Bevacizumab as Adjuvant Therapy in the Treatment of Keloid: A Randomized Clinical Trial
Abstract
Background. Despite the availability of numerous therapies, keloid treatment remains a challenging clinical issue. Intralesional triamcinolone has been established as an effective corticosteroid treatment for keloids, while sporadic reports suggest the efficacy of intralesional verapamil. This study aimed to evaluate the safety and efficacy of bevacizumab as an adjuvant therapy for keloid treatment. Methods. This randomized controlled trial involved 38 patients diagnosed with keloid according to clinical criteria. The study compared the effects of intralesional triamcinolone combined with bevacizumab injections with intralesional triamcinolone alone. Patients were randomly assigned to either the combination treatment group, which received intralesional triamHEXAL® (20 mg/ml, every two weeks for three months) plus Avastin® (2.5 mg/ml, every two weeks for two months), or the single treatment group, which received intralesional triamHEXAL® alone. The Vancouver Scar Scale (VSS) was used for serial photographic records of scar evaluation, with differences in VSS scores considered the primary outcome, and changes in height and patient satisfaction visual analog score (VAS) were secondary outcomes. Results. A total of 38 patients participated, with a mean age (SD) of 35.32 (14.02) years and 50% male. No significant differences in age, BMI, disease duration, gender, causing, family history, or site were observed between the two groups. The single treatment group exhibited a mean reduction of 0.60 (95% CI: (−1.18, −0.01); P = 0.045) in pigmentation score and a mean decrease of 1.37 (95% CI: (−2.68, −0.07); P = 0.039) in total score compared to the combination treatment group after three months of treatment. There was a significant reduction in keloid height in the combination group after the end of the treatment (P = 0.024). No significant differences in side effects were observed between the two groups. Conclusion. Our study demonstrates that bevacizumab can be considered an effective and safe adjuvant therapy option for keloid treatment, suggesting its potential as a promising treatment for the management of keloids. This trial is registered with IRCT20131119015455N5.
1. Introduction
Keloid scars are a cosmetic disorder resulting from aberrant wound healing processes [1, 2]. While the precise etiology of keloid formation remains unclear, existing evidence points to a genetic component in some instances, modulated by patient-specific and wound-related factors [3, 4]. Managing keloid scars poses a clinical challenge due to the absence of a universally effective therapy or standardized treatment regime. Local corticosteroid injections are commonly employed alongside other injectable treatments such as interferon (IFN), 5-fluorouracil (5-FU), bleomycin, and verapamil [3–5].
Despite the widespread use of corticosteroids, some patients exhibit poor responsiveness or encounter adverse effects like telangiectasia, linear hypopigmentation, subcutaneous tissue atrophy, necrosis, skin ulceration, or Cushing’s syndrome resulting from triamcinolone acetate overdose. These complications have prompted researchers to explore alternative therapeutic avenues [5–8].
One promising strategy involves the application of vascular endothelial growth factor (VEGF) inhibitors, which can restrict keratinocyte-derived VEGF activity or downregulate epidermal VEGF in keloid lesions [9]. VEGF is a potent proangiogenic factor facilitating endothelial cell survival, migration, and proliferation [10]. Previous studies have also demonstrated the expression of VEGF in keloid scars [10–13].
Bevacizumab (Avastin®) is a humanized anti-VEGF monoclonal IgG1 antibody approved by the U.S. Food and Drug Administration (FDA) for treating various metastatic malignancies, such as lung, breast, kidney, and brain tumors [14, 15]. Bevacizumab is also under investigation for off-label applications across diverse conditions [15–17].
A double-blind, randomized trial was conducted to assess the potential efficacy and safety of bevacizumab as an adjuvant therapy for keloid scars. This trial is the first controlled study to evaluate the role of bevacizumab in managing keloid scars. Bevacizumab was selected for its anti-angiogenic properties and ability to target VEGF, a key player in keloid development [2, 18]. This study aimed to ascertain the effectiveness and safety of combining bevacizumab with standard keloid treatments.
2. Materials and Methods
2.1. Trial Setting and Design
A double-masked, randomized controlled study was conducted at the outpatient dermatological clinic affiliated with the Isfahan University of Medical Sciences in Isfahan, Iran. The study was registered with the Iranian Registry of Clinical Trials (IRCT registration number: IRCT20131119015455N5) and received ethical approval from the Isfahan University of Medical Sciences Institutional Review Board. All procedures were performed by the Declaration of Helsinki and its later amendments (IR.MUI.MED.REC.1398.163). Eligible volunteers provided written informed consent after receiving a comprehensive explanation of the study’s details. Participants were informed of their right to withdraw from the study without affecting their treatment. Patients between the ages of 18 and 60 years who met the clinical diagnostic criteria for keloid were included, while those with a sensitivity to any drugs used during the study, systemic severe conditions (e.g., major surgery within 28 days, unhealed surgical wounds, severe hemorrhage, recent hemoptysis, uncontrolled hypertension, and severe arterial thromboembolism [19]), a history of intralesional injections in the past six months, infections near or at the injection site, pregnancy or breastfeeding, or unwillingness to continue treatment were excluded.
2.2. Interventions
The patients were divided into two treatment groups: single therapy and combined therapy group. The single treatment group received 20 mg/ml of intralesional triamHEXAL® (triamHEXAL® 40 mg, Grover, Germany) monthly for three months. In contrast, the combination group received 20 mg/ml intralesional triamHEXAL® monthly for three months plus 2.5 mg/ml of Avastin® (Genentech, Inc., South San Francisco, CA) monthly for two months (Figure 1).
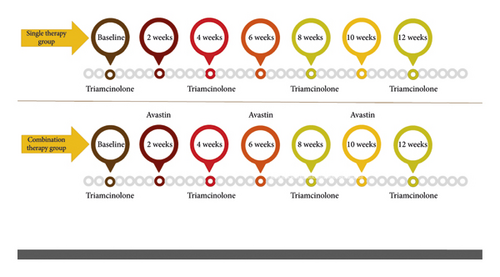
All participants were followed for 12 weeks, and one keloid scar per patient was selected using simple randomization. Injections were administered with an insulin syringe and a 27-gauge needle under aseptic conditions.
2.3. Outcome Assessment
Keloidal lesions were assessed using the Vancouver Scar Scale (VSS) at baseline, one month, and three months after completing the injection protocol. A single expert assessor conducted all measurements to minimize the risk of bias. The dermatologist and statistician were blinded to the medication type. The primary endpoint was the overall difference in VSS scores between the two groups, and the secondary endpoint included variations in the height of scars and patient satisfaction. Patient satisfaction is also measured by visual analog score (VAS).
2.4. Safety
Throughout the trial, patients were instructed to report any unexpected symptoms or complaints to the doctor. Adverse outcomes were documented using a structured checklist, and patients were queried about adverse effects at each appointment.
Bevacizumab has a maximum systemic cumulative dose that is dependent on the patient’s weight and the type of cancer being treated. For example, in metastatic cancers, the maximum dose is 10 mg/kg every two weeks, while in cervical cancer, it is 15 mg/kg every three weeks. There is no established maximum intralesional dosage for bevacizumab [20]. In our study, we administered a total of 7.5 mg of intralesional bevacizumab over two months, with each dose being 2.5 mg. This dosage is significantly lower than the maximum systemic dose. Our findings suggest that this dosage is safe for patients; however, further trials are needed to establish the maximum cumulative dose of intralesional bevacizumab.
2.5. Statistical Analysis
Continuous variables were presented as means (standard deviation (SD)), and categorical data were reported as numbers (percentage). Normality was assessed using statistical tests and graphical methods. The independent Student t-test and Mann–Whitney test were employed to compare means between the triamHEXAL® alone and triamHEXAL®+Avastin® groups for normally and nonnormally distributed data, respectively. Repeated measures ANOVA and Friedman tests were utilized to compare mean scores across time points for normal and nonnormal distributions, respectively. Categorical variables were compared using the chi-square test. Statistical analyses were conducted using SPSS for Windows, version 22 (SPSS Inc., New York, NY), with significance defined as P values below 0.05.
3. Results
The study included 38 participants, with an equal distribution of men and women, having a mean age of 35.32 (SD 14.02) years. The average age of keloid onset was 13.2 months, ranging from 2 weeks to 25 months. Participants were divided into two groups: a single therapy group (n = 19) and a combination therapy group (n = 19), with two patients in the latter group lost to follow-up. Initial characteristics such as age, BMI, illness duration, gender, cause, family history, and location were similar between the two groups, as detailed in Table 1.
| Total (n = 38) | TriamHEXAL (n = 19) | TriamHEXAL + Avastin (n = 19) | P | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (yr) | 35.32 | 14.02 | 38.00 | 15.38 | 32.63 | 12.34 | 0.243 |
| BMI (kg/m2) | 27.37 | 3.60 | 27.84 | 3.70 | 26.89 | 3.53 | 1.000 |
| Duration of disease (month) | 22.74 | 13.96 | 22.74 | 12.42 | 22.74 | 15.69 | 0.424 |
| N | % | N | % | N | % | ||
| Gender | |||||||
| Male | 19 | 50% | 10 | 52.6% | 9 | 47.4% | 0.746 |
| Female | 19 | 50% | 9 | 47.4% | 10 | 52.6% | |
| Cause | |||||||
| Acne | 15 | 39.5% | 8 | 53.3% | 7 | 46.7% | 0.740 |
| Surgery | 17 | 44.7% | 7 | 41.2% | 10 | 58.8% | |
| Burning | 4 | 10.5% | 3 | 75.0% | 1 | 25.0% | |
| Accident | 2 | 5.3% | 1 | 50.0% | 1 | 50.0% | |
| Family history | |||||||
| No | 31 | 81.6% | 15 | 48.4% | 16 | 51.6% | 1.000 |
| Yes | 7 | 18.4% | 4 | 57.1% | 3 | 42.9% | |
| Site | |||||||
| Trunk | 21 | 55.3% | 10 | 47.6% | 11 | 52.4% | 0.744 |
| Extremities | 17 | 44.7% | 9 | 52.9% | 8 | 47.1% | |
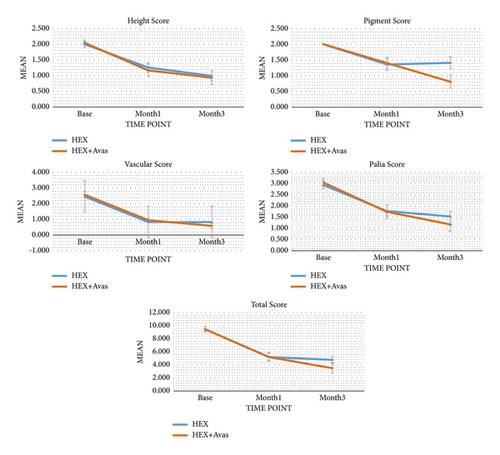
Clinical outcomes were assessed at various time points throughout the study and compared between the two groups. At baseline, there were no significant differences in mean scores between the two treatment groups (P > 0.05), as presented in Tables 2 and 3. However, all mean scores significantly decreased from baseline to three months after treatment (P < 0.05), as depicted in Table 2. The changes in mean scores from baseline to one month and three months posttreatment did not differ significantly between the two groups, except for the pigmentation score and total score, as outlined in Table 2 and Figures 2 and 3.
| Outcomes | Time points | TriamHEXAL | TriamHEXAL + Avastin | Changes | Pb | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean (95% CI) | |||
| Height | Baseline | 2.00 | 0.47 | 2.05 | 0.40 | −0.05 (−0.34, 0.24) | 0.817 |
| Month 1 | 1.26 | 0.65 | 1.18 | 0.81 | 0.09 (−0.41, 0.58) | 0.661 | |
| Baseline-month 1 | 0.74 | 0.56 | 0.82 | 0.64 | −0.09 (−0.49, 0.32) | 0.667 | |
| Month 3 | 1.00 | 0.67 | 0.94 | 0.90 | 0.06 (−0.47, 0.59) | 0.684 | |
| Baseline-month 3 | 1.00 | 0.47 | 1.06 | 0.75 | −0.06 (−0.49, 0.37) | 0.783 | |
| Pw | <0.001 | <0.001 | |||||
| Vascularity | Baseline | 2.47 | 0.77 | 2.58 | 0.77 | −0.11 (−0.61, 0.40) | 0.644 |
| Month 1 | 0.83 | 0.51 | 0.94 | 0.56 | −0.11 (−0.48, 0.26) | 0.660 | |
| Baseline-month 1 | 1.72 | 0.83 | 1.59 | 0.51 | 0.13 (−0.34, 0.61) | 0.570 | |
| Month 3 | 0.83 | 0.51 | 0.59 | 0.62 | 0.25 (−0.15, 0.64) | 0.245 | |
| Baseline-month 3 | 1.72 | 0.67 | 1.94 | 0.66 | −0.22 (−0.68, 0.24) | 0.337 | |
| Pw | <0.001 | <0.001 | |||||
| Pliability | Baseline | 2.95 | 0.78 | 3.05 | 0.78 | −0.11 (−0.62, 0.41) | 0.680 |
| Month 1 | 1.79 | 1.03 | 1.75 | 1.18 | 0.04 (−0.72, 0.80) | 0.917 | |
| Baseline-month 1 | 1.16 | 0.60 | 1.31 | 0.79 | −0.15 (−0.63, 0.33) | 0.517 | |
| Month 3 | 1.53 | 0.96 | 1.19 | 1.28 | 0.34 (−0.43, 1.11) | 0.378 | |
| Baseline-month 3 | 1.42 | 0.61 | 1.88 | 0.96 | −0.45 (−1.00, 0.09) | 0.098 | |
| Pw | <0.001 | <0.001 | |||||
| Pigmentation | Baseline | 2.00 | 0.00 | 2.00 | 0.00 | 0.00 | 1 |
| Month 1 | 1.37 | 0.83 | 1.41 | 0.71 | −0.04 (−0.57, 0.48) | 1 | |
| Baseline-month 1 | 0.63 | 0.83 | 0.59 | 0.71 | 0.04 (−0.48, 0.57) | 0.868 | |
| Month 3 | 1.42 | 0.84 | 0.82 | 0.88 | 0.60 (0.01, 1.18) | 0.066 | |
| Baseline-month 3 | 0.58 | 0.84 | 1.18 | 0.88 | −0.60 (−1.18, −0.01) | 0.045 | |
| Pw | 0.003 | <0.001 | |||||
| Total score | Baseline | 9.42 | 1.61 | 9.53 | 1.46 | −0.11 (−1.15, 0.94) | 0.835 |
| Month 1 | 5.21 | 2.46 | 5.18 | 2.79 | 0.03 (−1.74, 1.81) | 0.969 | |
| Baseline-month 1 | 4.21 | 2.02 | 4.35 | 1.62 | −0.14 (−1.39, 1.11) | 0.818 | |
| Month 3 | 4.74 | 2.42 | 3.47 | 3.14 | 1.27 (−0.62, 3.16) | 0.182 | |
| Baseline-month 3 | 4.68 | 1.77 | 6.06 | 2.08 | −1.37 (−2.68, −0.07) | 0.039 | |
| Pw | <0.001 | <0.001 | |||||
- Pb: P value obtained to compare means between treatment groups; Pw: P value obtained to compare means within treatment groups. The significance of values is <0.05.
| Outcomes | Time points | TriamHEXAL | TriamHEXAL + Avastin | Changes | Pb | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean 95% CI | |||
| VAS score | Month 1 | 5.68 | 2.11 | 6.47 | 2.35 | 0.79 (−2.30, 0.72) | 0.297 |
| Month 3 | 5.95 | 2.30 | 7.24 | 2.49 | 1.29 (−2.91, 0.33) | 0.116 | |
| Month 3-month 1 | 0.26 | 1.19 | 0.76 | 0.83 | 0.50 (−0.20, 1.21) | 0.158 | |
| P | 0.35 | <0.001 | |||||
| Height (mm) | Baseline | 2.84 | 1.25 | 3.58 | 1.57 | 0.74 (−1.67, 0.20) | 0.119 |
| Month 1 | 1.55 | 0.96 | 1.68 | 1.44 | 0.12 (−0.94, 0.69) | 0.760 | |
| Month 1-baseline | 1.29 | 0.85 | 1.74 | 1.03 | 0.45 (−1.09, 0.19) | 0.166 | |
| Month 3 | 1.37 | 1.00 | 1.21 | 1.53 | 0.16 (−0.70, 1.03) | 0.705 | |
| Month 3-baseline | 1.47 | 0.79 | 2.21 | 1.06 | 0.73 (−1.36, −0.10) | 0.024 | |
| Pw | <0.001 | <0.001 | |||||
- The significance of all values is <0.05.

Specifically, the mean change from baseline to three months after treatment for the pigmentation score in the single treatment group was 0.60 (95% CI: −1.18, −0.01; P = 0.045) lower than that in the combination treatment group. Similarly, the mean change from baseline to three months after treatment for the total score in the single treatment group was 1.37 (95% CI: −2.68, −0.07; P = 0.039) lower than that in the combination treatment group, as shown in Table 2.
Mean scores with standard errors for study time points by treatment groups are illustrated in Figure 4. Patient satisfaction was evaluated using a visual analog scale (VAS) score, which was significantly higher in the combination treatment group than in the single treatment group, as indicated in Table 3. Furthermore, there was a notable reduction in keloid height in the combination group three months after treatment completion (P = 0.024), as detailed in Table 3. No significant differences were observed in side effects between the combination treatment group and the single therapy group at the end of the three-month follow-up period. Overall, the findings suggest that combination therapy may offer more efficacy than single therapy in treating keloids, resulting in improved outcomes related to pigmentation, total score, patient satisfaction, and reduction in keloid height.
4. Discussion
To assess the efficacy of bevacizumab as an adjuvant therapy to intralesional triamcinolone for keloid treatment, a randomized controlled trial was conducted, monitoring patients for 12 weeks posttherapy completion. Results revealed significant scar height, vascularity, and pliability improvements in both treatment groups. However, the combination therapy group exhibited lesser scar pigmentation and overall scores. Additionally, the reduction in keloid height after three months was notably more significant in the combination therapy group than the single therapy group, with higher patient satisfaction scores.
These findings suggest that bevacizumab is comparably effective and safe as triamcinolone, making it a viable adjuvant treatment for keloids, particularly in cases where corticosteroids are contraindicated. While early stage keloids may benefit from bevacizumab’s anti-neoangiogenic effects, further research is needed to elucidate its impact on mature keloids.
Research on vascular endothelial growth factor (VEGF) inhibitors has explored their role in keloid formation, with bevacizumab potentially inhibiting endogenous VEGF to prevent keloid development. VEGF contributes to keloid formation by three distinct methods: (1) promoting fibroplasia or inflammation, (2) excessive tissue fibrosis through an imbalance in extracellular matrix metabolism [21, 22], and (3) VEGF as a proinflammatory cytokine released during the inflammatory phase of routine wound healing is a strong proangiogenic factor that can lead to aberrant wound healing and excessive tissue fibrosis [22–24].
Despite several animal research studies demonstrating the potential effectiveness of anti-VEGF in treating hypertrophic scares and keloid development, more human clinical trials are needed in this area [25, 26]. However, pathological investigations have shown that keloid tissue contains abnormal levels of VEGF [22, 27–29]. Salem et al. found a significant difference in VEGF expression between individuals with keloid lesions and controls, suggesting that VEGF may play a role in keloid formation [27]. Their findings imply that VEGF could be involved in developing keloids, and inhibiting these biological activities would be necessary in clinical treatment. However, the limited research sample might be a drawback.
Combining corticosteroids and VEGF inhibitors may synergistically reduce VEGF expression, as our study’s outcomes suggested. Pathological analysis by Jiang et al. suggested that VEGF overexpression may be associated with the invasive growth of keloids [30]. The eventual treatment cost of any keloid lesion is impacted by the location and form of the keloid; keloids on the back of the head, sternum, and chest require more treatment sessions and higher steroid doses; therefore, adjuvant therapies like bevacizumab that can reduce keloid height may be promising options for the treatment [31].
The cost-effectiveness of bevacizumab therapy for keloids is promising, considering its low required volume and potential impact on treatment sessions and overall costs. Notably, the observed differences in keloid pigmentation may stem from VEGF regulation and lesion growth control. This study stands out as the first randomized controlled trial to evaluate a novel adjuvant monoclonal therapy for keloids, employing a comparative approach to assess treatment efficacy.
4.1. Limitations
- (1)
Lack of Placebo Group. Future studies should consider including a placebo group to better assess the therapeutic efficacy of bevacizumab alone in treating keloids.
- (2)
Specific Participant Selection. The study focused on treatment-refractory individuals, limiting the generalizability of the findings. Including a broader range of participants would provide more comprehensive insights.
- (3)
Longer Follow-Up Period. Extending the follow-up period beyond three months would allow for a more thorough evaluation of keloid recurrence rates and long-term treatment outcomes.
- (4)
Continuous Monitoring of Bevacizumab Effects. Regular monitoring of bevacizumab concentrations and their effects on keloids throughout the study would enhance understanding of treatment mechanisms and optimize dosing strategies.
- (5)
Lack of Histological Examination. Incorporating histological examinations alongside clinical documentation would provide valuable insights into the underlying biological processes contributing to treatment outcomes.
5. Conclusion
This study provides evidence that bevacizumab may be a viable option for treating keloid disorder. However, further research is warranted to validate its effectiveness and safety, especially as a standalone therapy in diverse patient populations. Addressing these limitations in future studies will contribute to a more comprehensive understanding of bevacizumab’s role in managing keloid disorder.
Ethical Approval
This research was approved by the Ethics Committee of the Isfahan University of Medical Science (No. IR.MUI.MED.REC.1398.163), and at the Iranian Registry of Clinical Trials, the study was documented (https://www.irct.ir; IRCT registration number: IRCT20131119015455N5).
Consent
Written informed consent was obtained from the patients.
Disclosure
The abstract of this manuscript was presented as an E-poster in EADV congress-2023.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability
All data generated or analyzed during this study are included within the article.