Low Temperature, In Situ Polymerization of Vinyl Acetate in Silica Containing Emulsion Gels
Abstract
Vinyl acetate (VAc) was polymerized to about 90% conversion in 9 h at 40°C from the colloidal microstructure of the VAc/fumed silica/cetyltrimethylammonium bromide (CTAB) system. The glass transition (Tg) of poly(vinyl acetate) (PVAc) polymerized in these emulsion gels with silica was higher (Tg = 41°C) than those of PVAc made from bulk polymerization at 60°C (Tg = 31°C) and the weight average molar mass (Mw) was also larger (Mw about 300 kg/mol) than those from bulk polymerization (Mw = 125 kg/mol). Increased Mw, Tg, and lowered processing temperature for these composites could facilitate new applications for PVAc.
1. Introduction
Emulsion gels, composed of fumed silica, organic monomers, and surfactants, are attractive systems which can self-catalyze radical polymerizations at low temperature [1–4]. These emulsion gels can also be considered as concentrated emulsions, which form with or without silica [3]. These emulsion gels also seem to be very stable and promote the thermal decomposition of free radical initiators at low temperatures [4]. Here, “low temperatures” imply lower than those normally used for the thermally initiated free radical polymerizations. Free radical polymerization through an emulsion gel with silica is a promising approach for some vinyl monomers. Compared with the currently used methods for low temperature free radical polymerization, such as the use of ionic liquids [5], ϒ-ray irradiation [6], redox initiators [7], or metal catalysts [8], the polymerization promoted by emulsion gels is more eco-friendly and cost-effective [1].
The challenge in conducting low temperature free radical polymerizations is to facilitate initiator decomposition under mild conditions to produce radicals. 2,2′-azobis (isobutyronitrile) (AIBN) is a commonly used initiator for free radical reactions. Generally, AIBN requires elevated temperatures (about 60°C) [9, 10] to produce an adequate concentration of radicals for an in situ radical polymerization. Interestingly, low temperature decomposition of AIBN can be achieved by utilizing surfactant with or without silica [3]. The low temperature polymerization of emulsion gels containing iodine/iodide free CTAB and styrene [2] or methyl methacrylate (MMA) [1] has previously been reported. In the presence of iodine/iodide, the radical polymerization was found to be terminated [2, 11]. The degree of conversion of the emulsion gels with silica to polymers exceeded 90% in 24 h at room temperature (21°C) for styrene and in 4 h at 40°C for MMA.
Polystyrene (PS) and poly(methyl methacrylate) (PMMA) produced in emulsion gels are different from those of conventionally produced polymers in terms of their polymerization kinetics and morphologies. Especially in the presence of silica, styrene and MMA showed different polymerization kinetics due to different levels of inhibition due to silica in their emulsion gels. The silanols on fumed silica surfaces were found to act as a mild radical inhibitor, which affected polymerization kinetics [4]. The radical-inhibition effect can be minimized by covering the surface silanols with cationic surfactants, e.g., cetyltrimethylammonium bromide (CTAB) [4]. In silica-containing styrene emulsion gels, physically adsorbed styrene on silica surfaces can be easily blocked by CTAB, which covers most of the radical-inhibiting silanols on silica surfaces. In contrast, MMA can more effectively compete with CTAB for silica surface sites in MMA emulsion gels due to hydrogen bonding between MMA and silanols. As a result, polymerization conducted in styrene emulsion gels was faster than that in MMA emulsion gels [1, 2]. The resulting PS from emulsion gel polymerization with silica was of larger molar mass and smaller polydispersity compared to PS made by conventional techniques. Also, the PS/CTAB/silica composites synthesized from emulsion gels at room temperature were uniform, spherical, and monodispersed [2]. In contrast, PMMA/CTAB/silica composites were porous [1]. These observations suggest that the attributes of the polymer and the composite obtained from emulsion gel polymerization with silica are governed by the colloidal micro-structure, surface properties, and the monomer [1, 2].
Here, we report the low temperature polymerization of VAc. We also use this study to assist in a comprehensive understanding of emulsion gel polymerization of VAc. We find that the versatility of silica-containing emulsion gel polymerization of VAc can produce PVAc composites at lower reaction temperatures and with larger molar masses than normal.
2. Materials and Methods
2.1. Materials
Vinyl acetate (VAc, Sigma-Aldrich, USA) was purified by passing it through a basic alumina (Al2O3, Sisco Chemical Laboratories, India) column. 2,2′-azobis(isobutyronitrile) (AIBN, Paras Polymer and Chemicals, India) was purified by recrystallization from methanol. The purified samples were refrigerated while maintaining darkness to prevent photo-initiation. Cetyltrimethylammonium bromide (CTAB, Sigma H6269, USA) was used without further purification. The polymerization of styrene using Sigma H6269 CTAB was faster than H5882 CTAB which contained iodine/iodide impurities at different levels [2]. CAB-O-Sil M-5P hydrophilic fumed silica with the specific area 200 m2/g (Cabot Corporation, Tuscola, IL) was dried for 12 h at 300°C to minimize the moisture content and stored in a desiccator prior to use.
2.2. Preparation of Composites
For each sample, 2.00 mL of purified VAc and 0.04 g of AIBN were introduced into vials containing 0.105 g of fumed silica. The mixtures were stirred in a vortex mixer for 1 min. Aqueous CTAB solution, 0.80 mL, with a concentration of 0.2 M, was then introduced into each vial. Following 1 min of vortex mixing, the polymerization was continued at room temperature (30°C). About 90% conversion was observed in 240 h at room temperature. This extended time for the polymerization was inconvenient. Hence, to minimize this polymerization time, the reaction was conducted without stirring at 40°C in an oil bath. Sample vials were taken out hourly and the sample dried under ambient conditions for 24 h and then under vacuum for 24 h to remove water and unreacted monomers. The conversion efficiencies were calculated by dividing the mass of the polymer by the initial mass of monomer. About 90% conversion of VAc was observed in 9 h, at 40°C, which is about 1/30 of the time taken for room temperature polymerization in emulsion gels. Therefore, 40°C was considered as the desirable low temperature for silica-containing emulsion gel polymerization of VAc. To determine the effect of fumed silica and CTAB for the polymerization, a few control experiments as shown in Table 1 were also carried out at 40°C.
| Sample no. | Sample designation | Mass of fumed silica/g | Volume of VAc/mL | Mass of AIBN/g | Volume of CTAB/mL (CTAB concentration/M) | Sample appearance (after mixing) | Polymerization temperature/°C | Monomer conversion/% (polymerized time/h, temperature/°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | VAc/water | — | 2.00 | 0.04 | 0.80 (0.00) | Liquid-like | 40 | 0 (4, 40) |
| 2 | VAc/CTAB0.20 | — | 2.00 | 0.04 | 0.80 (0.20) | Liquid-like | 40, 60 |
|
| 3 | VAc/silica | 0.105 | 2.00 | 0.04 | — | Translucent, gel-like | 40, 60 |
|
| 4 | VAc/silica/water | 0.105 | 2.00 | 0.04 | 0.80 (0.00) | Opaque, gel-like | 40 | 0 (4, 40) |
| 5 | PVAc/silica/CTAB0.10 | 0.105 | 2.00 | 0.04 | 0.80 (0.10) | Opaque, white gel | 40 | 0 (5, 40) |
| 6 | PVAc/silica/CTAB0.20 | 0.105 | 2.00 | 0.04 | 0.80 (0.20) | Opaque, white gel | 30, 40, 60 |
|
| 7 | PVAc/silica/CTAB0.30 | 0.105 | 2.00 | 0.04 | 0.80 (0.30) | Opaque, white gel | 40 | 40 (5, 40) |
Similar control experiments were conducted for some samples (samples 2, 3, and 6) in Table 1, and for VAc/AIBN at 60°C, to study the effect of fumed silica and CTAB at 60°C, at which AIBN readily dissociates [9, 10]. These PVAc samples containing fumed silica and surfactant are referred to here as composites. The sample designation is used here indicated the processed temperature in parenthesis (e.g., PVAc/silica/CTAB0.20 (60°C)).
2.3. Characterization of Samples
A small amount of each composite was mixed well with toluene for 7 h and centrifuged for 20 min. The silica-free upper layer was poured into distilled water to precipitate the PVAc. The extracted polymers were washed several times with distilled water and dried under ambient conditions for 24 h and then under vacuum for 24 h.
Scanning electron micrographs of the gold sputtered composite samples were taken by a Carl Ziess EVO 18-research operated at an acceleration voltage of 10 kV. Molecular masses and thermal properties of the precipitated polymers were analyzed. Thermogravimetric analysis was done (TGA) using the TA Instruments SDT Q600 with a heating rate of 10°C/min from ambient to 800°C in N2 atmosphere (100 mL/min). Temperature-modulated differential scanning calorimetry (TMDSC) measurements were taken with a TA Instruments Model 2920 MDSC from −40°C to 120°C with a heating rate of 3°C/min and modulation amplitude of ±1.0°C for a period of 60 s under nitrogen gas flow (50 mL/min). Molecular masses were determined using gel permeation chromatography (GPC) in THF with a flow rate 0.5 mL/min and with Optilab DSP Interferometric Refractometer detector (Wyatt Technologies, Santa Barbara, CA).
3. Results and Discussion
3.1. Gel Stability and Polymerization
The VAc containing samples listed in Table 1 were all physically different before heating at 40°C. Samples 1 and 2, which did not contain fumed silica, were not gels; they were liquid-like. Samples 3 and 4, consisting of fumed silica, VAc, with or without water, were gel-like. Sample 3, made only with VAc and silica, was a translucent gel-like mixture due to the similarity of refractive indices of silica (1.46) [2] and VAc (1.40) [12]. Sample 4, containing VAc/silica/water, was an opaque gel-like mixture because VAc is slightly water soluble and acts as a co-surfactant to stabilize some water molecules in the sample. Sample 5−7 were the mixtures of VAc/silica/water with different concentrations of CTAB. These VAc mixtures became white when CTAB was added. VAc mixtures with 0.10 and 0.20 M CTAB concentrations were gel-like emulsions. The viscosity of the gel was high with 0.30 M CTAB. The formation of a gel in silica-organic solvent system is due to hydrogen bonding between silica particles which form fumed silica networks within the dispersed oil phases [2, 13]. The order of mixing is also important for the gelation [2]. In addition, the concentration of CTAB to form a stable VAc emulsion gel, which was 0.20 M, was smaller than that observed for styrene emulsion gels (2.0 M) [2], but closer to that for MMA emulsion gels (0.25 M) [1]. This observation suggested that the stability of emulsion gel with polar monomers can be sustained at smaller concentrations of CTAB, compared with non-polar monomers.
At 40°C, the decomposition of AIBN was very slow, virtually non-existent, without surfactant [3]. As a result, the samples 1, 3, and 4 did not show any polymerization at 40°C within 4 h, as CTAB was absent in those samples. Although CTAB was present in sample 2, the polymerization was still not observed at 40°C after 4 h. It is likely due to the lower reactivity of VAc compared with that of MMA where polymerization can be conducted at 40°C [1]. Interestingly, by adding 0.105 g fumed silica to VAc emulsion gels, the conversions of VAc for 0.1, 0.2, and 0.3 M CTAB samples were 0, 73, and 40%, respectively, in 5 h. It has been seen previously that the conversion is dependent on the surfactant amount [3]. The rates of polymerization (Rp) were found to be 0, 656 ± 33, and 375 ± 19 mmolL−1 h−1 for the emulsion gels containing 0.1, 0.2, and 0.3 M CTAB, respectively. It has been shown that fumed silica acts as a promoter for surfactant catalysis in emulsion gels, but silica alone does not catalyze AIBN dissociation [3]. In the presence of silica, the rate of polymerization for the emulsion gels containing 0.2 M CTAB (86%) was comparable with the one without silica (83%) at 60°C, where significant thermal decomposition of AIBN occurs in the absence of surfactant [9, 10]. Therefore, the catalytic effect of surfactant was not particularly impactful at 60°C. The polymerization was clearly enhanced as a result of the synergistic activity of fumed silica and surfactant molecules in emulsion gels [1, 2] at 40°C.
The observed decomposition of AIBN at lower temperatures is due to the formation of initiator-surfactant complexes [3]. The decomposition rate constant of AIBN and the rate of polymerization depend on the concentration of CTAB [3]. The surfactant concentration of 0.1 M CTAB is not only too small to catalyze the initiator decomposition at 40°C but also insufficient to block surface silanols on fumed silica [4]. The unblocked silanols on fumed silica reduced the rate of polymerization by inhibiting free radicals [4, 14, 15]. With increasing concentrations of CTAB more AIBN-CTAB complexes formed generating more radicals. In addition, more surface silanols are covered by CTAB minimizing surface inhibition. Both effects lead to faster polymerization rates in emulsion gels [4].
It has previously been shown that more than 90% conversion to polymer was achieved for styrene in 60 h3, and for MMA in 228 h, at room temperature [1]. This is consistent with the notion that in emulsion gels with silica, non-polar monomers polymerized faster than polar monomers at room temperature. Polar monomers, such as VAc and MMA, can adsorb onto the silica surface through hydrogen bonding, which can compete with CTAB for surface silica sites [1, 2, 4]. As a result, unblocked silanol groups on silica surfaces are more likely to inhibit polymerization of VAc or MMA. Styrene does not interact strongly with silica surfaces (e.g., silanols); therefore, CTAB covered the silica surfaces to prevent inhibition. In fact, it was previously observed that styrene-containing emulsion gels, with similar sample compositions to VAc, did not show an initial inhibition, at room temperature [3].
The decomposition of AIBN, at 40°C, was reported to be negligible in the AIBN initiated surfactant-free bulk polymerization [16]. However, both VAc and MMA polymerized at 40°C in the presence of fumed silica and CTAB. This suggests that surfactant catalyzed AIBN initiated polymerization at near ambient temperatures in the presence of fumed silica. Here, we report about 90% conversion through emulsion gels for VAc in 9 h compared to 90% conversion in 4 h for MMA at 40°C [1]. Compared to VAc, the higher reactivity of MMA monomers [17, 18] may be the reason for faster polymerization time with MMA in emulsion gels. The lower reactivity of VAc monomers [17–19] was confirmed the absence of polymerization in VAc emulsion gels without silica, when polymerization was observed in MMA samples with similar composition at 40°C in 4 h.
The polymerization rates of VAc in VAc/silica/AIBN, VAc/silica/CTAB/AIBN, VAc/CTAB/AIBN, and VAc/AIBN sample mixtures at 60°C were affected by surface inhibition. AIBN readily decomposes at 60°C [9, 10]. The VAc/silica/AIBN mixture showed the smallest conversion (66%) in 4 h, compared to other VAc samples. This is consistent with the notion that in the absence of CTAB, the initiator radicals produced in VAc/silica/AIBN were inhibited by the surface silanols of fumed silica [4]. This observation further confirmed that CTAB in silica-containing emulsion gels has blocked the surface inhibition sites. At 60°C, 90% conversion was reached within 5 h in emulsion gels with silica. At 40 and 60°C, the rates of polymerization were found to be 656 ± 33 and 630 ± 31 mmolL−1 h−1, respectively, for the silica containing emulsion gels (Figure S1, Supporting Information). Without CTAB, no significant amount of polymerization of VAc was observed below 60°C.
As evident by the control experiments at 40 and 60°C, the rates of monomer conversion were larger in the emulsion gels with silica compared to the samples without silica. This is due to large decomposition rate of AIBN in the presence of silica and CTAB. In emulsion gels with silica, room temperature decomposition rate constant of AIBN was found to be larger compared to emulsion gels without silica [20]. Therefore, at 40 and 60°C also, that of AIBN is expected to be larger in the presence of fumed silica and CTAB.
3.2. Composite Characterization
The PVAc/silica/CTAB0.20 composites polymerized at 30, 40, and 60°C were selected for SEM characterization, and the morphologies of those composites are shown in Figure 1. The morphologies of PVAc/silica/CTAB0.20 composites were reaction temperature dependent. At 30°C, PVAc/silica/CTAB showed a flake-like structure. Dense clusters were observed at 40°C, which could be due to encapsulated silica with PVAc chains and surfactant molecules. The colloids were destabilized in PVAc/silica/CTAB0.20 (60°C) composite.


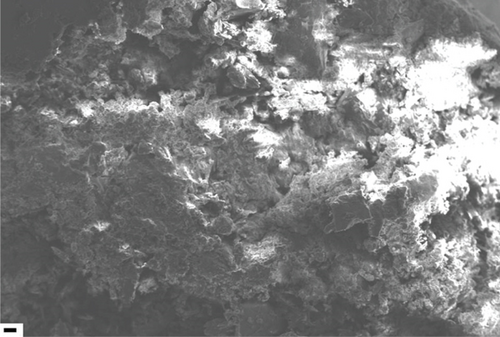
For comparison, the morphologies of PVAc/silica/CTAB and PMMA/silica/CTAB [1] composites obtained at 40°C were different, although both monomers are polar. PVAc composites showed clusters while the PMMA composites showed a porous structure with micron size holes, which formed due to continuous water regions in PMMA composite [1].
3.3. Thermal Analysis
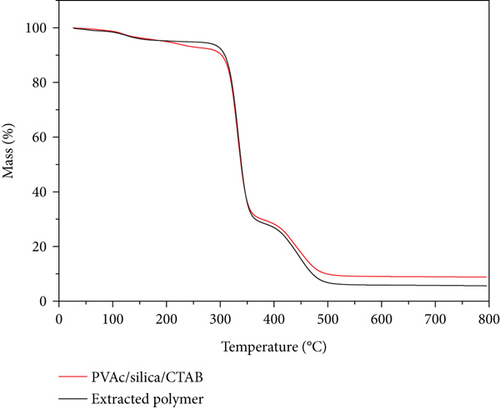
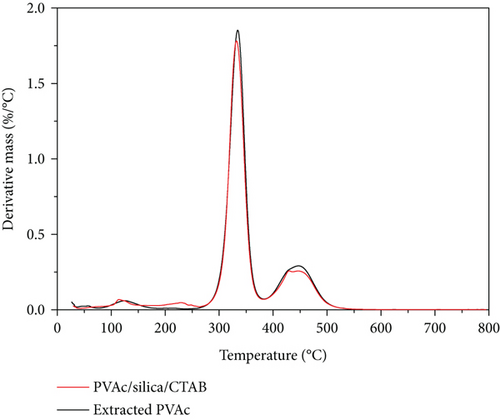
The TGA thermograms and derivative mass loss curves of PVAc/silica/CTAB0.20 (40°C) composite and its extracted PVAc are shown in Figure 2. The dried samples, assuming complete conversion (based on the original composition with less water), were estimated to contain 90% PVAc, 5% CTAB, and 5% fumed silica. The mass losses for PVAc were very close to those expected from the original compositions in the composite polymerized at 40°C. The mass loss near 100°C was due to the removal of water. PVAc showed two-step degradation. Pyrolysis of PVAc is a radical process [21], where the deacetylation occurs at first from the polymer side chain [22]. At the end of deacetylation, polymer backbone scission occurs [21, 23]. Extracted PVAc should decompose with 100% mass loss. However, a small amount of silica was observed in the extracted PVAc. PVAc chains H-bond onto silica surfaces [24, 25], and some of the silica particles may be not removed during the extraction as seen in the thermogram. Once the PVAc chains decomposed, the mass leveled off at higher temperatures, at an amount corresponding to the amount of silica in the sample. As seen in the derivative mass loss curves, the deacetylation of PVAc samples occurred at 335°C, while the backbone scission occurred at 448°C.
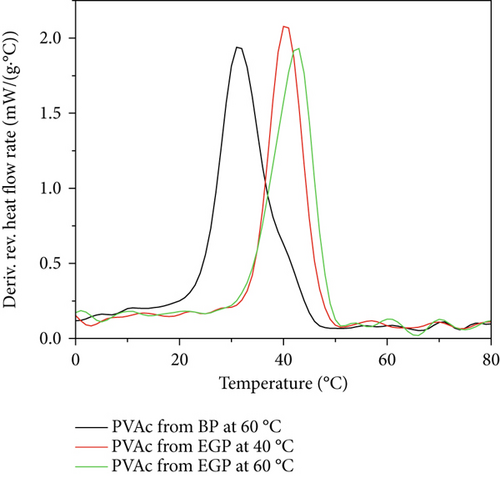
The TMDSC thermograms of extracted PVAc from PVAc/silica/CTAB0.20 (40°C), PVAc/silica/CTAB0.20 (60°C), and bulk polymerized VAc at 60°C are shown in Figure 3, and the Tg values of the extracted PVAc are shown in Table 2. Higher Tg values were observed for PVAc made from emulsion gel polymerization with silica than PVAc made from bulk polymerization. The extracted PVAc from PVAc/silica/CTAB0.20 showed Tg values slightly above 40°C, while PVAc from bulk polymerization at 60°C showed a Tg at 31°C. PVAc with increased Tg formed in silica-containing emulsion gels is not likely due to the presence of silica in extracted PVAc (as evident by TGA thermogram). This amount of silica is very small, about 5%. Previous studies have shown the dependence of PVAc Tg’s on the adsorbed amount of polymer [25]. Based on the amount of silica in the extracted sample, the corresponding adsorbed amount would be 95 mg PVAc/1 m2 silica. At compositions of 3 mg/m2, there was very little contribution from the silica bound and the Tg of “loosely-bound” only increased by less than 3°C. Our sample has about 30 times more polymer in it, so the effects from this small amount of silica will be negligible in the observed Tg [25]. Therefore, this increased Tg might be due to larger molar mass PVAc obtained in emulsion gels with silica, compared to bulk polymerization.
| Sample | Silica (g) | VAc (mL) | Aqueous CTAB solution (mL) (CTAB concentration/M) | Monomer conversion/% (polymerized time/h, temperature/°C) | Mw (kg/mol) | Polydispersity | Tg of extracted PVAc (°C) |
|---|---|---|---|---|---|---|---|
| PVAc/silica/CTAB0.20 (40°C) | 0.105 | 2.00 | 0.80 (0.20) | 90 (9, 40) | 364 | 2.6 | 41 |
| PVAc/silica/CTAB0.20 (60°C) | 0.105 | 2.00 | 0.80 (0.20) | 86 (4, 60) | 310 | 2.4 | 43 |
| PVAc/AIBN (60°C) | 0 | 2.00 | 0 | 84 (4, 60) | 125 | 1.6 | 31 |
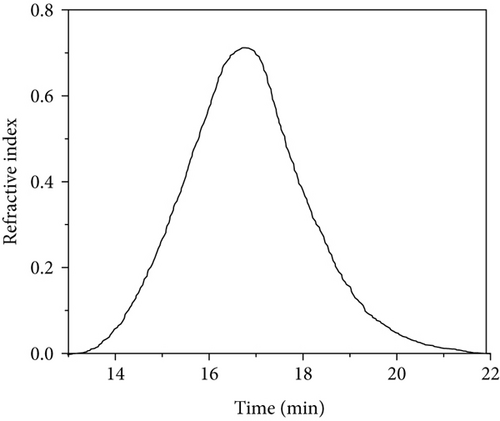
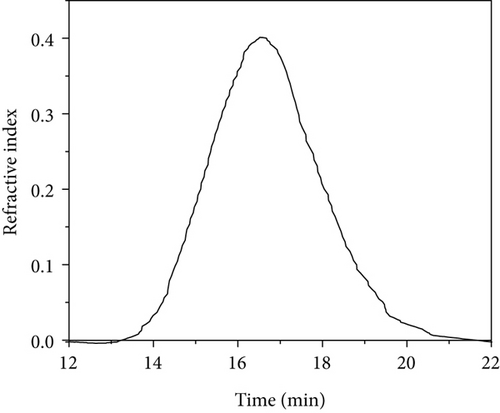
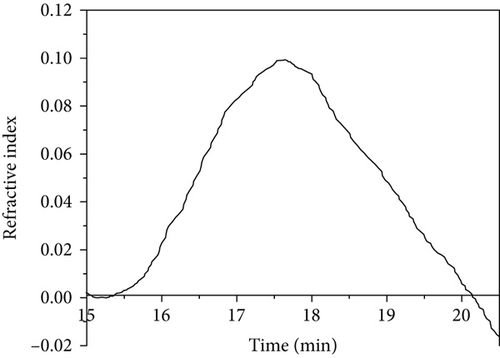
3.4. Molar Mass of the Polymers
The weight average molar masses and polydispersities of PVAc samples are shown in Table 2. GPC traces of PVAc samples are shown in Figure 4. PVAc obtained at 40°C showed a larger molar mass (364 kg/mol) than that at 60°C (310 kg/mol) with slightly different polydispersities. Bulk polymerization of VAc at 60°C showed significantly a smaller Mw (125 kg/mol) than emulsion gel polymerized samples. The results revealed that the obtained Tg values are comparable to the molar masses obtained where PVAc obtained from silica-containing emulsion gels showed higher Tg and higher molar mass values compared to the PVAc obtained from bulk polymerization. PVAc chain radicals undergo chain transfer to monomer [26]. In bulk, chain transfer to monomer reactions may be more pronounced than in emulsion gels resulting in higher molar mass PVAc in emulsion gels with silica.

Here, we report the polymerization of VAc at a considerably lower temperature with high molar mass and an elevated Tg. The PVAc/silica/CTAB composites are amphiphilic and can absorb both water and organic solvents for in situ encapsulation/immobilization of temperature sensitive molecules, such as proteins and enzymes, into the composite particles without damaging their bioactivities [2]. PVAc/silica/CTAB composite can be an effective substrate to prepare materials for immobilization of biomolecules at lower temperatures.
4. Conclusion
In this paper, we have reported an approach to polymerize VAc at a lower temperature, 40°C than normally required for AIBN initiation, via VAc/silica/CTAB/water/AIBN mixture. About 90% conversion was achieved at 40°C, in 9 h for VAc, and these PVAc/silica/CTAB composite were clustered as shown in SEM. Higher molar mass PVAc with elevated Tg were formed in the emulsion gels with silica compared to bulk polymerization. MMA also has been polymerized in emulsion gels with silica in 4 h, at 40°C [1], and PMMA/silica/CTAB composite was porous. The findings of this study provide a broad view on the properties of PVAc and the colloidal microstructures prepared at different temperatures.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Nazreen Zavahiar and Madhubhashini Maddumaarachchi acknowledge the financial support given by grants ASP/01/RE/SCI/2017/08 and ASP/01/RE/SCI/2021/06 from University of Sri Jayewardenepura, Sri Lanka, and Frank D. Blum acknowledges the Harrison I. Bartlett endowment. The authors also thank Mr. Ishan N. Jayalath from Oklahoma State University, USA, for assisting with GPC and DSC measurements.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.




