Effect of CCR2-V64I on the Susceptibility of Patients to Cancer
Abstract
Background. Results from the studies investigating the impact of CC chemokine receptor 2 (CCR2) polymorphism on the risk of cancers are diverse. An updated meta-analysis was conducted to access the relationship between cancer risk and CCR2-V64I polymorphism. Methods. We performed a meta-analysis using STATA 11.0 based on a comprehensive retrieval in WanFang Data, PubMed, China National Knowledge Infrastructure, EMBASE, and Web of Science databases up to January 20, 2023. Results. We included 23 studies in our analysis. Overall, we found CCR2-V64I polymorphism was remarkably related to cancer risk (OR = 1.39, 95% CI = 1.14–1.70, and P = 0.001 for A vs G; OR = 1.87, 95% CI = 1.30–2.70, and P = 0.001 for AA vs GG; OR = 1.35, 95% CI = 1.03–1.78, and P = 0.032 for GA vs GG; OR = 1.45, 95% CI = 1.11–1.90, and P = 0.006 for AA + GA vs GG; OR = 1.69, 95% CI = 1.20–2.37, and P = 0.003 for AA vs GA + GG). In the ethnicity subgroup analysis, the relevancy between CCR2-V64I polymorphism and an increased cancer risk was discovered among Asians (OR = 1.57, 95% CI = 1.30–1.91, and P < 0.001 for A vs G; OR = 2.30, 95% CI = 1.64–3.24, and P < 0.001 for AA vs GG; OR = 1.35, 95% CI = 1.10–1.67, and P = 0.005 for GA vs GG; OR = 1.52, 95% CI = 1.25–1.87, and P < 0.001 for AA + GA vs GG; OR = 2.21, 95% CI = 1.58–3.08, and P < 0.001 for AA vs GA + GG). In addition, the subgroup analysis in the light of cancer types demonstrated that CCR2-V64I polymorphism was strongly correlated with bladder cancer (OR = 3.04, 95% CI = 1.09–8.45, and P = 0.033 for AA vs GG; OR = 2.84, 95% CI = 1.07–7.09, and P = 0.035 for AA vs GA + GG) and oral cancer (OR = 1.83, 95% CI = 1.39-2.42, and P < 0.001 for A vs G; OR = 2.04, 95% CI = 1.47–2.85, and P < 0.001 for GA vs GG; OR = 2.03, 95% CI = 1.48–2.79, and P < 0.001 for AA + GA vs GG). Conclusion. The meta-analysis suggested that CCR2-V64I polymorphism might be a high-risk factor for cancers among Asians, especially for bladder and oral cancers.
1. Introduction
Chemokines are chemotactic cytokines generated by activated natural immunocytes, which could regulate the migration of immunocytes by interacting with chemokine receptors on the cell surface [1, 2]. Chemokines are known as an essential inflammatory response mediator [2]. Inflammation is crucial in the pathogenesis of cancers. CC chemokine ligand 2 (CCL2) is an important member of the CC chemokine family [3]. CCR2 is a key receptor for CCL2 and is related to carcinogenesis and angiogenesis [4, 5]. Carcinogenesis is an intricate process containing tumorigenesis, growth, and metastasis [6]. The binding of CCL2 and CCR2 facilitates tumor cell migration and attracts immunosuppressive cells into the cancer microenvironment, which accelerates the progression of tumors [7]. It was reported that CCL2-CCR2 signaling could recruit myeloid cells to stimulate an angiogenic switch [8, 9]. Moreover, the interplay between the vascular endothelial growth factor (VEGF) generated by tumor-associated macrophages (TAMs) and CCR2+ vascular endothelial cells could facilitate cancer angiogenesis [7]. As one of the extensively studied receptors, CCR2-V64I (rs1799864) is at codon 64 of CCR2.
Polymorphism refers to two or more discontinuous variations of genes that occur simultaneously or frequently in a certain biological population. In recent years, extensive research has been carried out on the single nucleotide polymorphism (SNP) of rs1799864 encoding isoleucine (ATC) in the place of valine (GTC). The rs1799864 polymorphism plays various roles in the progression of cancers. The effect of rs1799864 polymorphism in the risk of cancers has been widely researched by many studies, including cervical cancers, gastric cancers, bladder cancers, prostate cancers, and prostate cancers [10–17]. Nevertheless, these studies presented limited information owing to relatively small sample sizes and were unable to provide a consistent result. Two published meta-analyses have studied the correlation between the rs1799864 polymorphism and cancers in 2013. However, there were several articles studying the relationship after 2013 [13–22]. Therefore, we conducted an undated meta-analysis to study the effect of rs1799864 polymorphism on the development of cancers.
2. Materials and Methods
2.1. Paper Search and Selection
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [23] are applied in the study. Our meta-analysis carried out a comprehensive search in WanFang Data, PubMed, China National Knowledge Infrastructure, EMBASE, and Web of Science databases up to January 20, 2023. Search strategy was performed by the following words combination: “CC chemokine receptor 2,” or “CCR2,” or “rs1799864,” AND “polymorphism,” or “variation,” or “mutation,” or “SNP,” AND “cancer,” or “tumor,” or “carcinoma,” or “malignancy,” or “neoplasm.”
2.2. Inclusion and Exclusion Criteria
Literature that met the following criteria was enrolled: (1) the article was case-controlled, (2) the article was related to the correlation between the rs1799864 polymorphism and cancers, and (3) data presented by the article were enough to evaluate the relationship. Study that satisfied one of the following criteria was not included: (1) the type of article belongs to review, editorial, commentary, nonhuman study, or case report, (2) papers provided duplicated data were excluded, and (3) full text was unavailable.
2.3. Data Collection and Quality Evaluation
Two researchers fetched the following data independently from every eligible article in the light of the inclusion and exclusion criteria presented previously: the primary author’s name, publication year, country where study was researched, ethnicity, type of cancer, case groups and control groups counts, genotype distributions, genotyping methods, the control group source, and P value of Hardy–Weinberg equilibrium (HWE) in controls.
2.4. False-Positive Report Probability (FPRP) Analysis
We applied FPRP analysis [24] to evaluate the remarkable results. 0.5 was set as the cutoff value of the FPRP and performed the FPRP analysis at a prior probability level of 0.1 and an odds ratio (OR) of 1.5. Only a remarkable result with a FPRP value less than 0.5 was regarded as “noteworthy.”
2.5. Data Synthesis
P < 0.05 was regarded as significant in all statistical tests. STATA 11.0 was applied to obtain the pooled ORs and corresponding 95% confidence intervals (95% CIs). The pooled ORs along with 95% CIs were obtained for the allelic, homozygous, heterozygous, dominant, and recessive genetic models to assess the strength of the correlation between the rs1799864 polymorphism and cancers. The subgroup analyses were conducted in the light of ethnicity, cancer type, and source of controls to confirm whether these factors were related to the overall ORs. Cochran’s Q test and I2 statistics were applied in heterogeneity assessment. If the P > 0.10 and I2 < 50%, the fixed-effects model (the Mantel–Haenszel method) was performed for analysis. Otherwise, the random-effects model (the DerSimonian and Laird method) was used. Sensitivity analysis was carried out by omitting a single literature every time to assess the stability of the study. Begg’s test and Egger’s linear regression were implemented to discuss the publication bias.
3. Results
3.1. Included Papers and Paper Features
WanFang Data, PubMed, China National Knowledge Infrastructure, EMBASE, and Web of Science databases were retrieved to search relevant papers. Twenty-three studies [13–22, 25–37] involving 5344 cases and 6673 controls were ultimately included in the study. The flow of article selection is presented in Figure 1.
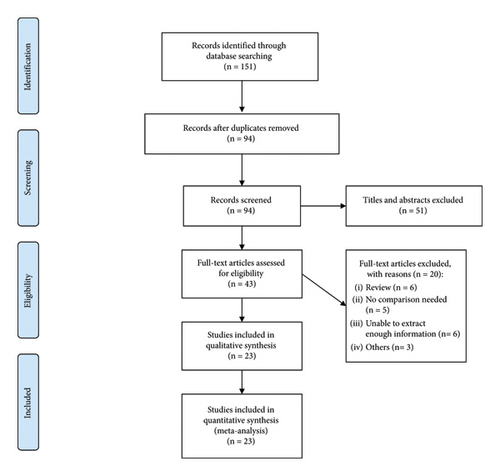
Among the 23 articles enrolled in our meta-analysis, 2 were about breast cancer [19, 25], 5 were about bladder cancer [13, 25, 28, 32, 37], 1 were about skin cancer [25], 3 were about cervical cancer [16, 26, 31], 1 was about gastric cancer [27], 1 was about endometrial cancer [29], 1 was about nonhodgkin lymphoma [30], 1 was about hepatocellular carcinoma [33], 2 were about oral cancer [34, 35], 3 were about prostate cancer [15, 17, 36], 1 was about renal cell carcinoma [14], 1 was about nonsmall cell lung cancer [18], 1 was about lung cancer [20], 1 was about colorectal cancer [21], and 1 was about ovarian cancer [22]. The article, which was researched by Zafiropoulos in 2004, studied three types of tumors, including breast cancer, bladder cancer, and skin cancer [25]. All articles demonstrated that the genotype distributions of controls were in compliance with HWE, except 3 articles [14, 16, 31]. The features of the enrolled articles are demonstrated in Table 1.
| Authors (year) | Country | Ethnicity | Cancer type | Case/control | Cases | Controls | Genotyping method | Source of controls | HWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | GG | GA | AA | ||||||||
| Zafiropoulos (2004) | Greece | European | BCa | 264/211 | 221 | 38 | 5 | 154 | 50 | 7 | PCR-RFLP | PB | 0.251 |
| Zafiropoulos (2004) | Greece | European | BCb | 68/148 | 51 | 16 | 1 | 115 | 33 | 0 | PCR-RFLP | PB | 0.127 |
| Zafiropoulos (2004) | Greece | European | SC | 110/362 | 74 | 32 | 4 | 271 | 87 | 4 | PCR-RFLP | PB | 0.303 |
| Ivansson (2007) | Sweden | European | CC | 1294/286 | 1054 | 228 | 12 | 217 | 61 | 8 | PCR | HB | 0.153 |
| Liou (2008) | Taiwan | Asian | GC | 177/217 | 109 | 59 | 9 | 138 | 71 | 8 | PCR-RFLP | HB | 0.760 |
| Vázquez-Lavista (2009) | Mexico | Others | BCb | 47/126 | 28 | 19 | 0 | 74 | 43 | 9 | PCR-RFLP | PB | 0.432 |
| Attar (2010) | Turkey | Asian | EC | 50/211 | 34 | 9 | 7 | 153 | 50 | 8 | PCR-RFLP | HB | 0.139 |
| Bracci (2010) | USA | American | NHL | 475/744 | 391 | 81 | 3 | 610 | 131 | 3 | PCR | PB | 0.147 |
| Chatterjee (2010) | South Africa | African | CC | 106/305 | 24 | 81 | 1 | 189 | 112 | 4 | PCR-SSP | HB | 0.005 |
| Chatterjee (2010) | South Africa | Mixed | CC | 340/1073 | 78 | 255 | 7 | 704 | 356 | 13 | PCR-SSP | HB | <0.001 |
| Narter (2010) | Turkey | Asian | BCb | 72/76 | 39 | 23 | 10 | 59 | 15 | 2 | PCR-RFLP | PB | 0.394 |
| Yeh (2010) | Taiwan | Asian | HCC | 102/344 | 66 | 31 | 5 | 276 | 61 | 7 | PCR-RFLP | HB | 0.106 |
| Chen (2011) | Taiwan | Asian | OCa | 216/344 | 142 | 67 | 7 | 276 | 61 | 7 | PCR-RFLP | HB | 0.106 |
| Bektas-Kayhan (2012) | Turkey | Asian | OCa | 129/140 | 88 | 35 | 6 | 112 | 24 | 4 | PCR-RFLP | HB | 0.07 |
| Kucukgergin (2012) | Turkey | Asian | PC | 156/152 | 101 | 44 | 11 | 120 | 30 | 2 | PCR-RFLP | HB | 0.936 |
| Singh (2012) | India | Asian | BCb | 200/200 | 128 | 62 | 10 | 126 | 70 | 4 | PCR-RFLP | HB | 0.104 |
| Kucukgergin (2012) | Turkey | Asian | BCb | 142/197 | 97 | 37 | 8 | 159 | 35 | 3 | PCR-RFLP | HB | 0.508 |
| Liu (2013) | China | Asian | RCC | 416/458 | 240 | 103 | 73 | 313 | 110 | 35 | PCR-RFLP | HB | <0.001 |
| Zambra (2013) | Brazil | Others | PC | 135/118 | 107 | 26 | 2 | 88 | 27 | 3 | PCR-RFLP | HB | 0.596 |
| Ding (2013) | China | Asian | CC | 40/60 | 11 | 5 | 24 | 23 | 19 | 18 | PCR-SSP | HB | 0.005 |
| Mandal (2015) | India | Asian | PC | 195/250 | 113 | 75 | 7 | 137 | 98 | 15 | PCR-RFLP | HB | 0.646 |
| Rafrafi (2015) | Tunisia | Others | NSCLC | 170/225 | 105 | 52 | 13 | 169 | 52 | 4 | PCR-RFLP | PB | 1.000 |
| Banin-Hirata (2016) | Brazil | Others | BCa | 118/180 | 91 | 25 | 2 | 140 | 37 | 3 | PCR-RFLP | NA | 0.760 |
| Bagci (2016) | Turkey | Asian | LC | 65/57 | 47 | 16 | 2 | 39 | 16 | 2 | PCR-RFLP | NA | 0.822 |
| Walczak (2017) | Poland | Others | CRC | 214/144 | 157 | 55 | 2 | 108 | 34 | 2 | PCR-RFLP | PB | 0.712 |
| Yildirim (2017) | Turkey | Asian | OCb | 43/45 | 19 | 21 | 3 | 27 | 15 | 3 | PCR | HB | 0.647 |
- BCa: breast cancer; BCb: bladder cancer; SC: skin cancer; CC: cervical cancer; GC: gastric cancer; EC: endometrial cancer; NHL: non-hodgkin lymphoma; HCC: hepatocellular carcinoma; OCa: oral cancer; PC: prostate cancer; RCC: renal cell carcinoma; NSCLC: non-small cell lung cancer; LC: lung cancer; CRC: colorectal cancer; OCb: ovarian cancer; NA: not available; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; PCR: polymerase chain reaction; PCR-SSP: polymerase chain reaction-sequence-specific primers; PB: population-based study; HB: hospital-based study.
3.2. Meta-Analysis Results
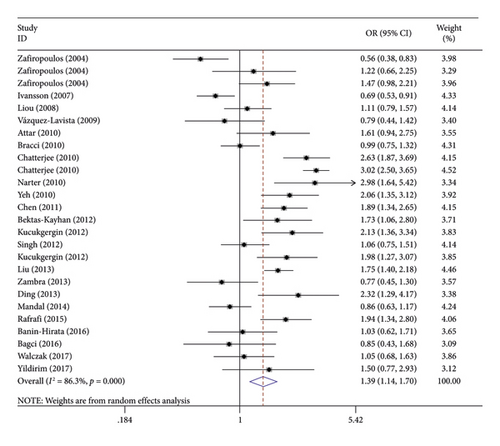
We discovered that the rs1799864 polymorphism was significantly related to the increased risk of cancers in all the studied models (Figure 2, OR = 1.39, 95% CI = 1.14–1.70, and P = 0.001 for A vs G; OR = 1.87, 95% CI = 1.30–2.70, and P = 0.001 for AA vs GG; OR = 1.35, 95% CI = 1.03–1.78, and P = 0.032 for GA vs GG; OR = 1.45, 95% CI = 1.11–1.90, and P = 0.006 for AA + GA vs GG; OR = 1.69, 95% CI = 1.20–2.37, and P = 0.003 for AA vs GA + GG; Table 2).
| No | A versus G | AA versus GG | GA versus GG | AA + GA versus GG | AA versus GA + GG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P(Z) | OR | (95% CI) | P(z) | OR | (95% CI) | P(z) | OR | (95% CI) | P(z) | OR | (95% CI) | P(z) | ||
| Overall | 26 | 1.39 | 1.14–1.70 | 0.001 | 1.87 | 1.30–2.70 | 0.001 | 1.35 | 1.03–1.78 | 0.032 | 1.45 | 1.11–1.90 | 0.006 | 1.69 | 1.20–2.37 | 0.003 |
| European | 4 | 0.89 | 0.57–1.38 | 0.603 | 0.98 | 0.26–3.71 | 0.982 | 0.86 | 0.58–1.26 | 0.436 | 0.87 | 0.56–1.34 | 0.522 | 0.99 | 0.28–3.48 | 0.990 |
| Asian | 14 | 1.57 | 1.30–1.91 | <0.001 | 2.30 | 1.64–3.24 | <0.001 | 1.35 | 1.10–1.67 | 0.005 | 1.52 | 1.25–1.87 | <0.001 | 2.21 | 1.58–3.08 | <0.001 |
| BCa | 2 | 0.75 | 0.41–1.36 | 0.336 | 0.62 | 0.23–1.64 | 0.332 | 0.73 | 0.38–1.41 | 0.343 | 0.72 | 0.37–1.41 | 0.341 | 0.67 | 0.25–1.78 | 0.421 |
| BCb | 5 | 1.42 | 0.93–2.18 | 0.107 | 3.04 | 1.09–8.45 | 0.033 | 1.29 | 0.91–1.83 | 0.160 | 1.41 | 0.93–2.13 | 0.109 | 2.84 | 1.07–7.49 | 0.035 |
| CC | 4 | 1.88 | 0.86–4.10 | 0.112 | 1.66 | 0.40–6.82 | 0.481 | 2.10 | 0.57–7.76 | 0.268 | 2.56 | 0.71–9.20 | 0.150 | 1.15 | 0.35–3.82 | 0.817 |
| OCa | 2 | 1.83 | 1.39–2.42 | <0.001 | 1.93 | 0.85–4.40 | 0.118 | 2.04 | 1.47–2.85 | <0.001 | 2.03 | 1.48–2.79 | <0.001 | 1.63 | 0.72–3.70 | 0.242 |
| PC | 3 | 1.12 | 0.61–2.06 | 0.717 | 1.23 | 0.25–6.08 | 0.801 | 1.08 | 0.70–1.69 | 0.722 | 1.11 | 0.62–1.98 | 0.718 | 1.20 | 0.27–5.29 | 0.812 |
| PB | 8 | 1.20 | 0.86–1.68 | 0.291 | 1.87 | 0.75–4.67 | 0.177 | 1.13 | 0.85–1.50 | 0.396 | 1.19 | 0.85–1.66 | 0.304 | 1.80 | 0.78–4.16 | 0.171 |
| HB | 16 | 1.55 | 1.20–2.01 | 0.001 | 1.95 | 1.27–2.99 | 0.002 | 1.52 | 1.02–2.25 | 0.038 | 1.68 | 1.16–2.43 | 0.006 | 1.70 | 1.14–2.54 | 0.009 |
- BCa: breast cancer; BCb: bladder cancer; CC: cervical cancer; OCa: oral cancer; PC: prostate cancer; PB: population-based study; HB: hospital-based study. The bold values given indicate that a significant relationship was discovered in the overall analyses and subgroup analyses.
In the ethnicity subgroup analysis, the results indicated that there was a remarkable correlation between the rs1799864 polymorphism and cancers among Asians (Table 2; OR = 1.57, 95% CI = 1.30–1.91, and P < 0.001 for A vs G; OR = 2.30, 95% CI = 1.64–3.24, and P < 0.001 for AA vs GG; OR = 1.35, 95% CI = 1.10–1.67, and P = 0.005 for GA vs GG; OR = 1.52, 95% CI = 1.25–1.87, and P < 0.001 for AA + GA vs GG; OR = 2.21, 95% CI = 1.58–3.08, and P < 0.001 for AA vs GA + GG) but not Europeans. In addition, the subgroup analyses were performed in the light of the kind of cancer and source of control groups. In the results of subgroup analyses presented in Table 2, a significant relationship was found in the bladder cancer subgroup (Table 2; OR = 3.04, 95% CI = 1.09–8.45, and P = 0.033 for AA vs GG; OR = 2.84, 95% CI = 1.07–7.09, and P = 0.035 for AA vs GA + GG), the oral cancer subgroup (Table 2; OR = 1.83, 95% CI = 1.39–2.42, and P < 0.001 for A vs G; OR = 2.04, 95% CI = 1.47–2.85, and P < 0.001 for GA vs GG; OR = 2.03, 95% CI = 1.48–2.79, and P < 0.001 for AA + GA vs GG), and the hospital-based (HB) control subgroup (Table 2; OR = 1.55, 95% CI = 1.20–2.01, and P = 0.001 for A vs G; OR = 1.95, 95% CI = 1.27–2.99, and P = 0.002 for AA vs GG; OR = 1.52, 95% CI = 1.02–2.25, and P = 0.038 for GA vs GG; OR = 1.68, 95% CI = 1.16–2.43, and P = 0.006 for AA + GA vs GG; OR = 1.70, 95% CI = 1.14–2.54, and P = 0.009 for AA vs GA + GG).
3.3. Sensitivity Analyses and Publication Bias
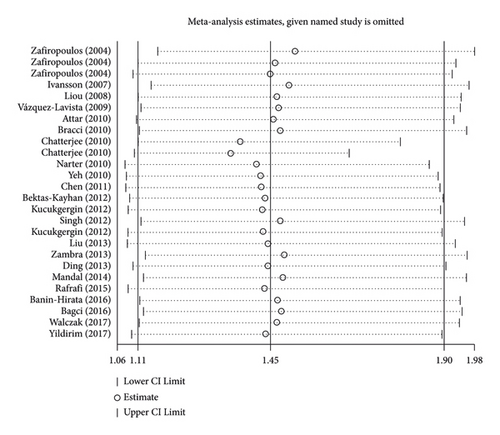
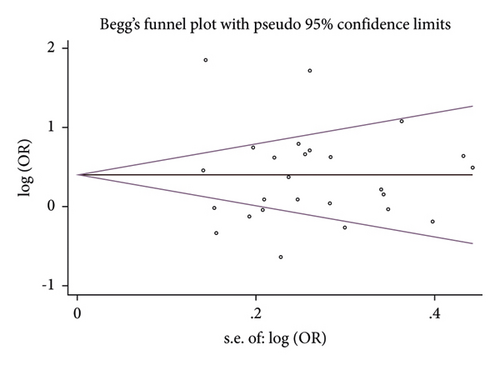
After excluding each article in turn, no material alteration was discovered in the combined ORs in the sensitivity analyses (Figure 3, AA + GA vs GG of rs1799864). Furthermore, no conspicuous publication bias was detected by the P value in the Egger test (allelic model: P = 0.219; homozygous model: P = 0.467; heterozygous model: P = 0.401; dominant model: P = 0.649; and recessive model: P = 0.309) and the almost symmetrical shape of Begg’s funnel plot (P > 0.05 under all the studied models; Figure 4, dominant model of rs1799864) for rs1799864 polymorphism.
3.4. FPRP Test Results
Furthermore, the FPRP tests were performed to investigate the remarkable relationships discovered in our meta-analysis. As demonstrated in Table 3, the FPRP values were mostly less than 0.50 in the remarkable findings, indicating that these remarkable correlations were “noteworthy” except the bladder cancer subgroup (Table 3; homozygous model: FPRP = 0.772 and recessive model: FPRP = 0.761).
| Variables | OR (95% CI) | Pa | Powerb | Prior probability | ||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.1 | 0.01 | 0.001 | 0.0001 | ||||
| A versus G | ||||||||
| Overall | 1.39 (1.14–1.70) | 0.001346 | 0.771 | 0.005 | 0.015 | 0.147 | 0.636 | 0.946 |
| Asian | 1.57 (1.30–1.91) | 0.000098 | 0.347 | 0.001 | 0.003 | 0.027 | 0.220 | 0.739 |
| HB | 1.55 (1.20–2.01) | 0.000949 | 0.402 | 0.007 | 0.021 | 0.189 | 0.702 | 0.959 |
| OCa | 1.83 (1.39–2.42) | 0.000023 | 0.082 | 0.001 | 0.002 | 0.027 | 0.216 | 0.734 |
| AA versus GG | ||||||||
| Overall | 1.87 (1.30–2.70) | 0.000838 | 0.120 | 0.021 | 0.059 | 0.409 | 0.875 | 0.986 |
| Asian | 2.30 (1.64–3.24) | 0.000002 | 0.007 | 0.001 | 0.002 | 0.025 | 0.207 | 0.724 |
| HB | 1.95 (1.27–2.99) | 0.002197 | 0.114 | 0.054 | 0.147 | 0.655 | 0.950 | 0.995 |
| BCb | 3.04 (1.09–8.45) | 0.033033 | 0.088 | 0.530 | 0.772 | 0.974 | 0.997 | 1.000 |
| GA versus GG | ||||||||
| Overall | 1.35 (1.03–1.78) | 0.033399 | 0.772 | 0.115 | 0.280 | 0.811 | 0.977 | 0.998 |
| Asian | 1.35 (1.10–1.67) | 0.005689 | 0.834 | 0.020 | 0.058 | 0.403 | 0.872 | 0.986 |
| HB | 1.52 (1.02–2.25) | 0.036405 | 0.474 | 0.187 | 0.409 | 0.884 | 0.987 | 0.999 |
| OCa | 2.04 (1.47–2.85) | 0.000029 | 0.036 | 0.002 | 0.007 | 0.075 | 0.450 | 0.891 |
| AA + GA versus GG | ||||||||
| Overall | 1.45 (1.11–1.90) | 0.007052 | 0.597 | 0.034 | 0.096 | 0.539 | 0.922 | 0.992 |
| Asian | 1.52 (1.251.87) | 0.000075 | 0.450 | <0.001 | 0.001 | 0.016 | 0.142 | 0.625 |
| HB | 1.68 (1.16–2.43) | 0.005871 | 0.274 | 0.060 | 0.162 | 0.680 | 0.955 | 0.995 |
| OCa | 2.03 (1.48–2.79) | 0.000013 | 0.031 | 0.001 | 0.004 | 0.039 | 0.291 | 0.804 |
| AA versus GA + GG | ||||||||
| Overall | 1.69 (1.20–2.37) | 0.002355 | 0.245 | 0.028 | 0.080 | 0.488 | 0.906 | 0.990 |
| Asian | 2.21 (1.58–3.08) | 0.000003 | 0.011 | 0.001 | 0.002 | 0.025 | 0.204 | 0.719 |
| HB | 1.70 (1.14–2.54) | 0.009594 | 0.271 | 0.096 | 0.242 | 0.778 | 0.973 | 0.997 |
| BCb | 2.84 (1.07–7.49) | 0.034889 | 0.098 | 0.515 | 0.761 | 0.972 | 0.997 | 1.000 |
- aChi-square test was adopted to calculate the genotype frequency distributions. bStatistical power was calculated using the number of observations in the subgroup and the OR and P values in this table. HB: hospital-based study; OCa: oral cancer; BCb: bladder cancer.
4. Discussion
CCL2 and CCR2 could be generated by various cells in the cancer environment, especially by the tumor cells. The combination of CCL2 and CCR2 is closely linked with the pathological angiogenesis, the growth of cancers, and the concentration of immunosuppressive cells. Besides, CCL2-CCR2 axis could facilitate the differentiation of mononuclear cells into metastasis-associated macrophages (MAMs), advancing the colonization and survival of metastatic cancer cells [7]. Recently, many research studies demonstrate that CCR2-V64I (rs1799864) is related to cancers [13–22, 25–39].
Up to now, the results produced by many articles focusing on the correlation between the rs1799864 polymorphism and the cancers are controversial. This may be related to the limitations of these articles, including small sample sizes, different ethnic groups, different control group sources, and different genotyping methods. Meta-analysis, as a useful method, could overcome these limitations to a certain extent and supply a more robust conclusion than any one study. Therefore, we conducted a meta-analysis included in 23 studies to study the role of rs1799864 polymorphism in the development of cancers. Our study reveals that the rs1799864 polymorphism is remarkably related to the increased risk of cancers.
In the subgroup analyses according to ethnicity, a statistically significant relationship between the rs1799864 polymorphism and cancers under all the studied genetic models was discovered in Asians but not in Europeans. The discovery indicates that the polymorphism might be related to an increased risk of cancers among Asians. This may be because the genetic characteristics are various in different ethnicities and people from different ethnicities have different genetic susceptibilities and living habits.
In the stratified analysis based on tumor type, the results demonstrated that the rs1799864 polymorphism could increase the risk of bladder and oral cancers. The possible reasons are as follows: first, this might be associated with the different microenvironments exposed by different tumor sites. Second, the biological activity of CCR2 receptor is altered by rs1799864 polymorphism, which might increase the risk of bladder and oral cancers. Moreover, the rs1799864 polymorphism would influence the gene half-life and expression level, which might result in the development of bladder and oral cancers. In the stratified analysis in the light of source of controls, a remarkable association was found under all the five studied genetic models in HB group but not in population-based (PB) group. The reason is unclear. We assume HB controls are more likely to develop carcinomas than PBs.
As far as we know, there have been two published meta-analyses [38, 39] studying the correlation between the polymorphism and cancers. Compared with these two articles, our study has many differences and highlights. First, 26 case-control studies from 23 enrolled papers were included in the updated meta-analysis, which contained several newly published articles because these two previous meta-analyses were conducted in 2013. Studies and samples in our article are much more than those in these two previous articles, suggesting that our results of the correlation between the rs1799864 polymorphism and cancers might be relatively more accurate. Second, the subgroup analyses were implemented by ethnicity, genotyping method, source of controls, and cancer types to research the potential origins of heterogeneity and to evaluate the study stability. Third, our meta-analysis included allele, homozygous, heterozygous, dominant, and recessive models. However, a related meta-analysis published previously written by Cho and Kim [38] assessed the association only under dominant genetic model and the other related meta-analysis written by Huang et al. [39] assessed the association only under homozygous, heterozygous, dominant, and recessive models. This may be due to the lack of relevant information in these two meta-analyses. In addition, the meta-analysis studied by Cho and Kim [38] found that there was no significant correlation between the rs1799864 polymorphism and cancers. On the contrary, we discovered the rs1799864 polymorphism was significantly correlated with cancers. This might be because our study contains more data and samples. Finally, we conducted the FPRP test, which suggested that the majority of remarkable results in our meta-analysis are robust. Therefore, to some degree, our present findings might be more comprehensive and precise.
There are still some limitations in our analysis. First, the cancer occurrence is usually thought to involve the latent interactions of gene-gene and gene-environment. Due to insufficient data, our study could not assess the interactions. Second, other related cancer risk factors such as age, gender, tobacco, alcohol, physical activity, and emotional state were not evaluated because of the lack of relevant data. In addition, publication bias could exist because negative results are more difficult to be published than the positive results. Finally, the FPRP test results demonstrate that the remarkable correlations of the bladder cancer subgroup analysis are not “noteworthy,” which might be because the enrolled articles associated with the bladder cancer are limited. The FPRP analysis result could indicate that the significant correlation between the rs1799864 polymorphism and bladder cancer requires more researches to verify. So, further studies with more data would be needed to observe the role of rs1799864 polymorphism in cancers.
5. Conclusion
In conclusion, our study discovered that the rs1799864 polymorphism was significantly related to an increased risk of cancers among Asians. Moreover, the polymorphism could increase the susceptibility of bladder and oral cancers. However, more relevant high-quality research studies with larger sample sizes concentrating on ethnicity or tumor type should be performed to verify our conclusions.
Conflicts of Interest
The authors declare that there are no conflicts of interest.