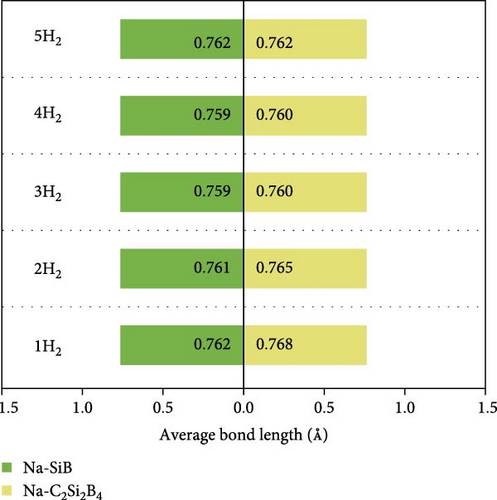
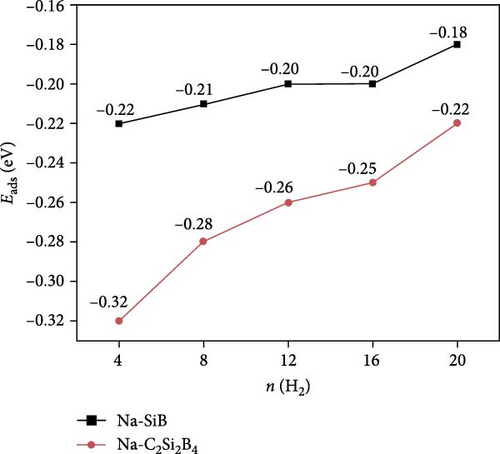
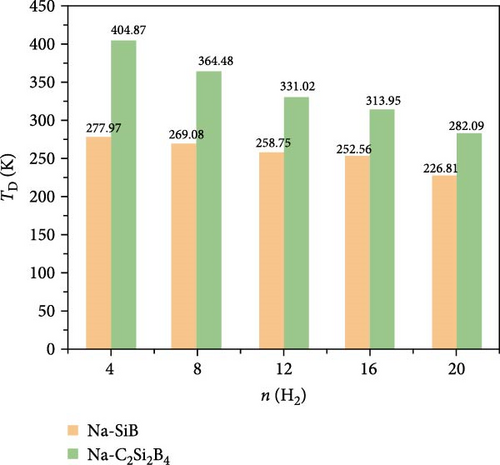
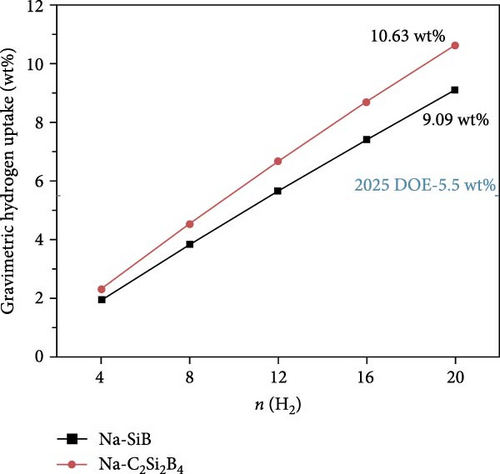
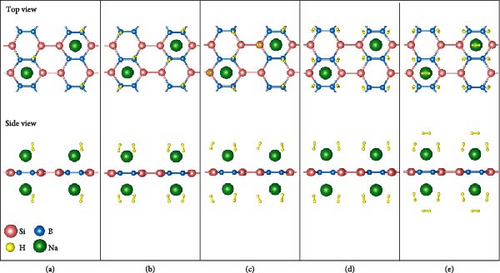
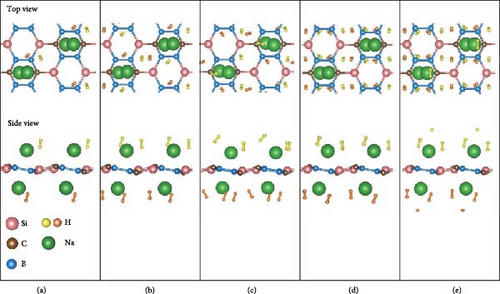
Next, based on the above stable 4Na-decorated SiB model, the adsorption process of H2 molecules was investigated systematically. The number of H2 molecules attached to the Na atom can be calculated by putting in H2 molecules one by one. Here, five H2 molecules can be added around each Na atom. The optimized structures with H2 adsorption are shown in Figure 4. In general, the adsorption model of the first H2 molecules is very important, which largely reveals the possible adsorption sites of H2 molecules around the Na atom. The detailed adsorption positions are as follows: the first H2 (1H2) molecule is positioned above Si and tilted toward the metal Na atom; the second H2 (2H2) molecule position is symmetric to the first H2 molecule position; the third H2 (3H2) molecule forms a triangular structure with the previous two H2 molecules; the fourth H2 (4H2) molecule forms a tetragonal structure with the previous three H2 molecules; the fifth H2 (5H2) molecule is located on the top of Na atom. Side and top views of these H2 adsorption locations described above are shown in Figure 4 and Figure S5. Combining Figure S5 with Figure 4, the placement of the H2 molecule can be observed more intuitively. As the number of H2 molecules increases, the magnitude of dH–H always remains around 0.76 Å (Figure 3(c)), indicating that H2 molecules gather around Na atoms by physical adsorption, which is favorable for the rapid release of H2 molecules. Within the range of 1H2 to 5H2 molecules for each Na atom, the calculated average H2 adsorption energies (i.e., −0.22 eV/H2 for 1H2, −0.21 eV/H2 for 2H2, −0.20 eV/H2 for 3H2, −0.20 eV/H2 for 4H2, and −0.18 eV/H2 for 5H2, respectively, refer to Figure 3(d)) are all in the ideal range for practical applications (i.e., −0.15 to −0.6 eV/H2). At the same time, although the value of dNa-H2 is slightly increased from 2.332 to 2.374 Å (Figure 3(b)), it is still less than the standard of stable H2 adsorption (<2.5 Å). In this case, the hydrogen gravimetric capacity is calculated as 9.09 wt% with the desorption temperature TD ~ 227 K (Figures 3(e) and 3(f)), exceeding the DOE target of 5.5 wt% and much higher than the liquefaction temperature of H2 (19 K).
As we know, the hydrogen gravimetric capacity (> 5.5 wt%) and the desorption temperature TD (near room temperature) are two important characteristic quantities to measure whether a material can be used for high-quality reversible hydrogen storage [41, 42]. In order to improve these two important performances of the whole system, enhancing the binding energy between the metal atom and substrate is the starting point. According to previous studies [43], doping specific atoms in the substrate is an effective method to increase the binding energy. Therefore, in the following calculations, the substitution of Si atom by C atom in the SiB substrate is studied.
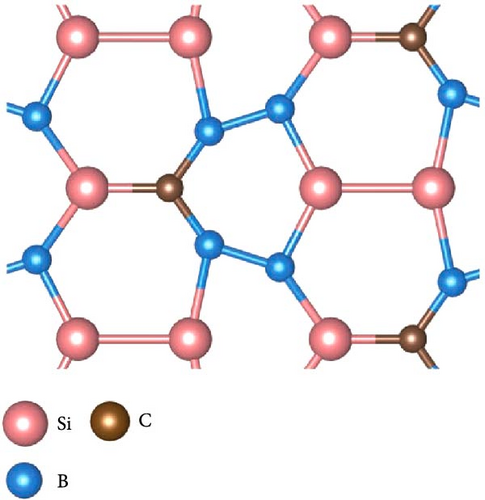
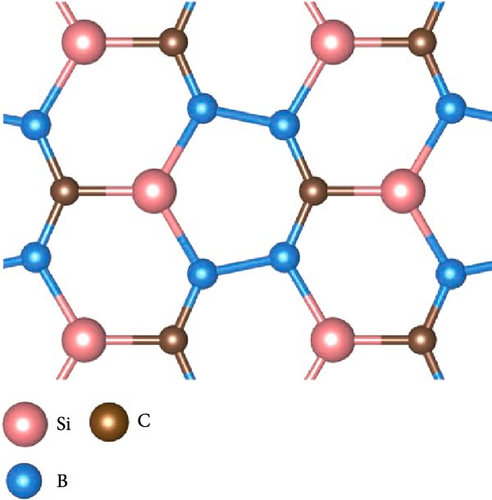
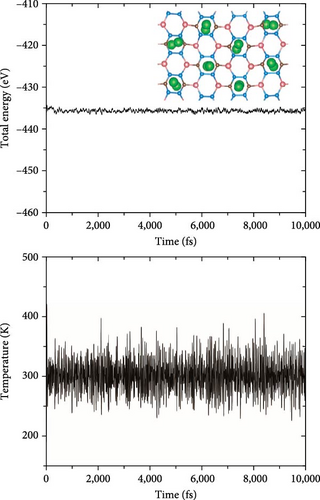
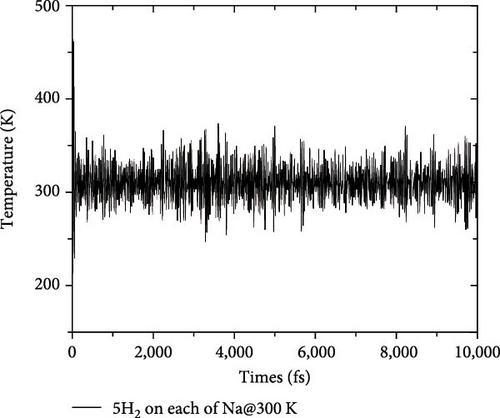
As shown in Figure 5, the structures in which Si atom is replaced by C atom have been checked. Here, three doping configurations are considered. First, the structure of 1 C atom replacing one Si atom (defined as type-I) was optimized, as shown in Figure 5(a). Because of the large difference in radius between C and Si atoms, the lattice symmetry of the system is severely broken, resulting in significant distortion of the original six-membered rings, including Si4B2 and Si2B4, which is in line with the previous research conclusions [38]. For the replacement of two Si atoms by C atoms, there are two possible doping modes defined as type-II and type-III, as shown in Figures 5(b) and 5(c). These two doping structures have relatively small symmetry distortion of six-membered rings compared to the type-I structure, because the CB ring or CSiB ring in type-II and type-III structures still has relatively high symmetry of six-membered rings. By comparing the energies of the different Na modification sites in these three doped structures, the Na modification at the hollow site H1 (above the C2B4 ring) in type-II doped structure (Figure 6(a)) has the lowest energy (~−2.6 eV), which is still larger than the cohesive energy of Na. Then, to detect the structural stability of C-doped SiB monolayer (C2Si2B4) with Na modification, AIMD simulations were performed at room temperature of 300 K. The total energy fluctuation with time and the temperature fluctuation with time are displayed in Figure 6(b). These curves show that although the temperature fluctuation is relatively drastic, the total energy variation range is small, indicating the thermal stable structure of the Na-modified C-doped SiB monolayer. In addition, due to the strong hybridization of 2p orbitals between SiB and C over a wide range of energies, as well as the hybridization of these 2p orbitals with Na’s s orbital near the Fermi level (Figure S4), the charge density difference presents the interesting charge channels along the Na-decorated C2B4 rings (Figure 6(c)).
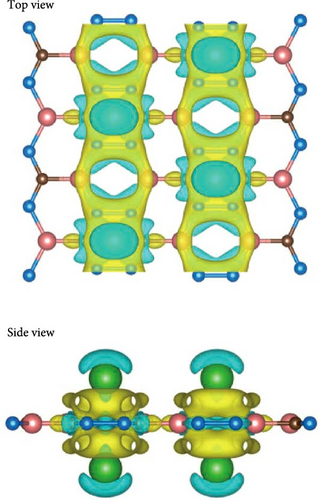
Compared with the case of Na-modified SiB without C doping, the binding energy of Na atom in C-doped structure (i.e., C2Si2B4) is slightly reduced (from −2.89 to −2.6 eV), because of the decreased charge transfer (from −0.798 to −0.783 e−/atom, refer to Table 2) between Na and the substrate. Surprisingly, however, when the system adsorbed H2 molecules (from the first H2 molecule to the fifth H2 molecule), the C-doped substrates are all folded (Figure 7). In this case, the charge transfer is increased from −0.806 to −0.812 e−/atom compared to H2 adsorption without C doping (Table 2), resulting in a stronger internal electric field. Based on this, the average adsorption energy of H2 has been significantly improved (i.e., −0.32 eV/H2 for 1H2, −0.28 eV/H2 for 2H2, −0.26 eV/H2 for 3H2, −0.25 eV/H2 for 4H2, and −0.22 eV/H2 for 5H2, respectively, refer to Figure 3(d)). The charge density difference of H2 adsorption is shown in Figure S6. With the folding of the structure, the charge distribution also presents a corresponding inclined distribution, as expected.
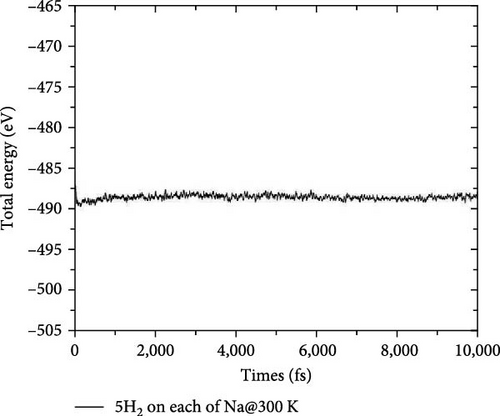
To test the thermal stability of Na-decorated C2Si2B4 at room temperature, AIMD simulations were performed at 300 K. As shown in Figure 8, the total energy variation of Na-decorated C2Si2B4 structure is within a reasonable range, which demonstrates the thermodynamic stability of C2Si2B4 with each Na adsorbing 5 H2 molecules under practical experimental conditions. In addition, as shown in Figures 3(a) and 3(b), the distances of Na to the substrate and to the H2 molecules are all smaller than those of the undoped ones, further verifying that doping indeed improves the related properties of hydrogen storage. However, the value of dH–H remains around 0.76 Å, as in the undoped case, indicating that the adsorption method of H2 is still physical adsorption, which is very favorable for the rapid release of H2. The corresponding desorption temperature TD reaches the desired near room temperature ~282 K (Figure 3(e)), indicating remarkable potential for reversible hydrogen storage. Here, the desorption temperature is a theoretical estimate, and the actual desorption temperature will be affected by environmental factors such as pressure. Moreover, due to the lighter atomic mass of C than Si, the storage capacity has also been increased from 9.09 to 10.63 wt% (Figure 3(f)), which is comparable to the strain effect [30]. It should be noted that the physical mechanism of increasing H2 gravimetric density in our work is completely different from the strain effect: our work is to enhance the internal electric field through substrate folding, thereby increasing the H2 adsorption energy, while the strain effect is mainly to increase the adsorption number of H2 molecules by expanding the H2 storage space. When the system continues to adsorb the sixth H2 molecule, it is found that there is no more space to accommodate the sixth H2 molecule. The distance between the sixth H2 molecule and Na atom is 4.435 Å, which belongs to the free state. Therefore, to further improve the storage capacity on this basis, it is necessary to introduce more space for H2 molecules, as suggested by Jiang et al., which is beyond this work.
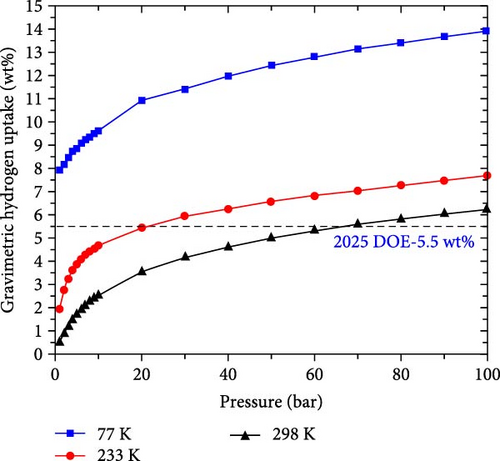
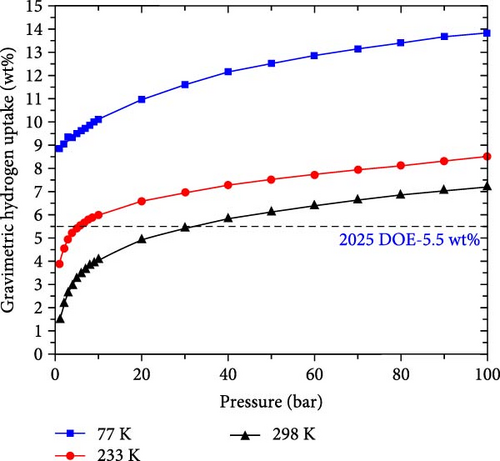
Furthermore, GCMC simulations were performed to simulate the actual conditions of hydrogen storage, mainly considering the influence of temperature and pressure. First, to make the simulation more accurate, the Dreiding force field parameters were fitted to the data calculated by DFT through the Morse equation [44] (Figure 9). The fitted parameters are listed in Table S2, where the rows of data corresponding to Na_1 and Na_2 denote the force field parameters before and after C doping, respectively. Then, based on these data, the isotherms of H2 adsorption on Na-modified SiB and C2Si2B4 at 77, 233, and 298 K are calculated as a function of pressure. As shown in Figure 10, at 77 K and 1 bar, the hydrogen adsorption rates of Na-modified SiB and C2Si2B4 are 7.93 and 8.87 wt%, respectively, both exceeding the target of 2025 DOE (5.5 wt%). With increasing pressure, the hydrogen adsorption rate of both Na-modified SiB and C2Si2B4 can be further improved to ~13.9 wt%. When the temperature is increased to 233 and 298 K, the hydrogen storage performance reaches 5.93 and 5.59 wt% at 30 bar (with 233 K) and 70 bar (with 298 K) for the Na-modified original SiB substrate, respectively. Furthermore, for Na-modified C2Si2B4 substrate, the target of 2025 DOE (5.5 wt%) can be achieved at relatively low pressure (5.55 wt% at 6 bar with 233 K, and 5.80 wt% at 40 bar with 298 K). In addition, for both Na-modified SiB and Na-modified C2Si2B4, the hydrogen adsorption rates can be increased to the desired 6.25 and 7.20 wt% at room temperature and 100 bar. Therefore, both Na-modified SiB and C2Si2B4 exhibit the excellent performance for H2 storage.
4. Conclusions
In summary, the hydrogen storage properties of Na-modified SiB and Na-modified C-doped SiB have been studied using first-principles calculation and GCMC simulations. Due to the large charge transfer between Na and the SiB monolayer, a strong internal electric field is formed, leading to a high binding energy (~−3.0 eV) between Na and SiB substrate, which is very favorable for hydrogen adsorption as well as stability of structural modification. Each Na can adsorb five H2 molecules with an adsorption energy of −0.18 eV/H2. The maximum 20H2 molecules can be adsorbed on the double-sided 4Na-decorated SiB monolayer, corresponding to the hydrogen gravimetric capacity of 9.09 wt%, exceeding the target of 2025 DOE (5.5 wt%). The calculated desorption temperature of H2 on the SiB monolayer is ~227 K. To improve the hydrogen gravimetric capacity and the desorption temperature, the Na-modified C-doped SiB has been investigated. It is found that due to the decrease of lattice symmetry and the uneven distribution of charge density, the substrate is distorted, which strengthens the internal electric field and increases the adsorption energy of H2 (−0.22 eV/H2 for 5H2). In this case, the corresponding gravimetric density as well as resolution temperature are increased to 10.63 wt% and 282 K (near room temperature), respectively. In addition, the GCMC simulation results are in good agreement with the DFT calculation results. All these results suggest that both the Na-modified SiB monolayer and the C-doped SiB monolayer can be treated as promising candidates for high-density hydrogen storage.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ Contributions
Fengyu Miao and Jie Li contributed equally to this work.
Acknowledgments
This work was supported by the Natural Science Foundation of Nanjing University of Posts and Telecommunications (grant nos. NY222167 and NY220005) and the open research fund of Key Laboratory of Quantum Materials and Devices (Southeast University), Ministry of Education.
Open Research
Data Availability
The data supporting the findings of this study are available within the article and its supplementary materials.




