Mathematical Modeling of the Transmission Dynamics of Gumboro Disease
Abstract
Gumboro disease is a viral poultry disease that causes immune suppression on the infected birds leading to poor production, mortality, and exposure to secondary infections, hence a major threat in the poultry industry worldwide. A mathematical model of the transmission dynamics of Gumboro disease is developed in this paper having four compartments of chicken population and one compartment of Gumboro pathogen population. The basic reproduction number Rog is derived, and the dynamical behaviors of both the disease-free equilibrium (DFE) and endemic equilibrium are analyzed using the ordinary differential equation theory. From the analysis, we found that the system exhibits an asymptotic stable DFE whenever Rog < 1 and an asymptotic stable EE whenever Rog > 1. The numerical simulation to verify the theoretical results was carried out using MATLAB ode45 solver, and the results were found to be consistent with the theoretical findings.
1. Introduction
Infectious bursal disease (IBD) popularly known as Gumboro is a viral poultry diseases that cause high morbidity and mortality, hence a major threat to the poultry industry due to high economic losses associated with it worldwide. Gumboro disease is associated with clinical disease symptoms such as depression, watery diarrhea, ruffled feathers, and dehydration [1].
Gumboro disease affects mostly young chickens around 3–6 weeks of age. Gumboro virus is extremely difficult to eradicate as it is hardy and can live in a great range of environmental conditions, and it is transmitted from one bird to another through feacal–oral route [2].
Generally, the poultry sector plays an important role in the growth of the economy as well as in poverty reduction. According to the Agricultural Sector Development Strategy 2010, in Kenya, each year, about 20 tonnes of poultry meat worth 3.5 billion Kenyan shillings and 1.3 billion eggs worth 9.7 billion Kenyan shillings are produced. This increased production of poultry is necessitated by the increased demand for quality protein especially in developing countries [3].
The IBD virus was observed 40 years ago with Kenya’s first case reported in 1991 in commercial birds on the Kenyan coast; the disease has remained to be a great threat to the commercial poultry industry not only in Kenya but also in the whole world [4].
Mathematical modeling over the years has become a very important tool that is used in the prediction, assessment, and control of various outbreaks. A number of these models that describe the impacts of preventive and control strategies on the transmission dynamics of various poultry infectious diseases have been developed. A study to investigate the impacts of quarantine and vaccination in controlling avian influenza disease was done by [5], and the results established that combining quarantine with vaccination is an effective strategy for control of the disease.
A mathematical model of Newcastle disease with optimal control having five compartments was formulated by [6]. The findings of the study showed that in the absence of control measures, the number of infected bird increased significantly and reduced significantly in the presence of control measure implying that the control measures were effective methods of controlling the disease.
Several mathematical models have been done to describe the dynamics of Gumboro disease; for instance, [7] formulated a model to investigate the impact of the environment in the spread of Gumboro infections while [8] described a model to describe the effects of vaccination and biosecurity measures in controlling Gumboro infection.
Although several Gumboro models have been developed, to the best of our knowledge, a Gumboro model with pathogen compartment has not been developed. In this paper, we formulate a Gumboro model capturing the pathogen compartment to study the dynamics of Gumboro disease.
2. Model Formulation
A model with Gumboro pathogen population Ng and the chicken population Nc is developed in this research. Thus, the total population at a given time (t) is N(t) = Nc(t) + Ng(t). Gumboro pathogen population Ng has one compartment consisting of concentration of Gumboro virus in the environment CV. The chicken population Nc is grouped into four compartments which consist of susceptible chicken S, birds that are at early stages of infection with Gumboro Eg, birds that are in acute stages of infection with Gumboro Ig, and those that will recover from Gumboro disease in both early and acute stages of infections simultaneously R. The model assumes that the bird population recruitment rate to the susceptible compartment will be Λ. The susceptible birds are infected with Gumboro at the rate of ωCV/τ2 + CV. Where ω is the contact rate of susceptible birds with an IBD virus-contaminated environment, τ2 is the IBD virus concentration in the environment with a 50% probability of Gumboro infections. All bird populations experience natural death at the rate η. Additionally, they die from Gumboro at the rate of μ. The Gumboro-infected birds in both stages of infection shed the virus to the environment at the rates ω1−2 which die at the rate α2, while σg and δg are recovery rates for birds at both early and acute stages of infection. Birds at the early stages of Gumboro infections move to the acute stages of infection at the rates ϕg.
2.1. Model Assumptions
- 1.
The bird species that are infected with Gumboro is chicken.
- 2.
Chickens are recruited into the system by birth or immigration.
2.2. Model Flow Chart and Equations
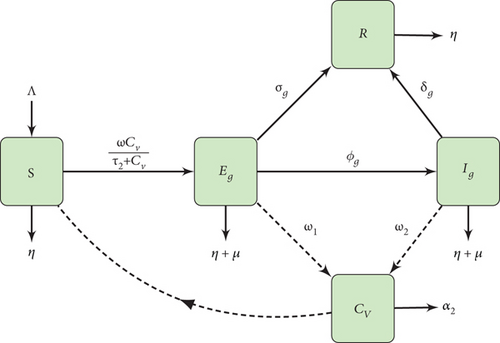
3. Basic Properties of the Model
In this section, we discuss the positivity and boundedness of the solutions of the model.
3.1. Positivity of the Solutions of the Model
Theorem 1. There exists a nonnegative solution set {S, Eg, Ig, R, CV}(t) of model (1) for all t > 0 given that the initial conditions S(0) > 0, Eg(0) ≥ 0, Ig(0) ≥ 0 R(0) ≥ 0, CV(0) ≥ 0 in have nonnegative values.
Proof 1. From the first equation of system (1), we have
Equation (2) can be expressed as
Separating the variables, we obtain
Integrating on both sides of Equation (4), we have lnS > −(γg + η)t + c1 or
From Equation (5), it is clear that S(0) = c for t = 0.
Therefore, and as t⟶∞, we have S(t) > 0∀t > 0.
Also from the second equation of system (1)
Solving the above equation by separation of variables, we have
Upon integrating on both sides, we have lnEg(t) ≥ −(σg + ϕg + η + μ)t + c:
Clearly for t = 0, c = Eg(0).
Thus, Equation (6) becomes
Applying the same method to other equations of the system (1), we get
. Thus (S(0) > 0, Eg(0) ≥ 0, Ig(0) ≥ 0, R(0) ≥ 0, CV(0) ≥ 0) for all t > 0.
3.2. Boundedness of the Solutions of the Model
Let be a feasible region in which the solutions of the total population are bounded, where ΩC is the feasible region of the solutions of the bird population and that of the Gumboro pathogen population is given by . We show that the solutions of the system (1) are bounded in the feasible region.
4. Analysis of the Model
4.1. Disease-Free Equilibrium (DFE) Point
4.2. Reproduction Number for Gumboro Model
where
c2 = σg + ϕg + η + μc5 = δg + η + μ
4.3. Local Stability of DFE
The DFE is locally asymptotically stable if all the real parts of the eigenvalues of the Jacobian matrix of the system (1) at the DFE are all negative.
According to Routh–Hurwitz criteria, Equation (17) has roots with negative real parts if a0 > 0, a1 > 0, a2 > 0, a3 > 0 and a1a2 − a0a3 > 0. Thus, the DFE of the system of Equation (1) is locally asymptotically stable if the following theorem holds.
Theorem 2. The DFE of the system of Equation (1) is locally asymptotically stable when R0g < 1 and unstable otherwise.
4.4. Global Stability of DFE
- 1.
is globally asymptotically stable (GAS)
Theorem 3. The DFE is a GAS equilibrium of system (18) provided that R0g < 1 and the assumptions in Equation (19) are met.
Proof 2. From Theorem 2, the DFE () is locally asymptotically stable when R0g < 1. Consider
From Equation (20), we have
Thus
Thus, the first and the second conditions in Equation (19) are satisfied since
4.5. Existence of the Endemic Equilibrium Point ()
Theorem 4. There exists a positive endemic equilibrium point for the system of Equation (1) provided that R0g > 1.
Proof 3. Letting S = S∗∗, , , R = R∗∗, and and setting the right-hand side of the equations of the system (1) equal to zero, we get
Explicitly solving for values of , we obtain
Thus, a positive exists if R0g > 1.
4.6. Local Stability of Endemic Equilibrium
We use the center manifold theory as described in [12] to assess the stability of the endemic equilibrium, , because evaluating the eigenvalues of the Jacobian matrix of system (1) at the endemic equilibrium is complicated. Theorem 4.1 in [12] outlines the procedure of analyzing the local stability of endemic equilibrium in a nutshell. The coefficients, a and b of the normal form, are two key quantities in expressing the system’s dynamics on the center manifold theory as described in Theorem 4.1 in [12]. According to part (iv) of Theorem 4.1 in [12], if a < 0 and b > 0, the endemic equilibrium is locally asymptotically stable for R0g > 1 but close to 1.
Theorem 5. The endemic equilibrium is locally asymptotically stable if R0g > 1
Proof 4. By using the center manifold theory, Theorem 4.1 in [12], we rename the variables in the system (1) as S = x1, Eg = x2, Ig = x3, R = x4, and CV = x5 such that . Further, by using , the system (1) can be written in the form dX/dt = F(X), with , as follows:
Suppose that ω = ω∗ is a bifurcation parameter when R0g = 1, solving for ω∗ for R0g = 1 from
The Jacobian matrix of the system (25) at with ω = ω∗ is given as
The Jacobian matrix (27) has zero eigenvalues close to ω = ω∗; thus, the center manifold theory is utilized to examine the dynamics of the system. Let , a right eigenvector associated with the Jacobian matrix (Equation (27)), be close to ω = ω∗, then
Solving system (28), we get
Also, let , a left eigenvector associated with the Jacobian matrix (Equation (27)), be close to ω = ω∗, such that
Solving system (30), we obtain
Using the formula described in [12], we compute a and b:
To get the bifurcation coefficient a, we first obtain the nonzero partial derivatives of the model system (25) evaluated at . Thus, it is evident that
Using Equations (29) and (31), Equation (33) can be written as
For the coefficient b, we have the following nonzero partial derivatives of the model system (25) evaluated at :
Hence,
Using Equations (29) and (31), Equation (35) can be written as
Since a < 0 and b > 0, it follows that the endemic equilibrium is locally asymptotically stable if R0g > 1.
4.7. Global Stability of the Endemic Equilibrium Point
Theorem 6. The endemic equilibrium point of the system (1) is GAS if R0g > 1.
Proof 5. We have shown in Section (4.5) that the endemic equilibrium point exists when R0g > 1. Using the Poincaré–Bendixson theorem, the global stability of the endemic equilibrium point is investigated [13]. It follows from Dulac’s multiplier, 1/SEgIgRCV, that
Due to the fact that Ω is positively invariant and the endemic equilibrium exists whenever R0g > 1, there are no periodic orbits in Ω according to Dulac’s criterion. From the Poincaré–Bendixson theorem, it follows that all solutions of the limiting system originating in Ω remain in Ω for all time t. The absence of periodic orbits suggests that whenever R0g > 1, the special endemic equilibrium of the Gumboro model is GAS.
5. Numerical Simulation
Numerical simulations to prove the theoretical results for the Gumboro disease mathematical model were carried out using MATLAB ode 45 solver. This was made possible by the use of some parameters in the literature and others that were estimated or assumed as shown in Table 1.
| Parameter | Value | Source |
|---|---|---|
| Λ | 10 | [7] |
| ω | 0.000143 (0.000143–0.0143)/day | [7] |
| τ2 | 0.009/day | Assumed |
| η | 0.0001543/day | [7] |
| μ | 0.032143/day | [7] |
| ω1 | 0.008 (0.008–0.08)/day | Assumed |
| ω2 | 0.009 (0.009–0.09)/day | Assumed |
| α2 | 0.0900982/day | Assumed |
| θg | 0.0039 (0.0039–0.39)/day | Assumed |
| σg | 0.0165/day | [8] |
| δg | 0.021429/day | [7] |
| ϕg | 0.033/day | [8] |
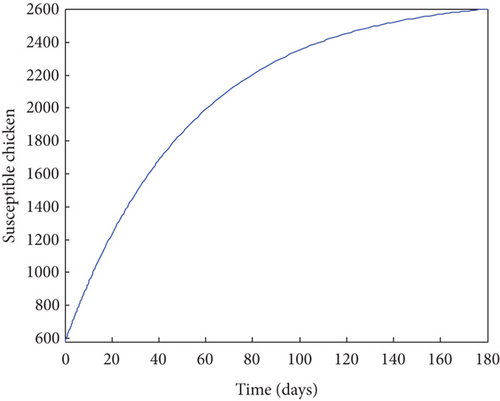
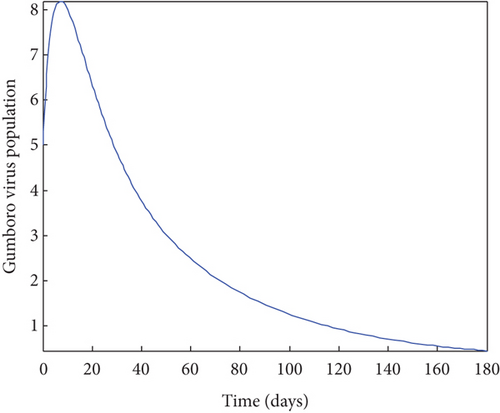
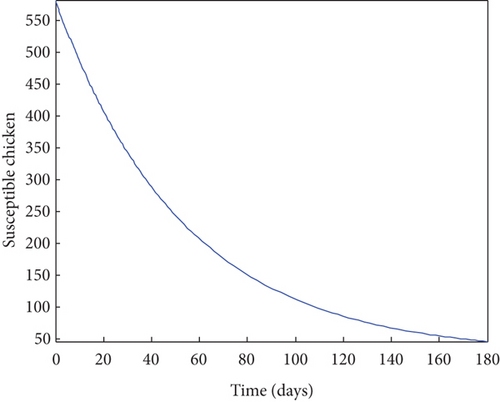
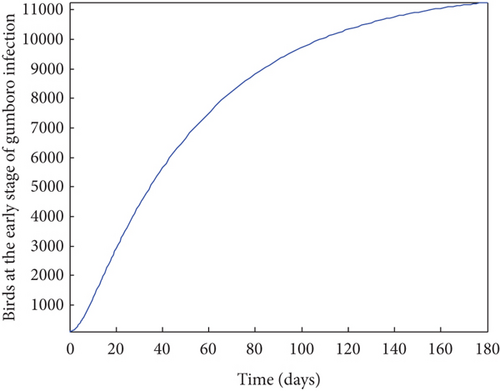
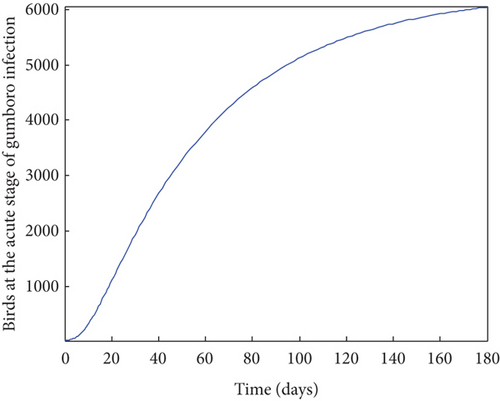
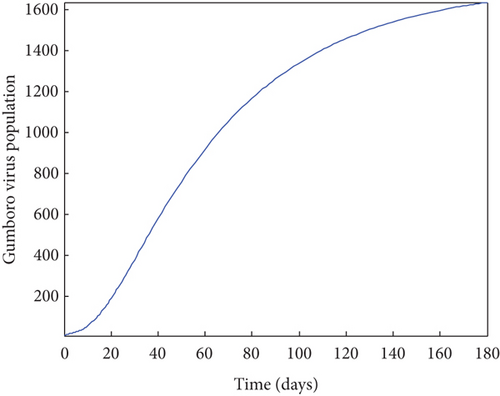
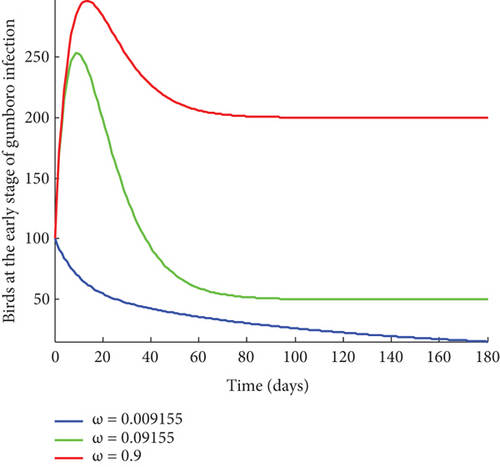
From Figure 2, it was shown that the number of birds at early stages of infection, acute stages of infection, and the pathogen population converges to zero while the susceptible birds’ population tends to a constant λ/η when R0g < 1 which implies that whenever Gumboro disease dies out, only the susceptible chicken would remain. Figure 3 also shows that birds at early and acute stages of infection together with the pathogen population tend towards the endemic equilibrium point when R0g > 1 while the susceptible birds converge to zero indicating that Gumboro disease remains endemic. From Figure 4, it was shown that birds at early stages of infection increase with the increase in the contact rate of susceptible to Gumboro disease-contaminated environment.


6. Conclusion
A mathematical model of Gumboro disease transmission dynamics was formulated in this paper. The disease-free and endemic equilibrium points were determined, and the reproduction number was derived. The findings showed that Gumboro disease dies out whenever R0g < 1 and persists in the chicken population whenever R0g > 1. Also, it was realized that minimizing the contact rate of chicken to a contaminated environment lowers cases of infections in a population. These numerical simulation findings were found to be in harmony with the theoretical stability analysis results.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All the authors collaborated in carrying out this study. Author I.C. designed the study, author J.S.M. wrote the first draft of the manuscript and performed the mathematical analysis of the study and the literature review, and author W.N.M. performed the numerical simulation. All authors read and approved the final manuscript.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors appreciate the ample time given by their respective universities in writing this manuscript.
Open Research
Data Availability Statement
All data is provided in this paper.




