Use of Tocolytic Agents in Preterm Labor: A Cross-Sectional Analysis from a Chinese Real-World Study from 2016 to 2021
Abstract
What Is Known and Objective. Tocolytic agents are used to prolong gestational age and prevent immediate preterm birth (PTB). This study aims to provide an overview of the use of tocolytics among patients with PTB in China through retrospectively analyzing trends in application, influencing factors, and inappropriate prescriptions. Methods. The prescription data of five tocolytic agents from 2016 to 2021 were extracted from the database of the Hospital Prescription Analysis Cooperation Project. Drug consumption was expressed as number of prescriptions, cost of prescriptions, and DDDs (defined daily doses). Pearson correlation analysis was used to examine the association between DDDs and DDC (defined daily cost). The appropriateness of prescriptions was analyzed in terms of drug dosage form, administration, clinical diagnosis, and combined medication. Results. The total number of tocolytic prescriptions and the total cost of tocolytic agents increased by 6.12% and 387.58%, respectively, over the six-year duration of the study. From 2016 to 2021, the ranking of the number of prescriptions and DDDs of tocolytic agents was magnesium sulfate > ritodrine > nifedipine > indomethacin > atosiban. During the study period, the cost of tocolytic agents increased significantly, which was mainly related to the increased costs of magnesium sulfate in 2017 and atosiban in 2018 and 2019. The ranking of DDCs was atosiban > ritodrine > magnesium sulfate > nifedipine = indomethacin from 2016 to 2021. For atosiban, the DDC was negatively correlated with the DDDs. Inappropriate prescription, which accounted for 14.84% of all prescriptions, was mainly manifested in the inappropriate selection of nifedipine dosage form, low frequency of nifedipine and indomethacin, and overdosing of ritodrine. Furthermore, 22.87% of tocolytic prescriptions remained active after 34 weeks of gestation, and 7.24% of the prescriptions authorized the use of combination drugs, with magnesium sulfate and nifedipine being the most commonly prescribed combination. What Is New and Conclusion. Magnesium sulfate, ritodrine, and nifedipine were the top three tocolytic agents. As the inappropriate use of tocolytic agents continues to persist, it is important to intensify efforts to ensure the safety and the appropriateness of maternal medication.
1. What Is Known and Objective
Preterm birth (PTB), which refers to delivery between 20+0 weeks and 36+6 weeks of gestation [1], is one of the important causes of infant complications and even death. In recent years, the incidence of premature delivery has been increasing worldwide. In 2021, the incidence of premature delivery in the United States increased to 10.49% [2]. From 2012 to 2018, the incidence of premature delivery in China increased at an annual rate of 1.3%, and the incidence of premature delivery in 2018 was 6.4% [3]. Most preterm infants are born at 32 to 36 weeks of gestation, about 10% at 28 to 32 weeks of gestation (excluding 32 weeks), and about 5% at less than 28 weeks of gestation [4]. Preterm infant mortality is inversely proportional to birth weight and gestational age, that is, the lower the birth weight or the smaller the gestational age, the higher the mortality rate [5]. There are many risk factors for PTB, such as maternal age, socioeconomic status, underlying diseases, and pregnancy-related complications [6]. Factors that affect the risk of premature death include the prematurity degree [5], congenital abnormalities [7], intrauterine growth restriction [8], and insufficient neonatal care [9]. According to the 2014 Guidelines for the Clinical Diagnosis and Treatment of PTB [10] and the 2016 Supplementary Notice of the ACOG Committee Management of Preterm Labor [11], tocolytic agents are used to prevent immediate PTB before 34 weeks of gestation, which allows for the continued maturation of the fetus and the transport of pregnant women to hospitals to rescue premature infants. The commonly used tocolytics are magnesium sulfate, calcium channel blockers (such as nifedipine), cyclooxygenase (COX) inhibitors (such as indomethacin), β-adrenergic agonists (such as ritodrine), and oxytocin receptor antagonists (such as atosiban). The 2016 ACOG committee guidelines for the management of preterm labor recommend calcium channel blockers, β-adrenergic agonists, and cyclooxygenase (COX) inhibitors as first-line tocolytic treatments. Among them, ritodrine is the only medication authorized by the US Food and Drug Administration (FDA) for the purpose of inhibiting uterine contractions. However, due to serious adverse events caused by ritodrine, the drug’s manufacturer voluntarily withdrew it from the US market [12]. In China, ritodrine is widely used to prevent PTB. Currently, there are no studies showing the use of different types of tocolytic agents in China. In this study, we analyzed tocolytic prescriptions for PTB patients from 86 hospitals across nine Chinese cities from 2016 to 2021, aiming to understand their current use status in China.
2. Materials and Methods
2.1. Data Sources
In this study, all data originated from the Hospital Prescription Analysis Cooperation Project database, administered by the Hospital Pharmacy Professional Committee of the Chinese Pharmaceutical Association. This database encompassed information gathered from nine cities, including Beijing, Shanghai, Guangzhou, Chengdu, Hangzhou, Tianjin, Zhengzhou, Shenyang, and Harbin. From each quarter, a random selection of outpatient prescriptions and inpatient medical orders spanning 10 working days was extracted from the respective hospital information systems and subsequently incorporated into the project’s prescription database. The information of inpatient medical orders from 86 hospitals (Table S1) in nine cities was extracted from this database for a total of 240 working days from 2016 to 2021. The extracted information included region, time, patient code, prescription number, name of the department, reimbursement, source of prescription, drug code, generic name, trade name, drug strength, route of administration, number of prescriptions, number of drugs taken, prescription cost, drug price, drug usage (frequency of medication), single dosage, patient age, and primary diagnosis.
2.2. Inclusion and Exclusion Criteria
The extracted prescription data were cleaned. The inclusion criteria were (1) women aged 18 and above; (2) women at 20 to 36+6 weeks of gestation; (3) women with “premature delivery” or “abortion” or “premature rupture of membranes” or “pregnancy” as the primary diagnosis; and (4) prescriptions with “magnesium sulfate” or “nifedipine” or “indomethacin” or “ritodrine” or “atosiban” as the primary generic drug name. The exclusion criteria were (1) women who terminated the pregnancy (including stillbirth, fetal arrest, spontaneous abortion, medical abortion, and ectopic pregnancy); (2) prescriptions with “magnesium sulfate” as the primary generic drug name and women with “eclampsia” or “hypertension” or “high blood pressure” as the primary diagnosis; (3) prescriptions with “nifedipine” as the primary generic drug name and women with “eclampsia” or “hypertension” or “high blood pressure” as the primary diagnosis; and (4) prescriptions with “indomethacin” as the primary generic drug name and women with “inflammation” or “fever” as the primary diagnosis.
2.3. Analysis of Rationality
Prescriptions with complete information on frequency of usage, single dosage, or total dosage were evaluated according to the drug instructions and off-label uses. Magnesium sulfate is administered as an intravenous drip, and the total dose in 24 hours cannot exceed 30 g. Nifedipine (immediate-release) is given orally at 10–20 mg every 6 hours [10, 13]. Initially, indomethacin is administered as a loading dose, rectally or orally between 50 and 100 mg. Subsequently, a maintenance dose of 25 mg is given every 6 hours [10, 11]. However, its usage is strictly limited to no more than 3 days and should be prescribed before the 32nd week of gestation. In contrast, ritodrine is administered initially as an intravenous drip, followed by oral maintenance. The initial oral dosage of ritodrine is 10 mg every 2 hours for the first 24 hours, and then 10 to 20 mg every 4 to 6 hours, with a total daily dosage not exceeding 120 mg. Atosiban is administered as an intravenous drip, and the total dose in 24 hours cannot exceed 330 mg.
Inappropriate prescriptions include inappropriate selection of drug dosage forms, overdosing, underdosing, low-frequency, and high-frequency. Inappropriate selection of drug dosage forms refers to the situation where nifedipine intended for the treatment of preterm labor should be in its immediate-release form [13]. Using the sustained-release or controlled-release form instead would constitute inappropriate selection of drug dosage forms. Overdosing refers to a dose higher than the recommended dosage, while underdosing refers to a dose lower than the recommended dosage. High-frequency medication refers to a situation where the daily frequency of dosing exceeds the recommended number, whereas low-frequency medication occurs when the daily frequency of dosing is less than the recommended.
All prescription combinations and clinical diagnoses were checked to determine whether the prescribing of medications was appropriate. The combined use of two or more tocolytic agents is considered inappropriate. Prescriptions with gestational weeks exceeding 34 weeks in the “original diagnosis” category are considered to have inappropriate clinical diagnoses.
2.4. Statistical Methods
Data classification and data analysis were performed using Excel 2021 (Microsoft, Redmond, WA, USA) and SPSS 25 (IBM, Armonk, NY, USA) software packages. The defined daily dose (DDD) refers to the average daily dose established for a specific drug to treat its primary indication in adults, and these DDD values are derived from data formulated by the World Health Organization (WHO) [14]. The defined daily dose (DDD) was defined as the consumption of a drug in DDD and calculated as the total dosage of a drug/DDD [15]. The larger the DDDs are, the greater the tendency they have to reflect the clinical selection of the drug. The defined daily cost (DDC) was defined as the average daily expense of a specific drug used by a patient and calculated as the annual sales amount of a drug/DDDs. The larger the DDC, the heavier the economic burden caused to the patient.
2.5. Ethics Statement
This study protocol was approved by the Ethics Committee of the Obstetrics and Gynecology Hospital affiliated with Fudan University (approval number: 2023-01). This was a retrospective study with an informed consent exemption. Patient consent was not needed.
3. Results
3.1. Patient Characteristics
The data of 54,497 tocolytic agents were extracted from the database, and the data of 39,842 tocolytic agents remained after excluding the ineligible data. Data with the same prescription number (6,604) were combined into one prescription, and finally 33,129 prescriptions were included in this study. In brief, 95.75% of patients were aged 18 to 39 years (mostly 30 to 39 years old, followed by 18 to 29 years) (Table 1). More than half of prescriptions were distributed in non-first-tier cities, but the mean number of prescriptions in hospitals in first-tier cities (446 prescriptions/hospital) was higher than that in non-first-tier cities (351 prescriptions/hospital) (Tables 1 and S2).
| Subgroup | 2016 (n = 5182) | 2017 (n = 5272) | 2018 (n = 5230) | 2019 (n = 6241) | 2020 (n = 5705) | 2021 (n = 5499) | Total | Chi-square value | P value |
|---|---|---|---|---|---|---|---|---|---|
| Age group, n (%) | 227.916 | <0.001 | |||||||
| 18–29 years | 2470 (47.66%) | 2299 (43.61%) | 2161 (41.32%) | 2589 (41.48%) | 2106 (36.91%) | 2077 (37.77%) | 13702 (41.36%) | ||
| 30–39 years | 2534 (48.90%) | 2722 (51.63%) | 2781 (53.17%) | 3394 (54.38%) | 3395 (59.51%) | 3194 (58.08%) | 18020 (54.39%) | ||
| 40–49 years | 177 (3.42%) | 246 (4.67%) | 283 (5.41%) | 256 (4.10%) | 203 (3.56%) | 219 (3.98%) | 1384 (4.18%) | ||
| ≥50 years | 1 (0.02%) | 5 (0.09%) | 5 (0.10%) | 2 (0.03%) | 1 (0.02%) | 9 (0.16%) | 23 (0.07%) | ||
| Average age (mean ± SD) | 30.39 ± 4.74 | 30.80 ± 5.06 | 30.97 ± 5.03 | 30.79 ± 4.63 | 31.09 ± 4.56 | 31.02 ± 4.71 | 30.85 ± 4.79 | ||
| Region, n (%) | 241.485 | <0.001 | |||||||
| First-tier cities | |||||||||
| Beijing | 375 (7.24%) | 277 (5.25%) | 329 (6.29%) | 385 (6.17%) | 291 (5.10%) | 325 (5.91%) | 1982 (5.98%) | ||
| Shanghai | 838 (16.17%) | 773 (14.66%) | 552 (10.55%) | 1083 (17.35%) | 750 (13.15%) | 518 (9.42%) | 4514 (13.63%) | ||
| Guangzhou | 1243 (23.99%) | 1277 (24.22%) | 1120 (21.41%) | 1366 (21.89%) | 1222 (21.42%) | 1105 (20.09%) | 7333 (22.13%) | ||
| Total | 2456 (47.39%) | 2327 (44.14%) | 2001 (38.26%) | 2834 (45.41%) | 2263 (39.67%) | 1948 (35.42%) | 13829 (41.74%) | ||
| Non-first-tier cities | |||||||||
| Chengdu | 830 (16.02%) | 748 (14.19%) | 838 (16.02%) | 750 (12.02%) | 612 (10.73%) | 614 (11.17%) | 4392 (13.26%) | ||
| Harbin | 445 (8.59%) | 434 (8.23%) | 450 (8.60%) | 520 (8.33%) | 477 (8.36%) | 527 (9.58%) | 2853 (8.61%) | ||
| Hangzhou | 679 (13.10%) | 699 (13.26%) | 729 (13.94%) | 678 (10.86%) | 832 (14.58%) | 794 (14.44%) | 4411 (13.31%) | ||
| Shenyang | 57 (1.10%) | 49 (0.93%) | 49 (0.94%) | 46 (0.74%) | 143 (2.51%) | 178 (3.24%) | 522 (1.58%) | ||
| Tianjin | 239 (4.61%) | 366 (6.94%) | 465 (8.89%) | 548 (8.78%) | 430 (7.54%) | 449 (8.17%) | 2497 (7.54%) | ||
| Zhengzhou | 476 (9.19%) | 649 (12.31%) | 698 (13.35%) | 865 (13.86%) | 948 (16.62%) | 989 (17.99%) | 4625 (13.96%) | ||
| Total | 2726 (52.61%) | 2945 (55.86%) | 3229 (61.74%) | 3407 (54.59%) | 3442 (60.33%) | 3551 (64.58%) | 19300 (58.26%) | ||
- P values of chi-square tests across subgroups are provided.
3.2. Number of Prescriptions for Tocolytics
The total number of prescriptions for tocolytics was the highest in 2019, with an increase of 20.44% from 2016 to 2019, a slight decrease after 2019, and a decrease of 11.89% from 2019 to 2021 (Table S3). From 2016 to 2021, the number of tocolytic prescriptions was ranked as magnesium sulfate > ritodrine > nifedipine >indomethacin and atosiban (Figure 1). From 2018 to 2019, the number of prescriptions for atosiban underwent a significant surge, exhibiting a substantial growth of 185.71% in 2018 and a remarkable jump of 871.43% in 2019, when compared to 2017 (Figure 1, Table S3).
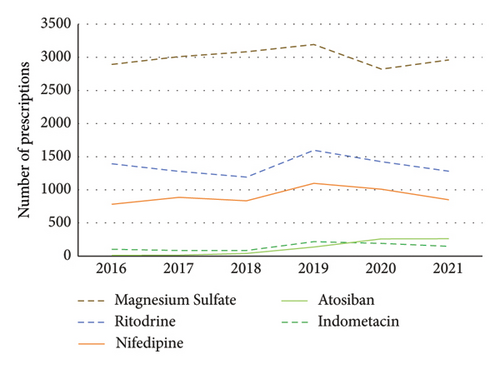
3.3. Prescription Cost of Tocolytics
The prescription costs of nifedipine and indomethacin were lowest from 2016 to 2021. The prescription cost of tocolytic agents was ranked as ritodrine > atosiban > magnesium sulfate in 2016. The number of magnesium sulfate prescriptions increased by 4.08% in 2017, whereas the cost of magnesium sulfate increased by 691.11%. From 2018 to 2019, there was a substantial leap in the prescription cost of atosiban, experiencing a rise of 104.21% in 2018 and a steep jump of 558.23% in 2019 compared to 2017 (Table S4). The prescription cost of tocolytic agents was ranked as atosiban > magnesium sulfate > ritodrine from 2018 to 2021 (Figure 2).
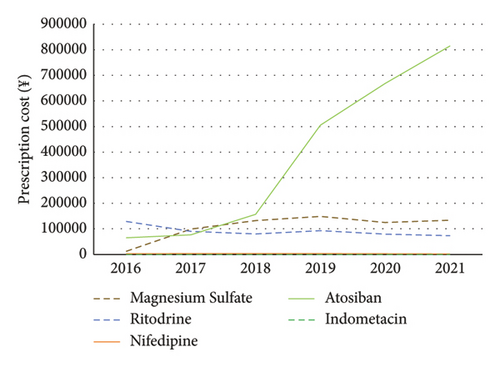
3.4. Daily Defined Doses for Tocolytics
From 2016 to 2021, the ranking of DDDs was magnesium sulfate > ritodrine >nifedipine > indomethacin > atosiban (Figure 3).
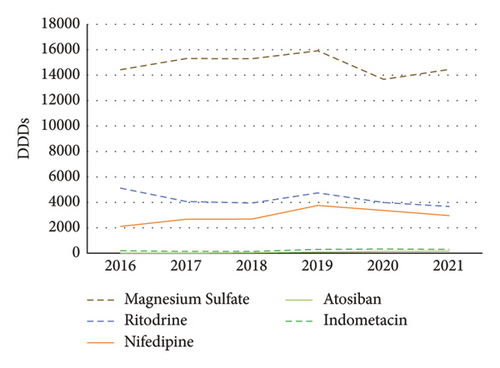
3.5. Daily Defined Cost of Tocolytics
From 2016 to 2021, the ranking of DDC was atosiban > ritodrine > magnesium sulfate > nifedipine = indomethacin (Figure 4).
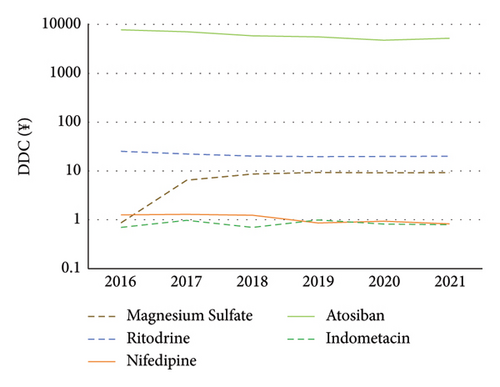
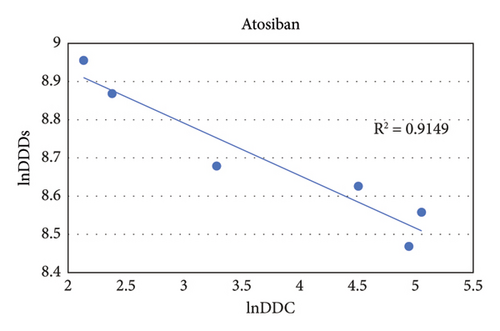
3.6. Relationship between Daily Defined Doses and Daily Defined Cost of Atosiban
From 2016 to 2021, the DDC of atosiban gradually decreased, while the DDDs continued to rise. The natural logarithm (ln) value of DDC for atosiban exhibits a negative correlation with the ln value of DDDs (R2 = 0.9149, P < 0.01) (Figures 5 and S1).
3.7. Appropriate Evaluation of the Use of Tocolytic Agents
3.7.1. Analysis of Drug Dosage Form and Administration
The drug dosage form and administration of 33,129 prescriptions included in this study were analyzed (Table 2). The inappropriate prescriptions of nifedipine were mainly due to the inappropriate selection of the dosage form. Among them, prescriptions using nifedipine controlled-release tablets or nifedipine sustained-release tablets account for 52.32% of the total nifedipine prescriptions. The second issue was “low-frequency” with nifedipine, where the daily dosing frequency is less than four times, such as once, twice, or three times a day, accounting for 29.05% of the total nifedipine prescriptions. The inappropriate prescriptions of indomethacin were mainly characterized by low-frequency, which means that the daily dosing frequency of indomethacin was less than four times, such as once, twice, or three times a day. These prescriptions account for 17.27% of the total indomethacin prescriptions. The inappropriate prescriptions of ritodrine were mainly overdosing, which refers to the single oral dose of ritodrine tablets being 40 mg or 60 mg, accounting for 2.45% of the total ritodrine prescriptions.
| Total number of prescriptions | Dosage and admin instructions missing | Low-frequency | Overdosing | Underdosing | |
|---|---|---|---|---|---|
| Magnesium sulfate | 17956 | 3583 (19.95%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Ritodrine | 8166 | 2968 (36.35%) | 0 (0.00%) | 200 (2.45%) | 0 (0.00%) |
| Nifedipine | 5457 | 681 (12.48%) | 1585 (29.05%) | 101 (1.85%) | 13 (0.24%) |
| Indomethacin | 828 | 339 (40.94%) | 143 (17.27%) | 20 (2.42%) | 0 (0.00%) |
| Atosiban | 722 | 108 (14.96%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
3.7.2. Analysis of Combination
In this study, combination drugs (2400, 7.24%) consisted of two, three, and four tocolytic agents used in combination (Table 3). The most commonly used combined tocolytic agents were magnesium sulfate and nifedipine (3.01%); magnesium sulfate and ritodrine (1.92%); nifedipine and ritodrine (0.83%); ritodrine and indomethacin (0.37%); magnesium sulfate and indomethacin (0.32%); magnesium sulfate and atosiban (0.20%); magnesium sulfate, nifedipine, and ritodrine (0.14%); and atosiban and ritodrine (0.09%).
| Scheme type | Drug combination | Number of prescriptions | Proportion of prescriptions (%) |
|---|---|---|---|
| Combination of two drugs | Magnesium sulfate + indomethacin | 105 | 0.32 |
| Magnesium sulfate + nifedipine | 996 | 3.01 | |
| Magnesium sulfate + atosiban | 65 | 0.20 | |
| Magnesium sulfate + ritodrine | 636 | 1.92 | |
| Indomethacin + nifedipine | 20 | 0.06 | |
| Indomethacin + atosiban | 9 | 0.03 | |
| Indomethacin + ritodrine | 123 | 0.37 | |
| Nifedipine + atosiban | 35 | 0.11 | |
| Nifedipine + ritodrine | 276 | 0.83 | |
| Atosiban + ritodrine | 31 | 0.09 | |
| Combination of three drugs | Magnesium sulfate + indomethacin + nifedipine | 11 | 0.03 |
| Magnesium sulfate + indomethacin + ritodrine | 7 | 0.02 | |
| Magnesium sulfate + nifedipine + atosiban | 5 | 0.02 | |
| Magnesium sulfate + nifedipine + ritodrine | 46 | 0.14 | |
| Magnesium sulfate + atosiban + ritodrine | 6 | 0.02 | |
| Indomethacin + nifedipine + ritodrine | 12 | 0.04 | |
| Indomethacin + atosiban + ritodrine | 5 | 0.02 | |
| Nifedipine + atosiban + ritodrine | 7 | 0.02 | |
| Combination of four drugs | Magnesium sulfate + indomethacin + nifedipine + ritodrine | 2 | 0.01 |
| Magnesium sulfate + atosiban + nifedipine + ritodrine | 3 | 0.01 | |
3.7.3. Analysis of Gestational Week
The gestational age was documented in 7,770 of 33,129 prescriptions, including 1,777 prescriptions at 34 to 36+6 weeks of gestation, accounting for 22.87% of prescriptions (Table 4). In brief, 12.92% of prescriptions for tocolytic agents were prescribed for more than 34 weeks of pregnancy in first-tier cities and 34.59% in non-first-tier cities, and the proportion in non-first-tier cities was higher than that in first-tier cities.
| Gestational week | First-tier cities, n (%) | Non-first-tier cities, n (%) | Total, n (%) |
|---|---|---|---|
| 20–27+6 | 1573 (37.43) | 644 (18.05) | 2217 (28.53) |
| 28–33+6 | 2086 (49.64) | 1690 (47.37) | 3776 (48.60) |
| 34–36+6 | 543 (12.92) | 1234 (34.59) | 1777 (22.87) |
| Total | 4202 (100.00) | 3568 (100.00) | 7770 (100.00) |
4. Discussion
Because the mortality rate of preterm infants is closely related to the gestational age at birth, the lower the gestational age at birth, the higher the mortality rate [5, 16, 17]. In light of this information, it has been reported that tocolytic agents, including magnesium sulfate, indomethacin, nifedipine, ritodrine, and atosiban, can delay delivery for 48 hours more effectively than placebo/no tocolytic agent, and the difference is statistically significant [18, 19]. In this study, we investigated real-world drug treatment patterns of Chinese patients with risk of PTB by performing big data analysis of tocolytic prescriptions. The data covered the major regions of China, mainly from tertiary-level hospitals, and represented as well as demonstrated the use of tocolytic agents in China.
4.1. Demographic Characteristics of Patients
Studies have shown that the relationship between the incidence of PTB and maternal age follows a “U-shaped” pattern, with a higher occurrence rate of PTB in younger and older pregnant women [20–22], the lowest occurrence rate of PTB in pregnant women aged 30–34 years (5.7%), and the highest occurrence rate of PTB in pregnant women aged 40 years or older (7.8%) [21]. In China, there is an increasing trend in the proportion of older maternal age (≥35 years), rising from 10.6% in 2016 to 16.7% in 2017 and 15.9% in 2018 [3]. In the United States, there has been a steady increase in pregnant women aged 45 years or older, rising from 0.19% in 2009 to 0.26% in 2020 [23]. The likelihood of PTB among pregnant women aged 45 years or older is significantly higher compared to those under 45 years (16.5% vs 8.3%) [23]. In summary, the annual increase in the proportion of older pregnant women is accompanied by a growing potential for preterm delivery. Therefore, it is inevitable that the proportion of older pregnant women experiencing PTB will also increase. This is also confirmed by the findings of this study, which show an increasing trend in the use of tocolytic therapy among older pregnant women (for example, those aged 50 or above) (Table 1). In this study, the average age of PTB patients was 30.85 years, with 77.66% of patients aged between 20 and 34 years old (Table S2). This may be due to the larger number of pregnant women between 20 and 34 years old, so even if the incidence of PTB is lower in this age group, there will still be a large number of patients who experience PTB. Systematic reviews have reported that PTB is related to environmental factors, such as fine particulate matter, ozone, and high temperature [24–26]. Exposure to ozone and high fine particulate matter levels may have significant impacts on the cardiovascular system through oxidative stress and inflammatory responses, leading to endothelial dysfunction and arterial vasoconstriction, which in turn increases placental vascular resistance, leading to an increased risk of PTB [27]. The relationship between temperature and PTB is U-shaped, with both high and extremely low temperatures increasing the risk of PTB [28]. The impact of low temperatures is primarily explained by changes in hemodynamics, while the effects of high temperatures are mainly attributed to alterations in immunity and inflammation. Low temperatures are associated with peripheral vasoconstriction during pregnancy, which may alter uteroplacental perfusion and have adverse effects on the developing fetus [28]. Exposure to excessive heat can trigger the secretion of cytokines, including prostaglandins, oxytocin, and heat shock proteins. This can potentially upset the intricate cytokine equilibrium at the interface between mother and fetus, ultimately precipitating the activation of the delivery mechanism prematurely [28]. In this study, the average number of prescriptions for PTB patients in first-tier cities was higher than that in second-tier cities, which may be associated with the different environmental characteristics of the cities [29]. Another study has demonstrated that the rate of intrauterine transfer of preterm infants differs greatly across different geographical regions, and the intrauterine transfer of preterm infants to well-equipped medical institutions can significantly improve survival outcomes [30]. Therefore, it cannot be excluded that PTB patients in second-tier cities were transferred to medical institutions in first-tier cities.
4.2. Pharmacoeconomic Evaluation
DDC can guide the market price of drugs, which is an important determinant of drug consumption [31]. In order to improve the affordability of medicines, the Chinese government has implemented various measures, including establishing a basic medical insurance system, conducting price negotiations, and executing centralized drug procurement policies, to reduce drug prices. Previous studies have shown a negative correlation between DDC and DDDs during the process of price reduction for drugs [15, 32, 33]. Although atosiban in this study is the safest tocolytic agent approved so far, its sales are very low due to its far higher price than other tocolytic agents. The DDC of atosiban decreased significantly in 2018, which may be related to the donation of atosiban by social welfare projects and the market entry of domestic generic drugs. From January 2018 to December 2019, the Beijing Renze Foundation launched the “Yibao Patient Assistance Program,” which provided atosiban injections free-of-charge to financially challenged women who were experiencing a threatened PTB. In March 2018, generic atosiban was approved for marketing in China. Compared with patent-protected drugs, the prices of domestic generic drugs are significantly lower. In 2019, the atosiban injectable was listed in the National Medical Insurance Drug List (implemented on January 1, 2020), and the DDC of atosiban further decreased in 2020. During the period from 2018 to 2020, social welfare funds, generic drugs, and the national medical insurance policy contributed to a notable decline in the cost of atosiban. Consequently, the percentage of prescriptions rose from 0.21% in 2016 to 4.76% in 2021. As expected, the DDC and DDDs of atosiban in this study were negatively correlated.
4.3. Drug Characteristics
4.3.1. Analysis and Evaluation of Tocolytic Agents
The utilization of magnesium sulfate, as a tocolytic agent, is controversial due to conflicting research results in preterm women. Some studies have shown that magnesium sulfate fails to decrease the birth rate within 48 hours or the incidence of PTB within 7 days compared to placebo/no treatment [34, 35]. However, a recent study has shown that, compared to placebo/no treatment, magnesium sulfate can reduce the risk of PTB within 48 hours [36]. Whether it is effective in reducing the risk of PTB within 7 days and improving neonatal outcomes still requires further research [36]. The ACOG obstetric practices and maternal-fetal medical considerations suggest that magnesium sulfate may be used for short-term extension of pregnancy (up to 48 hours) [37]. The 2019 Society of Obstetricians and Gynecologists of Canada clinical practice guidelines recommend considering antenatal use of magnesium sulfate for fetal neuroprotection in women who are about to give birth prematurely (≤33+6 weeks of gestation) [38]. This study found that the number of prescriptions and DDDs of magnesium sulfate were the highest among this population, suggesting that magnesium sulfate is the most commonly prescribed tocolytic agent in clinical settings and also functions as a means for fetal neuroprotection.
A meta-analysis comparing the efficacy, safety, and adverse events of tocolytic agents concluded that nifedipine is the preferred choice in the absence of contraindications within 48 hours of onset [19]. In this meta-analysis, the efficacy and safety of ritodrine was lower than that of nifedipine [19], and ritodrine was most likely to cause adverse events [39]. Another network meta-analysis revealed that the most effective tocolytic agents for delaying PTB by 48 hours and/or 7 days were calcium channel blockers (such as nifedipine), oxytocin receptor antagonists (such as atosiban), and combination tocolytics (such as nifedipine combined with magnesium sulfate) [36]. Both meta-analyses showed that nifedipine was superior to ritodrine in efficacy and had a lower incidence of adverse reactions. At the same time, PTB-related guidelines in many countries tend to choose nifedipine or atosiban as the preferred first-line treatment options [40], while ritodrine has been withdrawn from the market in many countries due to its severe adverse reactions. In China, our study revealed that the number of prescriptions and the frequency of ritodrine use in the real-world clinical settings were higher than those of nifedipine. There are likely two reasons for this observation. Firstly, the Chinese guidelines for PTB recommend ritodrine as one of the first-line medications for inhibiting uterine contractions during preterm labor. Secondly, the medication instructions for ritodrine explicitly state that its approved indication is the prevention of PTB after 20 weeks of gestation. Conversely, the medication instructions for nifedipine lack such a specific indication. Consequently, the administration of nifedipine for the prevention of PTB constitutes an off-label use. In addition, a survey conducted among obstetricians indicated that in clinical settings, ritodrine tops the list as the preferred tocolytic agent, followed closely by nifedipine as the second-best option [41]. Therefore, we speculate that ritodrine is prescribed more often than nifedipine, which may also be related to the preference of obstetricians for ritodrine.
A study has shown that COX inhibitors (such as indomethacin) are comparable to β-agonists, although they are less effective than other tocolytic agents in delaying delivery for 48 hours and/or 7 days [36]. Another study based on a small sample concluded that COX inhibitors may be superior to β-agonists and comparable to magnesium sulfate and calcium channel blockers in delaying delivery for 48 hours [42]. The common features of these two studies are the relatively small number of included trials related to COX inhibitors and the very low quality of evidence. Our study found that indomethacin had the lowest average daily cost in the real world, but its prescription number and DDDs were much lower than those of magnesium sulfate, ritodrine, and nifedipine, indicating that clinicians rarely prescribe indomethacin for PTB patients. This may be due to the lack of high-quality and large-sample data showing the efficacy and safety of indomethacin for PTB. Furthermore, indomethacin has the capacity to readily traverse the maternal-placental barrier, and its administration during late pregnancy has been associated with a heightened risk of untimely closure of the ductus arteriosus and a condition known as oligohydramnios, which involves a decrease in amniotic fluid volume. Therefore, it is only prescribed before 32 weeks of gestation, and the World Health Organization (WHO) Guideline Development Group considers restricting its use for PTB before 28 weeks of gestation [13].
A meta-analysis has reported that atosiban can delay PTB within 48 hours and/or 7 days [36]. The study included a relatively large number of trials on atosiban, and the quality of evidence was moderate. Compared with other tocolytic agents, the probability of halting treatment due to adverse events is the lowest with atosiban [36]. Furthermore, the product label for atosiban clearly indicates its use for delaying PTB, but its price is much higher than that of other tocolytic agents, resulting in low prescription number and DDDs.
4.3.2. Inappropriate Prescriptions
(1) Inappropriate Dosage Form and Administration. In terms of inappropriate dosage form, the World Health Organization (WHO) clearly recommends the immediate-release nifedipine for the suppression of uterine contractions in preterm labor [13]. This is because the immediate-release tablets were typically used in clinical trials where nifedipine is employed as a tocolytic agent, facilitating the monitoring of blood pressure and cardiotocography [43, 44]. In terms of administration, both nifedipine and indomethacin were characterized by low-frequency, whereas ritodrine was characterized by overdosing. Theoretically, these problems can be avoided by using a preprescription review system [45]. Therefore, to reduce inappropriate prescriptions, pharmacists should routinely update the administration in the system.
(2) Inappropriate Combination of Medications. The 2014 Clinical Diagnosis and Treatment Guidelines for Premature Birth [10], the European Association of Perinatal Medicine (EAPM) [46], ACOG [1, 11], and WHO [13] do not recommend combining tocolytic agents. Among them, the Clinical Diagnosis and Treatment Guidelines for Premature Birth and the EAPM contend that the combination of two or more tocolytic agents may increase the incidence of adverse events, so their use should be avoided as much as possible [10, 46]. The ACOG believes that, due to potential serious maternal complications, β-adrenergic agonists (such as ritodrine) and calcium channel blockers (such as nifedipine) should be used cautiously in combination with magnesium sulfate. However, indomethacin can be combined with magnesium sulfate before 32 weeks of gestation [11]. The WHO believes that combination therapy does not have more benefits than monotherapy, so it only recommends monotherapy [13]. The 2019 Society of Obstetricians and Gynecologists of Canada guidelines state that if nifedipine is used for PTB or hypertension, there is no contraindication for magnesium sulfate in fetal neuroprotection [38]. A recent meta-analysis found that the combination of tocolytic agents, including magnesium sulfate with ritodrine (most common), nifedipine with ritodrine, indomethacin with ritodrine, and nifedipine with atosiban, was effective in delaying PTB but was more likely to result in discontinuation of treatment due to adverse events [36].
In this study, two-drug combination regimens accounted for 96.33% of all combination prescriptions, of which magnesium sulfate combined with nifedipine accounted for 59.99%, magnesium sulfate combined with ritodrine accounted for 17.83%, and magnesium sulfate combined with indomethacin accounted for 2.88%. Three-drug combination regimens accounted for 3.53%, and four-drug combination regimens accounted for 0.14%.
(3) Inappropriate Clinical Diagnosis. A previous study has reported that the use of tocolytic agents after 34 weeks of gestation can significantly prolong the gestational period, but although there is a trend toward improvement in the completion of steroid use and neonatal composite outcome, there is no statistical difference in these parameters [47]. The EAPM [46], ACOG [11], and WHO [13] have set 34+0 weeks of gestation as the upper gestational age for the use of tocolytic agents because the incidence and mortality of perinatal complications after this gestational age are low enough to not cause maternal and fetal complications and to incur related costs to suppress premature labor and to delay delivery in the short term [11]. Our study found that the proportion of prescriptions for tocolytic agents in pregnancy after 34 weeks was 22.87%, and the proportion of prescriptions in non-first-tier cities was higher than that in first-tier cities. It could potentially be linked to the fact that there are fewer hospitals with neonatal intensive care units in non-first-tier cities than in first-tier cities, and for pregnant women who must be transferred to hospitals in order to rescue preterm infants, contraction inhibitors are used to prolong the pregnancy and to reduce the possibility of premature infants needing urgent care.
5. What Is New and Conclusion
This study found that magnesium sulfate is the main type of medication prescribed for PTB patients, followed by nifedipine and ritodrine. Drug donation programs from social welfare organizations, domestically produced generic drugs, and national medical insurance systems substantially alleviate the financial burden on patients. Due to inappropriate dosage form, administration, combined medication, and lack of consideration for gestational age, more efforts are needed to ensure the appropriateness of prescriptions.
6. Limitations
Despite the large amount of information included in this study, there are some limitations. First, the study randomly collected data for only 10 working days every quarter, resulting in a small number of atosiban prescriptions, which did not reflect the actual use of atosiban. Second, due to the incomplete clinical diagnosis of some women in this study, after excluding prescriptions that failed to document the week of gestation (76.55% of all prescriptions did not record the week of gestation), the rational rate of prescriptions may be inconsistent with the real-world rate of prescriptions when we judged whether the gestational age of using tocolytic agents was reasonable. Third, due to the lack of usage and dosage information of some data in this study, the rationality evaluation may not be consistent with the real-world situation. Therefore, further efforts are needed to standardize the quality of patient medical data and to carry out a more comprehensive analysis of the rationality of medication use in PTB patients.
Abbreviations
-
- DDDs:
-
- Defined daily doses
-
- DDC:
-
- Defined daily cost
-
- COX:
-
- Cyclooxygenase
-
- ACOG:
-
- American College of Obstetricians and Gynecologists
-
- WHO:
-
- World Health Organization
-
- PTB:
-
- Preterm birth.
Ethical Approval
This study protocol was approved by the Ethics Committee of the Obstetrics and Gynecology Hospital affiliated with Fudan University (approval number: 2023-01, date: 2023-1-26).
Consent
This was a retrospective study with an informed consent exemption. Patient consent was not needed.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors’ Contributions
Haoran Liu conceived and designed the study, analyzed the data, and wrote and revised the manuscript. Xianli Wang conceived and designed the study, provided guidance for this study, critically revised the paper, and approved the final version to be published.
Acknowledgments
This work was financially supported by Pharmaceutical Evaluation Professional Committee of China Research Hospital Association (grant no. Y2022FH-YWPJ01-206), Shanghai Hospital Association (grant no. X2022119), Shanghai Municipal Health Commission (grant no. 202140065), Shanghai Clinical Research Center for Gynecological Diseases (grant no. 22MC1940200), and Shanghai Urogenital System Diseases Research Center (grant no. 2022ZZ01012).
Open Research
Data Availability
The data are available from the corresponding author on reasonable request.




