Enhanced Expression and Bioactivity Assessment of Recombinant SUMO-Protease-1 in E. coli BL21 (DE3) via Cleavage of His6-SMT3-SDF-1 Fusion Protein
Abstract
Background. The SUMO fusion system is advantageous in improving the solubility and correct folding of proteins that are difficult to produce. SUMO-protease-1 (Ulp1) is a key enzyme in this system, and its proper expression and purification in Escherichia coli BL21 (DE3) are critical to its efficiency. Objective. To optimize Ulp1 expression and purification using His6-SUMO-SDF-1 as a model, aiming to improve efficiency across similar fusion proteins. Methods. We provided a codon-optimized Ulp1 gene into the pET28a vector. Essential culture factors, including IPTG concentration, incubation length of time, and temperature, were precisely adjusted. Ulp1 was purified using Ni-NTA affinity chromatography. A cleavage activity assay of recombinant Ulp1 is carried out using the His6-SUMO-SDF-1 fusion protein to demonstrate how well the optimized settings work. Furthermore, the activity of recombinant SDF-1 was evaluated using the scratch wound healing assay. Results. Ulp1 expression was optimized in E. coli using the pET28a vector, with optimum conditions including 0.1 mM IPTG, 28°C incubation temperature, and an overnight period of time. Recombinant Ulp1 was purified using Ni-NTA affinity chromatography, resulting in high purity. Enzymatic activity was shown by the effective cleavage of the His6-SUMO-SDF-1 fusion protein. In the scratch wound-healing assay, recombinant SDF-1 increased cell migration following cleavage, confirming that biological activity was retained. These findings illustrate Ulp1’s efficient synthesis and effectiveness for prospective applications in biology. Conclusion. This study successfully showed the expression and purification of the recombinant Ulp1 enzyme, showing the most suitable growing conditions for productive protein production. Ulp1’s functionality was validated by its specific cleavage of the His6-SUMO-SDF-1 fusion protein, as well as the fragmented SDF-1 purified and its biological activity in promoting cell migration. These findings highlight Ulp1’s potential in protein processing as well as its application in biotechnological and biological research.
1. Introduction
SUMO proteases regulate various biological activities, including protein deSUMOylation, cell function, stability, and gene transcription factors, impacting cellular antioxidant capabilities [1–3]. They are crucial in protein dynamics during chromosomal segregation and cell division [4–6]. The use of SUMO proteases, particularly Ulp-1, in the SUMO fusion system has proven effective for expressing difficult proteins [7].
The SUMO fusion system significantly improves the expression, stability, and purification of recombinant proteins [8–10]. By utilizing SUMO as a fusion partner, this method enhances the solubility and yield of a variety of recombinant proteins, protecting peptides from degradation during expression in hosts like E. coli [11–14]. The His6-SMT3-fusion protein method offers notable advantages for therapeutic applications, especially in treating infectious diseases. This method ensures the natural production of proteins, minimizing side effects and allergic reactions [15, 16]. Optimal production of the Ulp1 enzyme, a specific protease that cleaves the bond between the SUMO tag and the target protein, is essential for this process. Ulp1’s specificity ensures the target protein is released without unwanted sequences, maintaining its natural structure and function.
Using the His6-SMT3-fusion protein method enhances the solubility of naturally insoluble target proteins, facilitates their production and purification, and improves protein stability, leading to higher yields [15]. The His6 tag allows straightforward purification using affinity chromatography, resulting in higher purity of the final product. Post-purification, Ulp1 enzyme provides precise cleavage, crucial for maintaining the therapeutic proteins′ natural structure and function. Optimal production of the Ulp1 enzyme in the E. coli BL21 (DE3) system is vital due to this strain’s rapid growth, well-understood genetics, and significant recombinant protein expression capabilities. Implementing the His6-SMT3-fusion protein method in this system can significantly advance the production of therapeutic recombinant proteins, improving the efficiency and cost-effectiveness of manufacturing high-quality proteins and holding promise for developing more effective treatments for infectious diseases.
Stromal cell-derived factor 1 (SDF-1), also known as CXCL12, is a chemokine with a pivotal role in treating infectious diseases due to its multiple therapeutic functions [17, 18]. SDF-1 is a potent chemoattractant, recruiting T cells, B cells, and monocytes to infection sites, thereby enhancing the immune response [19]. It also mobilizes hematopoietic stem cells from the bone marrow to the peripheral blood, aiding in immune cell regeneration and recovery from infections [17, 20]. In addition, SDF-1 promotes tissue repair and regeneration, crucial for healing tissue damage caused by infections, and has potential direct antimicrobial effects against certain pathogens [18, 21]. Furthermore, SDF-1 helps modulate and reduce excessive inflammation, minimizing tissue damage and improving patient outcomes [22]. Using the His6-SMT3-fusion protein method for producing recombinant SDF-1 ensures high solubility, stability, and purity, which are essential for its therapeutic efficacy. This method allows the production of SDF-1 in its natural form without unwanted sequences, maintaining its functional integrity. Therefore, the His6-SMT3-fusion protein system is justified as an effective approach for generating recombinant SDF-1 for therapeutic applications in infectious diseases.
This study focuses on enhancing Ulp1 production and purification in E. coli BL21 (DE3), assessing the cleavage efficiency of the His6-SUMO (His6-SMT3)-SDF-1 fusion protein. The model fusion system used in this study is a critical step toward restoring the native structure and function of proteins relevant to infectious diseases. The biological activity of SDF-1 postcleavage demonstrates Ulp1’s functional efficacy in protein processing.
The primary goal of this study is to optimize the production and purification of recombinant SUMO protease in E. coli BL21 (DE3), advancing the SUMO fusion system for therapeutically relevant proteins. This work provides opportunities for future studies to enhance this system, potentially leading to more effective and economical techniques for manufacturing superior quality therapeutic proteins.
2. Materials and Methods
2.1. Materials
The following materials were used in the extraction, purification, and refolding of His-tagged recombinant Ulp1. Affinity chromatography was performed by gravity flow purification of His-tagged proteins under native conditions using Ni-NTA (nickel-nitrilotriacetic acid) resin (Qiagen). Ion exchange chromatography was performed using a gravity column packed with ArgPure SP Sepharose Fast Flow resin (ARG Biotech Co., Iran). Bovine serum albumin (BSA) was used as a protein standard, and all solvents were of analytical grade and purchased from Sigma-Aldrich. Specifically, the materials included NaH2PO4, NaCl, imidazole, Tris-HCl, L-arginine, glycerol, EDTA (all from Sigma-Aldrich), and IPTG (isopropyl-beta-D-thiogalactopyranoside) from CinnaGen Co., Iran. Human dermal fibroblasts (NCBI C646) were obtained from the National Cell Bank of Iran (Pasteur Institute, Iran).
2.2. Bacterial Strain and Plasmid
To optimize Ulp1 production in E. coli, a codon-optimized DNA sequence for recombinant SUMO protease expression in E. coli BL21 (DE3) was constructed, which included an amino-terminal 6xHis tag and a stop codon for effective purification and detection. This fragment was produced by General Biol, China, using the pET28a expression plasmid. The E. coli BL21 (DE3) strain was selected as the host for transformation with the recombinant pET28a plasmid. This strain is well known for its ability to produce recombinant proteins, especially those that are difficult to express [23–25]. The thoughtfully designed and synthesized construct, combined with the selection of a suitable E. coli strain, served as the foundation for our following expression efforts, which intended to optimize the synthesis of soluble and active recombinant Ulp1 for broader applications in protein biochemistry.
2.3. Transforming pET28a-Ulp1 into Competent E. coli BL21 (DE3)
E. coli BL21 (DE3) cells were transformed with pET28a-Ulp1 using heat shock. A single positive colony was grown in 10 mL of LB broth with 50 μg/mL kanamycin at 37°C and 180 rpm overnight. This preculture was then grown in 200 mL of LB medium at a 1 : 100 ratio and incubated under the same conditions until the absorption of optical density (OD 600) reached 0.6. For optimal expression settings, temperatures of 16°C and 28°C were examined, as well as incubation times of 4 hours and overnight. IPTG concentrations of 0.05, 0.1, and 0.5 mM were tested to find the most efficacious concentration. Following induction, cells were collected by centrifugation at 7000 × g for 8 min and kept at −20°C for future study.
2.4. Extraction, Purification, and Refolding of His-Tagged Recombinant Ulp1
A series of steps were used to extract the Ulp1 protein. Initially, cell pellets held at −20°C were frozen-thawed and then lysed with a lysis buffer and a specified quantity of lysozyme (5 mg per gram of pellet). Sonication was then performed with an ultrasonic probe set to 40% amplitude for 30 s at 30-s intervals, followed by five cycles. The cell lysates were then centrifuged at 8000 × g for 20 min at 4°C, and the supernatants were carefully transferred into fresh tubes and immediately stored on ice for further purification.
Three buffers were prepared to purify His-tagged Ulp1 under native conditions: binding buffer (50 mM NaH2PO4, 350 mM NaCl, and a concentration of 20 mM imidazole, pH 8), wash buffer (50 mM NaH2PO4, 350 mM NaCl, and 10–30 mM imidazole, pH 8), and elution buffer (50 mM NaH2PO4, 350 mM NaCl, and 200 mM imidazole, pH 8). The Ni-NTA column was equilibrated with binding buffer before the supernatant was loaded onto it. After the cell extract had entered the column completely, it was washed five times with wash buffer before the His-tag fusion protein was eluted in four fractions of one milliliter of elution buffer.
To restore the native structure and function of the protein, purified proteins were refolded by gradually dissolving them in a refolding buffer (50 mM Tris-HCL, 500 mM L-arginine, 10% glycerol, and 2 mM EDTA, pH 8).
2.5. Production of the Recombinant His6-SMT3-SDF-1 System
Similar to Ulp1, the His6-SMT3-SDF-1 gene was codon optimized for expression in E. coli and synthesized. A 6xHis tag was inserted at the N-terminal end for purification, followed by a stop codon. To enhance protein expression, the synthesized gene was cloned into the pET28a vector (General Biol., China).
E. coli BL21 (DE3) cells were transformed using a recombinant plasmid encoding the His6-SMT3-SDF-1 gene. The transformed cells were grown under optimal conditions for protein expression (200 mL of medium, 28°C, and 1 mM IPTG for 4 h). The recombinant His6-SMT3-SDF-1 protein was isolated with His-tag affinity chromatography. The stages included cell lysis (with lysis buffer and sonication), binding of the His-tagged protein to a Ni-NTA column, washing to remove nonspecifically bound proteins, and elution of the His6-SMT3-SDF-1 protein. The purity and integrity of recombinant proteins were verified using SDS-PAGE.
2.6. Cleavage Assay for Recombinant Ulp1 Activity
To assess the protease activity of recombinant Ulp1, a cleavage assay was performed with pure His6-SMT3-SDF-1 as a substrate. Ulp1 was incubated with His6-SMT3-SDF-1 under ideal reaction conditions to assess the enzyme’s efficiency and specificity. SDS-PAGE was used to separate and visualize cleaved and uncleaved proteins in the reaction mixtures. Control reactions without Ulp1 were included to ensure that the observed cleavage was exclusively due to Ulp1’s enzymatic activity.
2.7. SDF-1 Purification by Cation Exchange Chromatography
The SDF-1 was separated from the His6-SMT3 segment using cation exchange chromatography. This chromatographic approach is very effective because most chemokines, including SDF-1, have a positive charge. Under basic buffer conditions, the positively charged chemokine binds to the negatively charged cation exchange resin, whereas the negatively charged and slightly acidic His6-SMT3 (theoretical pI 5.7) does not.
2.8. The Biological Activity of Purified SDF-1
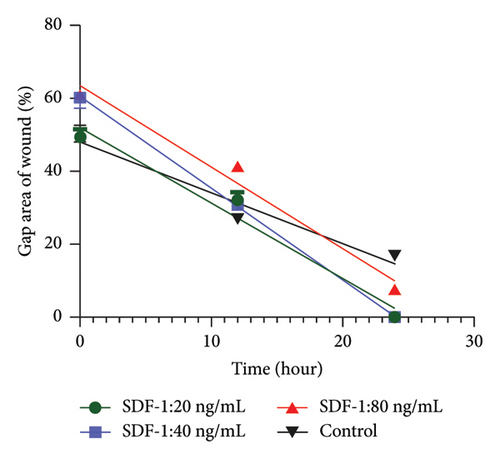
The biological activity of pure SDF-1 on human dermal fibroblast (HDF) cell migration was assessed using a scratch wound healing assay, which is a common approach for evaluating cell migration and proliferation in wound healing. SDF-1’s impact was studied at three concentrations (20, 40, and 80 ng/mL). A control group that received no SDF-1 treatment was included for baseline comparison. Photographs of each scratch were taken immediately after the scratch, as well as 12 and 24 h later, to provide critical visual data for assessing cell migration rates and extents at various SDF-1 concentrations. The images were analyzed with ImageJ2 software to allow for a thorough investigation of how SDF-1 impacts HDF cell migration, revealing light on its potential in skin wound healing and regeneration.
3. Results
3.1. Optimized Expression Conditions for Ulp1
In order to optimize the expression conditions for Ulp1 in E. coli BL21 (DE3) cells, we examined the effects of different incubation times, temperatures, and IPTG concentrations. The findings demonstrated that the most significant expression of Ulp1 occurred under certain conditions, i.e., cells were induced with 0.1 mM IPTG and incubated at 28°C overnight. This particular condition beat others in terms of protein yield, which was an important finding in our study.
We discovered that temperature has a significant impact on the expression of Ulp1 and that an incubation temperature of 28°C was optimal for increasing protein expression. This observation was supported by the distinct bands shown on the SDS-PAGE gels, which were most visible under these circumstances. Short-term expression (after 4 h) of Ulp1 was shown to be high at 28°C across multiple IPTG doses, particularly at 0.05 mM, 0.1 mM, and 1 mM. However, with 0.5 and 1 mM IPTG, this rate decreased, indicating a complex connection between IPTG concentration and expression efficiency. In contrast, prolonged incubation (overnight) at 16°C resulted in high expression rates at all IPTG doses, with the exception of 0.5 mM, which was moderate. This suggested that longer incubation could improve efficiency at lower temperatures. Most notably, overnight incubation at 28°C with 0.1 mM IPTG resulted in very high expression rates, indicating that temperature and IPTG concentration are ideal for Ulp1 expression (Figure 1). These conditions were considered the gold standard in our approach, providing a dependable and efficient way for Ulp1 synthesis. This approach improves our understanding of protein expression dynamics and lays the path for future biochemical and structural investigations of Ulp1, which could lead to improvements in this field of study.
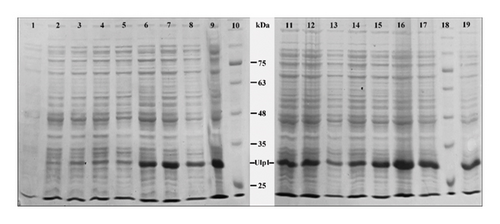
3.2. Efficient Purification of Ulp1 and His6-SMT3-SDF-1
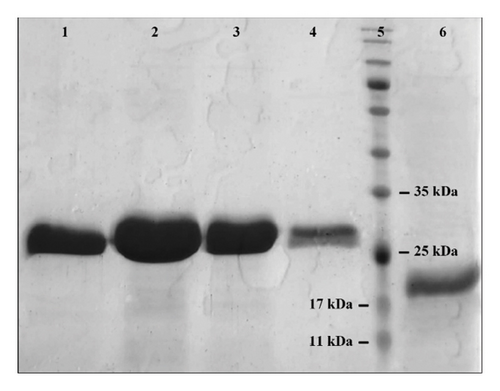
The recombinant Ulp1 and His6-SMT3-SDF-1 proteins were successfully purified using Ni-NTA affinity chromatography under native conditions. The His6-SMT3-SDF-1 gene was optimized for production by E. coli BL21 and cloned into a pET28a vector, which included a 6xHis tag for purification. E. coli BL21 (DE3) cells efficiently produced the proteins when cultured under optimal conditions (200 mL medium at 28°C, induced with 0.1 mM IPTG overnight). The purification process included cell lysis, binding of His-tagged proteins to a Ni-NTA column, washing to remove unattached proteins, and eluting the target proteins. SDS-PAGE evaluation verified the purification process, with distinct bands indicating high purity and integrity (Figure 2). This optimized approach demonstrates the efficacy of affinity chromatography in purifying recombinant proteins, paving the way for future functional investigations. Under optimized expression conditions, the average yield of purified Ulp1 from E. coli BL21 (DE3) cultures was determined to be approximately 40 mg of Ulp1 per liter of culture. This represents a doubling of yield compared to previously reported expressions in similar studies [26].
3.3. His6-SMT3-SDF-1 Cleavage Confirms Ulp1 Proteolytic Activity
The optimization of Ulp1 enzyme activity and the determination of enzyme activity time were performed to ensure the complete digestion of the fusion protein. However, the specific data related to this optimization have not been provided. The findings of the protease activity assay using recombinant Ulp1 showed that the His6-SMT3-SDF-1 substrate was successfully cleaved. After incubating Ulp1 at 0.5 mg/mL for three hours under optimum conditions (pH 8.0, 30°C), deactivate the enzyme by heating it to 55°C for 15 minutes. SDS-PAGE analysis revealed separate bands corresponding to the cleavage products (Figure 3). This was opposed to the control experiment, which lacked Ulp1 and showed no such cleavage, indicating that the exhibited substrate cleavage was triggered by Ulp1’s enzymatic activity. The theoretical molecular weight of SUMO tag is approximately 11 kDa. However, their higher apparent molecular weight of 10–20 kDa observed on SDS-PAGE can be attributed to the unique properties of SUMO proteins. These properties likely affect their electrophoretic mobility, causing them to migrate more slowly than expected based on their actual size [27, 28]. The theoretical molecular weight of SDF-1 is approximately 8 kDa. The result of this experiment confirms Ulp1’s effective proteolytic function under specific conditions.
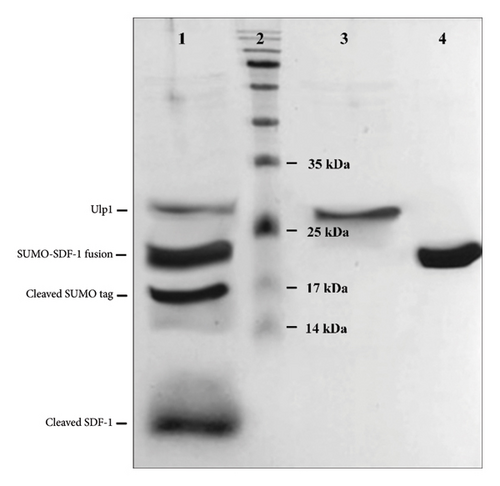
The yield of the cleaved product, after purification of the his6-SMT3-SDF-1 fusion protein and subsequent cleavage by Ulp1, was approximately 8 mg/L. These yields are indicative of the effectiveness of our expression and purification strategy, which includes the use of IPTG inducers and low-temperature growth conditions.
The cleavage efficiency of purified Ulp1 was rigorously assessed through SDS-PAGE analysis combined with densitometry to quantify the relative amounts of substrate before and after cleavage. The Ulp1 displayed a cleavage efficiency of approximately 95% under optimized conditions. This high efficiency underscores the robustness of our expression and purification protocol.
3.4. The Biological Activity of Purified SDF-1
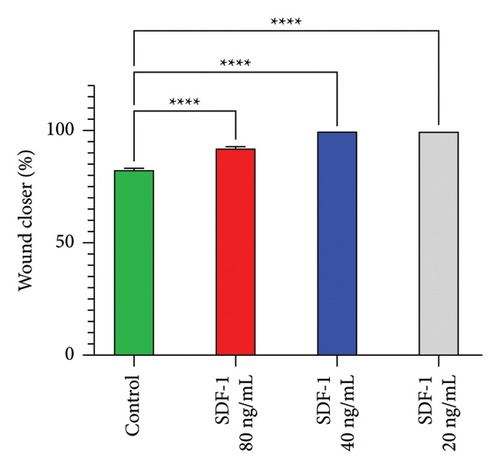
The effect of different concentrations of SDF-1 on human dermal fibroblast (HDF) cell migration was investigated using the scratch wound healing assay. The primary goal of the study was to assess SDF-1 activity and determine the most effective concentration that significantly improves HDF migration during wound repair. The effects of SDF-1 at concentrations of 20, 40, and 80 ng/ml were evaluated in triplicate at 0, 12, and 24 hours. Surprisingly, at a concentration of 20 ng/mL, a significant increase in wound closure rate was observed, indicating a strong promoting effect of SDF-1 on HDF migration. The findings illustrate the impact of SDF-1 in promoting efficient wound healing, confirming the biological activity of recombinant SDF-1 (Figure 4). The ANOVA results revealed significant differences in group means, with a remarkably low P value of less than 0.0001. These statistical findings support the significant impact of varying SDF-1 concentrations on HDF migration and highlight the potential therapeutic implications for tissue repair interventions.
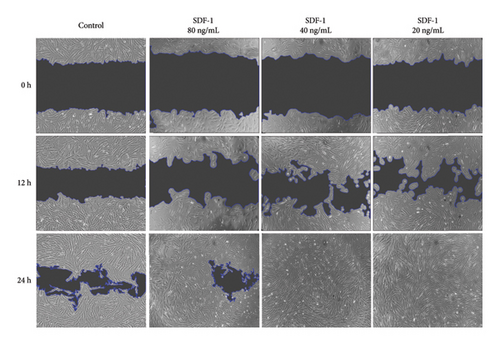
4. Discussion
This study optimized the expression of recombinant SUMO-protease-1 in E. coli BL21 (DE3) to create a straightforward, low-cost approach for manufacturing active recombinant Ulp1 and SDF-1 on a laboratory scale. It also aimed to determine the biological activity of these proteins by cleaving the His6-SMT3-SDF-1 fusion system. The results demonstrated that SUMO-protease-1 expression levels increased under optimal circumstances, which is important for protein purification applications. In our investigation to optimize Ulp1 expression in E. coli BL21 (DE3), we studied the effect of IPTG concentration and incubation conditions, noting parallels with previous research, particularly those conducted by Wang et al. [12, 29].
We utilized the pET28a vector and a purification method for His-tagged proteins under native conditions for producing the enzyme Ulp1. This approach offers significant advantages over a similar study that employed the pET27b vector and purification under denaturing conditions [30]. While denaturing conditions can solubilize insoluble proteins and increase purification efficiency, they disrupt the tertiary and quaternary structures of proteins, necessitating refolding steps that are not always successful. Conversely, purification under native conditions is simpler, faster, and maintains proteins in their active, natural state, which is crucial for biological and research applications. Notably, in our study, inclusion bodies were minimized and the protein remained soluble, which is a substantial advantage over the similar study. This demonstrates that our method not only preserves the active form of the protein but also facilitates the production of soluble and usable protein for practical applications. Therefore, for applications requiring active proteins, our method of purification under native conditions is far superior and more efficient compared to the denaturing method.
In this study, Ulp1 was successfully expressed using the pET28a vector in E. coli BL21 (DE3), with significant protein expression observed at various time intervals (Figure 1). Ulp1 was predominantly in a soluble form, with minimal inclusion body formation, indicating reduced toxicity and simplifying the purification process.
This contrasts with the findings by Lau et al., where Ulp1 expression in E. coli was reported to be toxic, resulting in significant inclusion body formation [31]. One of the main ways to assess the toxicity of a recombinant protein, such as Ulp1, is by monitoring the formation of inclusion bodies. When a protein is toxic to the host cells, it often misfolds and aggregates into these inclusion bodies. Their presence indicates that the cells are under stress, struggling to manage the misfolded proteins, which can reduce overall cell health and protein yield [32].
The absence of significant inclusion body formation and cellular toxicity marks a substantial improvement. This demonstrates a more efficient and cost-effective method for producing active Ulp1, providing a practical advantage for large-scale production and application.
Lower IPTG amounts, specifically 0.1 mM IPTG in our study, have been found to successfully increase protein expression with minimal deleterious effects on cellular health [29, 33, 34]. This is in contrast to a similar study that used 1 mM IPTG, a 10-fold higher concentration [30]. Ulp1 has also exhibited great cleavage efficiency on SUMO fusion proteins [35, 36]. As a result, by utilizing lower IPTG quantities, it is possible to strike a compromise between protein expression efficiency and cellular health while maintaining Ulp1’s cleavage efficiency on SUMO fusion proteins. Our findings are consistent with the broader scientific understanding that lower IPTG doses successfully balance protein expression efficiency and cellular health while maintaining the cleavage efficiency of Ulp1 on SUMO fusion proteins. This was demonstrated by our optimal expression conditions of 0.1 mM IPTG and 28°C incubation, which resulted in a substantial increase in Ulp1 yield, as confirmed by different SDS-PAGE band patterns (Figure 4).
The effects of various SDF-1 concentrations on HDF cell migration were examined using the scratch wound healing test. Several studies have shown that SDF-1 plays a crucial role in cell migration and tissue regeneration [37–39]. Studies have shown that combining SDF-1α with PTH promotes stromal cell migration and periodontal tissue regeneration [40]. Research suggests that SDF-1α promotes tissue healing by attracting stem and progenitor cells and directing monocyte development toward a protissue formation phenotype [41]. Pasquier et al. found that varying SDF-1 concentrations affect the balance of adhesion and migration in cancer cells, with 50 and 100 ng/mL leading to increased migration [42]. The objective of this study was to determine SDF-1 activity and establish the optimal concentration of SDF-1 that significantly improves HDF migration during wound repair at concentrations of 20, 40, and 80 ng/mL. Notably, at a concentration of 20 ng/mL, the wound closure rate increased significantly, demonstrating that SDF-1 has a strong stimulatory effect on HDF migration.
The effective cleavage of His6-SMT3-SDF-1 by recombinant Ulp1 in our study verifies its proteolytic activity and also correlates with earlier research, highlighting the functional integrity of Ulp1 and variety in proteolytic processes [7, 30]. This is important because it confirms Ulp1’s enzymatic functioning, which is required for protein purification applications. Previous research has demonstrated the ability of Ulp1 to cleave the SUMO tag without leaving any additional amino acids, as well as its function in acquiring natural N-terminal target proteins by cleaving SUMO fusion proteins [43]. Furthermore, Ulp1’s stability in a variety of buffer and temperature conditions increases its utility [12, 44]. Our experimental results support these inferences. Incubating His6-SMT3-SDF-1 with recombinant Ulp1 at 20 μg under optimum circumstances (30°C for three hours) resulted in prominent bands on SDS-PAGE. The absence of such cleavage in the control experiment, which lacked recombinant Ulp1, demonstrated that the substrate cleavage was cut by recombinant Ulp1. This not only demonstrates its effective proteolytic function under certain conditions but also adds to current evidence by providing practical insights into its use in biochemical research.
In conclusion, the optimized expression and confirmed biological activity of SUMO-protease-1 in E. coli BL21 (DE3) represent a significant step forward in the field of protein expression. Our findings provide the framework for future protein purification applications, which may have an impact on cost efficiency and process optimization. This study advances our understanding of protein expression in bacterial systems and reveals how these proteins can be used in a variety of biotechnological and biomedicinal applications. The adjustment of expression conditions, as well as effective purification procedures and biological activity assessment, are crucial steps towards realizing these proteins’ full potential for real-world applications.
Disclosure
This article is the result of Farzad Sadri’s dissertation work done in the comprehensive research laboratory of Birjand University of Medical Sciences.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Sadri, F., developed, designed, and conducted recombinant SUMO-protease-1 expression experiments, collected and analyzed data, and contributed significantly to manuscript drafting. Safarpour, H., collaborated on evaluating biological activity, notably cleavage analysis, and helped to optimize the approach and revise the text. Ansari, Z., helped optimize expression conditions and provide critical criticism for the research and publication. Ebrahimi, Y., optimized expression conditions and contributed considerably to the study’s intellectual content by assessing and interpreting the data. Feriedouni M. supervised the overall direction of the project, ensuring that the design and analysis were all integrated, as well as leading paper preparation and journal communication.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.




