Mathematical Modeling of Coccidiosis Dynamics in Chickens with Some Control Strategies
Abstract
Coccidiosis is an infectious disease caused by the Eimeria species. The species can infect a bird’s digestive system, severely slow down its growth, and is a serious economic burden for chickens. A mathematical model for the transmission dynamics of coccidiosis disease in chickens in the presence of control interventions has been formulated and analyzed to gain insights into the dynamics of the disease in the population. Three control interventions, namely vaccination, sanitation, and treatment, are implemented. The study intends to assess the effects of these control interventions in coccidiosis transmission dynamics. Using the theory of differential equations, the invariant set of the model was derived, and the model’s solution was found to be mathematically and biologically significant. Analytical methods are employed to establish equilibrium solutions and investigate the stability of the model system’s equilibria, while numerical simulations illustrate the analytical results. The effective reproduction number is obtained using the next-generation matrix method, and the local stability of the equilibria of the model is established. The disease-free equilibrium is proved to be locally stable when the effective reproduction number is less than unity. Also, the nature of the bifurcation and its implications for disease prevention are investigated through the application of the center manifold theory. On the other hand, sensitivity analysis is carried out to investigate the parameters that impact the transmission of coccidiosis disease using the normalized forward sensitivity index. The parameters that have a greater influence on the effective reproduction number should be targeted for control purposes to lessen the spread of disease. Furthermore, numerical simulation is performed to investigate the contribution of each control intervention.
1. Introduction
Coccidiosis is a poultry disease that is triggered by a protozoan parasite of the genus Eimeria that invades a bird’s digestive tract and significantly slows its growth. Worldwide, the poultry industry is among the main suppliers of animal protein as it provides both eggs and meat [1]. Any pathogen that undermines the effectiveness of a poultry production sector could endanger world food security. The chicken can be affected by seven species of the genus Eimeria, each with different pathogenic properties and targeting a particular intestinal location. The seven species are Eimeria acervulina, Eimeria brunetti, Eimeria, maxima, Eimeria mitis, Eimeria necatrix, Eimeria praecox, and Eimeria tenella. The model of transmission begins with the ingestion of sporulated oocysts (the infection form of the parasite). Depending on the species, the infection can result in nutrient deficiencies, reduced rates of growth, and, in the case of the most pathogenic species, increased mortality. According to the literature, though seven species can affect chicken, E. tenella is considered to be the most pathogenic [2, 3]. The infected chicken has ruffled feathers and symptoms of depression or drowsiness. Furthermore, feed and water consumption are reduced, and feces are watery, whitish, and occasionally bloody. This causes dehydration, impaired weight gain, and, in the absence of treatment, death may occur [4, 5]. The risk of developing clinical coccidiosis is greatest between 1 and 3 weeks of chicken’s life. Mortality and morbidity rates for chickens between the ages of 10 and 30 days and 35 and 60 days may exceed 80% [6]. Coccidiosis control and prevention are based on the use of vaccines, natural feed additives, prophylactic anticoccidial drugs, and improved farm handling practices. Some beneficial practices include facility cleaning and disinfection, proper ventilation, and safe drinking water, all of which helped to contribute of keeping litter conditions that limit oocyst sporulation [7, 8].
To help identify crucial intervention points and reduce disease-related mortality, mathematical modeling has emerged as a crucial tool. Researchers can forecast a phenomenon’s future by using mathematical models. For example, we can obtain a wealth of knowledge regarding the transmission dynamics, eradication, and future spread of infectious or viral diseases in society through the use of mathematical models and simulations of these diseases [9, 10]. Recently, few mathematical models have been made in the area of coccidiosis. For example, Kachanova et al. [11] conducted an experimental model of coccidiosis by E. tenella in broiler chicken. The findings showed that the infected chicken gained much less weight each day than the noninfected bird. The outcomes also demonstrated that the dose of infection affects the quantity of oocysts expelled with feces. Also, Zou et al. [6] construct a principle component analysis-based model for assessing chicken coccidiosis resistance. The findings suggested that a better parameter for assessing an individual biological resistance to coccidiosis illness may be the infection index (II). Further, Johnston et al. [12] developed a model of the chicken infection caused by E. maxima and E. praecox within the host. Assumptions about the quantity of available host cells, the average life expectancy of these cells, and the age structure inside the host-cell population were made and the mathematical models combined with experimental data to test whether these conditions could reproduce the crowding effect in the two species were studied. The findings revealed that, at very low infectious doses, experimental data indicated that crowding occurred during in vivo infections. However, none of the models could accurately simulate crowding at the same dosages while preserving accurate estimations of the dynamics of the enterocyte pool. Despite the studies that have been conducted to curb coccidiosis, the disease remains one of the most common parasite infections in the poultry. Coccidiosis causes an annual loss to the poultry business of over $3 billion worldwide, of which more than 70% is attributable to chickens’ stunted growth and lower feed conversion rates [6, 13]. To the best of our knowledge, no mathematical model has attempted to examine the role of treatment, vaccination, and sanitation or cleaning of the environment as a control strategy in the transmission dynamics of coccidiosis disease. Vaccination is administered to protect the susceptible chicken from coccidiosis for a sufficient period. The current vaccines available in the market for coccidiosis pathogens include live virulent, live attenuated, and subunit vaccines [1]. Environmental hygiene and sanitation involve raising people’s awareness about biosecurity measures to free the environment from coccidiosis pathogens. This is attained by ensuring cleanliness in the chicken’s yard and its surroundings to prevent indirect transmission of the pathogens to the chicken from the unhygienic environment [7]. Therefore, relying on several clinical and epidemiological studies conducted on the transmission of coccidiosis disease in chickens, in this study, we developed a mathematical model to examine the role of treatment, vaccination, and sanitation in the transmission dynamics of coccidiosis in chickens. The implementation of these three control strategies will contribute to the reduction and possible elimination of coccidiosis in the chicken population. The current study will add up knowledge to the existing literature and provide a cornerstone for further research on poultry infectious diseases.
2. Model Description and Formulation
The chicken population at any time t, denoted by N(t) is divided into five subpopulations based on the disease status as follows; susceptible chickens who are at risk of acquiring coccidiosis disease, S(t), vaccinated chicken, V(t), chicken infected with a coccidiosis disease who are capable of transmitting the infection to susceptible chicken, I(t), and the recovered chicken, R(t). The number of coccidiosis viruses in a contaminated environment is denoted by H(t). It is assumed that chickens are recruited at a constant rate Λ. Susceptible chickens are vaccinated at rate τ in which vaccinated chickens who did not respond to vaccination wane out at the rate η and become susceptible again. It is also assumed that a susceptible chicken can be infected with coccidiosis if it comes into effective contact with an infectious chicken at a force of infection λ = βI + αH, where β denotes transmission rate for infectious chicken and α denotes ingestion rate of coccidiosis pathogen by chicken. Infected chicken shed pathogens into the environment at a rate ϵ. The term pathogens refers to infectious agents that course infection and illness in the chickens. These pathogens are shed from an infected chicken through its feces, containing oocysts. These oocysts can spread and contaminate the environment including water, food, and litter [14]. A newly infected chicken can progress to the infectious chicken class at rate γ. Sanitation is very important as it leads to the death of the coccidiosis pathogen. By so doing, the number of infections may significantly be reduced. Furthermore, it is assumed that the infected chicken in the I(t) compartment receives treatment at a rate θ. Moreover, all recovered chickens acquire temporary immunity, which wanes out at the rate ψ and becomes susceptible again. The disease-induced mortality occurs in the compartment, I(t) at rate δ. The pathogens deplete naturally at a rate μh or by sanitation measures at the rate ρ. All chickens in different subgroups experience natural death at a rate μ. The model flow diagram is in Figure 1.
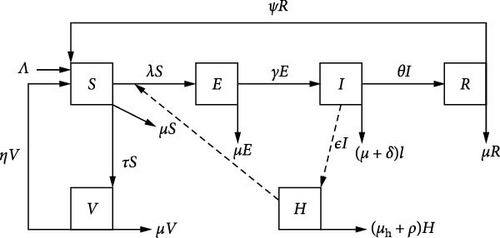
The initial conditions of the model system (1) are S(0) ≥ 0, V(0) ≥ 0, E(0) ≥ 0, I(0) ≥ 0, R(0) ≥ 0, and H(0) ≥ 0.
3. Model Analysis
3.1. Invariant Region
Since the model system (1) deals with human beings, it is assumed that all parameters and variables in the model are nonnegative for all t ≥ 0. The discussion on the invariant region involves the description of the region in which the solution of the system makes epidemiological sense. We state and prove the following theorem.
Theorem 1. The region is positively invariant under the flow induced by the model system (1).
Proof. Consider the population size: N(t) = S(t) + V(t) + E(t) + I(t) + R(t) + H(t) and the rate of change given by:
From Equation (2) we have:
Solving Equation (3) for N yields implying that as t → ∞, where c is the constant of integration. Therefore, the feasible region solution set of the system enters the region Ω. Thus, the region Ω is a positively invariant set under the flow induced by the model and the model is well-posed and also it is biologically meaningful.
3.2. Positivity of the Solutions
In this section, we describe the positivity of the solution of the model system.
Theorem 2. The solution set (S(0), V(0), E(0), I(0), R(0)H(0)) > 0} of the model system (1) is positive for all t ≥ 0.
Proof. Consider the first equation of the model system (1):
It follows that
Integrating Equation (5) by separation of variables, we obtain:
Applying the initial conditions S(0), in Equation (6) gives:
Similarly, the remaining other equations in the model system (1) give the following results:
Therefore, the solutions of the model are positive for all values of t > 0.
3.3. Existence of Equilibrium
3.4. Disease-Free Equilibrium (DFE) Point
3.5. The Effective Reproduction Number
The reproduction number that is used to investigate whether an infection introduced into a population will be eliminated or become endemic is obtained. It is defined as the expected number of secondary cases produced in a completely susceptible population by a typical infectious individual during its period of infectiousness [15, 16].
3.6. Endemic Equilibrium Point
Therefore, it can be noted that the model system (1) has a unique endemic equilibrium point when Re > 1.
3.7. Local Stability of the DFE
From Equation (38), C0 is positive if Re < 1. Hence, we have the following result.
Theorem 3. The DFE point is locally asymptotically stable if Re < 1 and unstable if the inequality is reversed.
3.8. Global Stability of DFE
For global stability, the spread of the infection is independent of the initial size of the population. By using the comparison method [20], we establish the following theorem.
Theorem 4. The DFE is globally asymptotically stable if Re < 1 and unstable if the inequality is reversed.
Proof. Let F and V be the Jacobian matrices of Fi and Vi defined as and ; 1 ≤ i, j ≤ m, where x is the number of individuals in each compartment, x0 is a DFE, Fi is the rate of appearance of new infections in compartment i, , in which is the transfer rate of individuals into compartment i and is the rate of transfer of individuals out of compartment i. The rate of change of variables representing the infected components of the model system (1) can be rewritten as follows:
The matrix (F − V) is given by:
The eigenvalues of matrix (43) are established from the characteristic equation |J2(G) − λI| = 0.
The polynomial obtained in matrix (44) is given by:
By Routh–Hurwitz criteria, the third-order polynomial Equation (45) has all roots in the open left half-plane if D0, D1, D2 are positive and D1D2 > D0 [16]. It is clear that D2 is positive, and D1 is positive if a6(a3 + a4) + a3a4 > βγS0. Also, from Equation (46) D0 is positive if Re < 1. This result signifies that the eigenvalues of matrix (F − V) have negative real parts if Re < 1. Therefore, system (1) is stable for Re < 1. It follows that (E0, I0, H0) → (0, 0, 0) as t → ∞. Furthermore, examining the first, second, third, fourth, fifth, and sixth equations of system (9), gives S = Λa2/a1a2 − ητ, and V = τΛ/a1a2 − ητ whenever E0 = I0 = H0 = 0. Therefore, by employing the comparison method [21] it follows that (S0, V0, E0, I0, R0) → (Λa2/a1a2 − ητ, τΛ/a1a2 − ητ, 0, 0, 0, 0) as t → ∞ for Re < 1. Hence, E0 is globally asymptotically stable whenever Re < 1. Thus, the system will come to a DFE point from any starting point.
3.8.1. The Impact of Intervention Strategies
The effects of implementing intervention strategies are investigated. Since, the interventions have been invested in controlling pneumonia disease, it is very important to understand the benefits of implementing these interventions. The three intervention strategies’ contribution is observed by comparing the basic reproduction number and the effective reproduction number.
This indicates that the value of the numerator is less than the value of the denominator. Consequently, it implies that k < 1. This demonstrates that vaccination, treatment, and sanitation effectively curb initial disease transmission and halt-epidemic spread.
3.9. Bifurcation Analysis
To establish the conditions for the occurrence of forward or backward bifurcations, the Castillo-Chavez and Song Theorem 4.1 in [22] is used. The theorem is provided below for easy reference since it is important for providing the local stability of the endemic equilibrium point around R0 = 1.
Theorem 5. Consider the following general system of ordinary differential equations with a parameter ϕ such that
- (1)
is the linearization matrix of system (63) around the equilibrium 0 with ϕ evaluated at 0.
- (2)
Zero is a simple eigenvalue of A, and all other eigenvalues of A have negative real parts.
- (3)
Matrix A has a nonnegative right eigenvector ω and a left eigenvector v corresponding to the zero eigenvalue.
Let fk be the kth components of f and
The local dynamics of the system around the equilibrium point are totally determined by the signs of a and b for |ϕ| < 1,
- (i)
a > 0, b > 0. When ϕ < 0, 0 is locally asymptotically stable and there exists a positive unstable equilibrium; when 0 < ϕ < 1, 0 is unstable and there exists a negative, locally asymptotically stable equilibrium.
- (ii)
a < 0, b < 0. When ϕ < 0, 0 is unstable; when 0 < ϕ < 1, 0 is asymptotically stable equilibrium, and there exists a positive unstable equilibrium.
- (iii)
a > 0, b < 0. When ϕ < 0, 0 is unstable, and a positive unstable equilibrium appears.
- (iv)
a < 0, b > 0. When ϕ changes from positive to negative, 0 changes its stability from stable to unstable. Correspondingly, a negative unstable equilibrium becomes positive and locally asymptotically stable.
Particularly, if a > 0, and b > 0, then, a subcritical (or backward) bifurcation occurs at ϕ = 0.
Theorem 6. The unique endemic equilibrium is ensured, and by Theorem 5, the model is locally asymptotically stable for R0 > 1. Moreover, according to Theorem 5 item (i), the model experiences backward bifurcation when a > 0. This is true only if v1 < 0, and a1a2 < ητ are present; otherwise, forward bifurcation occurs.
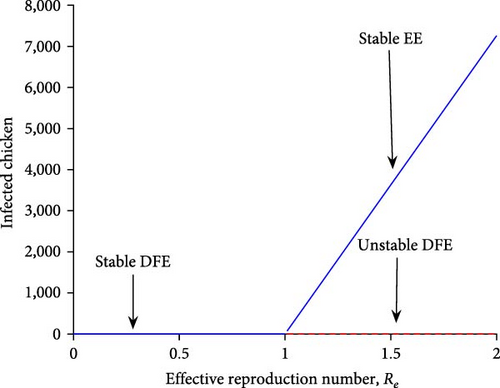
The model system (1) exhibits forward bifurcation at R0 = 1 when a < 0 and b > 0. Biologically, this scenario demonstrated that the model system (1) is globally asymptotically stable at a DFE point when R0 < 1, and has a distinct endemic equilibrium point for R0 > 1. When R0 is close to one the unique endemic equilibrium point is locally asymptotically stable. When R0 falls below one, that is R0 < 1 no endemicity exists and the disease continues to decline, and as R0 increases to above one, that is R0 > 1, the disease invades the population. Furthermore, when a > 0 and b > 0, the model system (1) exhibits a backward bifurcation, as R0 approaches one, the disease prevalence rapidly increases giving rise to a situation in which the DFE co-exists with the endemic equilibrium. The biological implication of backward bifurcation is that the classical requirements of having the reproduction number less than unity, although necessary, is no longer sufficient for disease eradication. Based on Equation (15), The model system (1) exhibit a forward bifurcation as shown in Figure 2.
| Parameters | Sensitivity index for Re < 1 | Sensitivity index for R0 > 1 |
|---|---|---|
| Λ | + 1 | +1 |
| β | +0.0805 | + 0.7820 |
| α | +0.2180 | +0.9195 |
| μ | −0.04383 | −0.0944 |
| γ | +0.3733 | +0.3733 |
| θ | −0.1068 | — |
| δ | −0.2137 | −0.2392 |
| η | +0.0042 | — |
| τ | −0.0118 | — |
| ϵ | +0.1111 | +0.7999 |
| ρ | −0.2127 | — |
| μh | −0.0053 | −0.9195 |
| Parameter | Descriptions | Values | Source |
|---|---|---|---|
| Λh | Chicken recruitment rate | 0.001/day | Assumed |
| β | Transmission rate for infectious chicken | 0.7/day | Assumed |
| α | Eimeria transmission rate due to the chicken-to-environment interaction | 0.8/day | Assumed |
| μ | Nature death rate of a chicken | 1.37 − 5.48 × 10−4/day | [27] |
| γ | Chicken incubation rate | 0.09/day | Assumed |
| θ | Chicken recover rate/treatment rate | 0.01/day | Assumed |
| ψ | Immunity waning rate for the recovered chicken | 0.09/day | Assumed |
| δ | Chicken death rate due to coccidiosis | 0.02/day | Assumed |
| η | Waning rate of vaccinated chicken | 0.03/day | Assumed |
| τ | Rate at which susceptible chicken are vaccinated | 0.001/day | Assumed |
| μh | Parasite natural death rate | 0.001/day | [13] |
| ρ | Eimeria death rate due to sanitation measures | 0.04/day | Assumed |
| ϵ | Parasite shedding rate to the environment by infectious chicken | 0.4/day | [13] |
3.10. Global Stability of the Endemic Equilibrium Point
Then, if S = S∗, V = V∗, E = E∗, I = I∗, R = R∗, H = H∗, the function (75) becomes less than or equal to zero and for all S, V, E, I, R, H > 0, following the approach of Korobeinikov [23]. Hence, the largest compact invariant set in {S, V, E, I, R, H} where is the singleton {S∗, V∗, E∗, I∗, R∗, H∗} which is the endemic equilibrium point of the model system (1). It is implied that {S∗, V∗, E∗, I∗, R∗, H∗} is globally asymptotically stable in the interior region of {, V, E, I, R, H} by Lasalle’s invariant principle [24]. As a result, the following theorem is provided:
Theorem 7. If R0 > 1, then the coccidiosis model (1) has a unique endemic equilibrium point S∗, V∗, E∗, I∗, R∗, H∗ which is globally asymptotically stable in S, V, E, I, R, H.
3.11. Sensitivity Analysis
Sensitivity indices measure the relative change in a state variable when a parameter change [25]. Initial disease transmission is directly related to the reproduction number. The sensitivity analysis is performed to determine which model parameters are the most important to disease transmission and prevalence (the parameters that are the most sensitive with respect to the initial transmission of the disease). In this study, we use it to discover parameters that have a high impact on reproduction number, and should be targeted by intervention strategies [26]. If a variable is a differentiable function of the parameter, the sensitivity indices may be alternatively defined using partial derivatives [25]. We intend to know how each parameter affects the effective reproduction number Re. We use the formula of the normalized sensitivity index of a variable for Re to achieve our goal.
Applying the normalized forward sensitivity index defined in Equations (77) and (78) yields to the results in Table 1. The parameter values in Table 2 are used to determine the sensitivity indices.
3.11.1. Interpretation of Sensitivity Indices
The sensitivity indices are presented in Table 1, when R0 > 1 (in the absence of intervention) and when Re < 1 (in the presence of interventions). Parameters that have positive sensitivity indices in Table 1, indicated that if their values are increased while keeping other values constant, reproduction number increases. These parameters have a greater influence on spreading the coccidiosis infection. But the parameters with negative sensitivity indices, show that if their values are increased while keeping the rest parameters fixed, reproduction number decreases. The most sensitive parameter is Λ with positive sensitivity indices. Other parameters with greater positive sensitivity indices are β, α, and γ. These parameters have an impact on reproduction number, and should be targeted for intervention strategies. These findings can be used to inform the development of intervention strategies to control coccidiosis infection. For example, interventions that target reducing the rate of transmission, such as vaccination or improved hygiene, are likely to be most effective.
3.12. Numerical Analysis
In this section, the model system (1) was numerically solved using MATLAB software and the Runge–Kutta fourth-order approach. To support previously analytical findings, various graphical representations are provided and discussed. Since many parameters were not publicly available, we collected a few from the literature and others were assumed for demonstration. For simulation, the study employed the initial values of the parameters from Table 2. The following initial values for the subpopulation were assumed as follows: S(0) = 400, V = 300, E = 100, I = 100, R = 80, and H = 5, 000.
3.13. Effects of Interventions on Infected Chickens
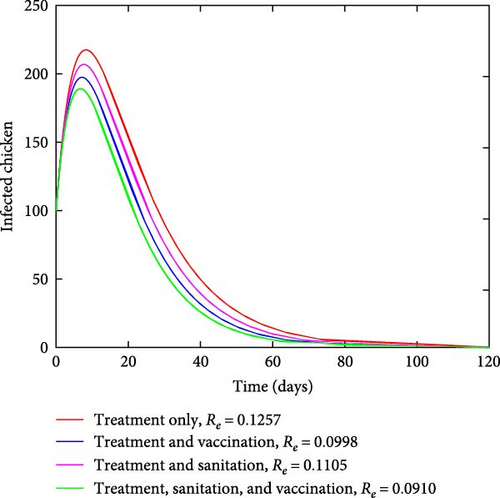
Figure 3 depicts how the number of exposed and infected chickens changes over time. It can be observed from Figures 3(a) and 3(b) that the number of exposed and infected chickens decreases as time increases when there are interventions and increases when there are no interventions. This finding implies that the prevention measures of treatment, vaccination, and sanitation have a positive impact on reducing the spread of coccidiosis in the population.
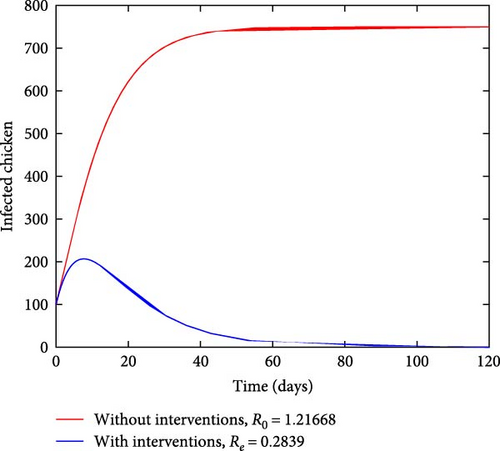
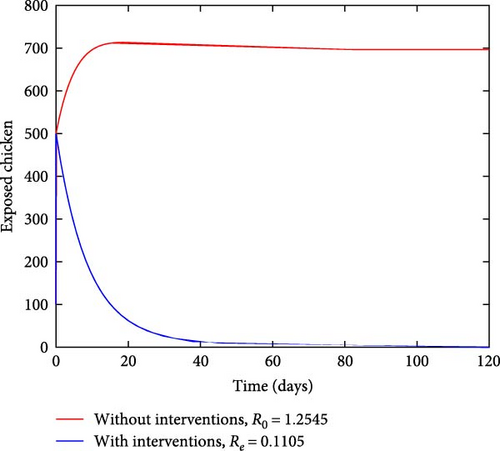
Figure 4 depicts how the number of exposed and infected chickens changes over time. It can be observed from Figures 4(a) and 4(b) that the number of exposed and infected chickens decreases as time increases when there are interventions and increases when there are no interventions. This finding implies that the prevention measures of treatment, vaccination, and sanitation have a positive impact on reducing the spread of coccidiosis in the population.
3.14. Effect of Varying Some Parameter Values
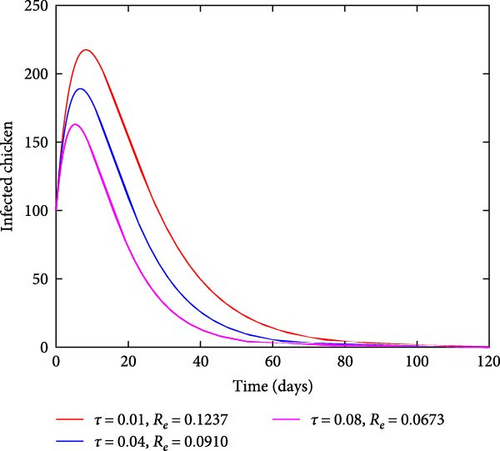
Figure 5 illustrates the effects of varying treatment rates. As the rate of treating the infected chicken increases, the number of infected chickens also reduces, as shown in Figure 5(a). On the other hand, by increasing the treatment rates, the number of recovered chickens increases with time as shown in Figure 5(b). This result indicates that treating the affected chicken has a significant effect on curbing coccidiosis as it reduces the number of sick chickens and increases the number of recovered individuals in the population.
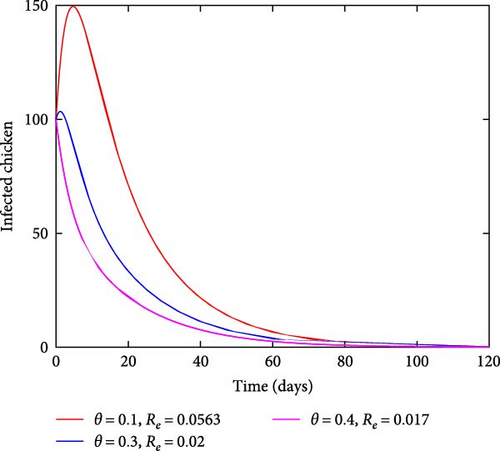
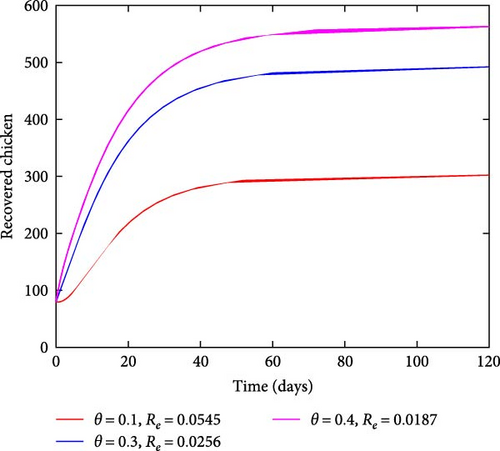
Figure 6 shows the impact of sanitation on infected chickens and Eimeria pathogen population. The findings show that when sanitation rates increase, the number of both infected chickens and pathogens decreases as time passes as shown in Figures 6(a) and 6(b). This finding demonstrated that cleanliness has a major role in the complete eradication of coccidiosis disease in the population.
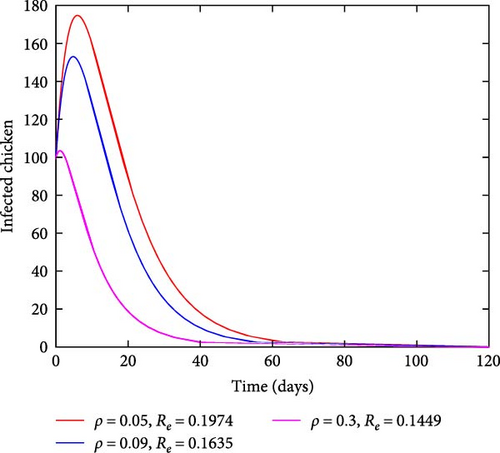
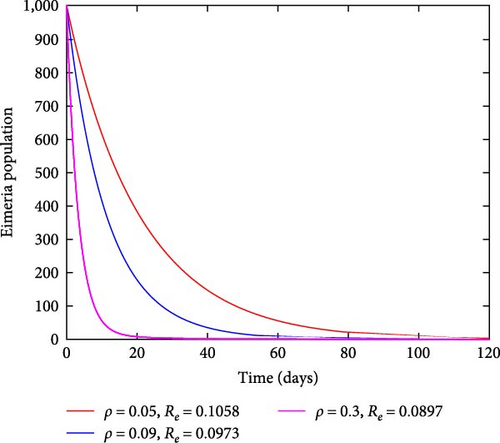
In this instance, the focus was to evaluate the contribution made by the sanitation strategy toward eradicating the coccidiosis epidemic in the community. Figure 7 findings demonstrate that when sanitation rates increase, the number of infected chickens significantly decreases. In this strategy, efforts like adequate waste disposal and hygienic practice follow-up may be practiced with the main objective of limiting the spread of this disease in the community.
4. Conclusion
A deterministic model with control interventions for chicken coccidiosis disease is derived and analyzed to investigate the effect of each control intervention for controlling this disease. The effective reproduction number and equilibria of the model are derived. The stability analysis of the DFE is derived, and it is stable when the reproduction number is less than unity. Also, the contribution of the control interventions is assessed by comparing the basic reproduction number (R0) and effective reproduction number (Re), whereby it is observed that Re < R0. This result implies that the control interventions (vaccination, sanitation, and treatment) reduce the transmission rate of the disease. On the other hand, sensitivity analysis carried out on the model parameters shows that: the control parameters (θ (treatment), ρ (sanitation), and τ (vaccination)) are the essential parameters for effective control of the disease, as they have negative indices. Numerical analysis of the model system (1) is carried out to illustrate the effectiveness of the control interventions. The results show that as the rate of the control parameters increases, the number of infected chicken and coccidiosis pathogens decreases. The results further show that applying the control interventions singly or in combination reduces the infection rate, though the combination has the most significant results. The model discussed in this paper is not exhaustive. As a result, the model’s underlying assumptions can be adjusted to include the aspect of the age structure of the chicken, the role of seasonal variations on the transmission dynamics of coccidiosis in the chicken population, or optimal and cost-effectiveness.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Y. Liana would like to appreciate the support provided by the College of Business Education, Tanzania. M. Swai is grateful for the support provided by the Open University of Tanzania.
Open Research
Data Availability
All data and materials that support the findings of this research study are included in the paper.




