Adverse Reaction Signals Mining of Vonoprazan: A Pharmacovigilance Study Based on FAERS
Abstract
Background. Vonoprazan is a novel selective and noncompetitive oxidative-reduction state proton pump inhibitor (PPI), known as a potassium-competitive acid blocker (P-CAB). Due to its novelty, the clinical application of vonoprazan is relatively limited, and its long-term safety and adverse effects still need to be further studied and confirmed. Methods. We used the data from the FDA adverse eventreporting system (FAERS) from 2015 to 2022. Disproportionality method including calculation of the reporting odds ratio (ROR) and calculation of the information component (IC) was used to detect potential adverse drug reactions (ADRs). In preferred terms, the significant signals are ranked in the following order: Plateletcrit increased (ROR 1447.3 IC 36.62), benign duodenal neoplasm (ROR 11157.84 IC 36.40), gallbladder volvulus (ROR 964.77 IC 36.20), myopathy endocrine (ROR 723.58 IC 35.88), pernio-like erythema (ROR 723.58 IC 35.88), septic coagulopathy (ROR 723.58 IC 35.88), and so on. In SOCs, the significant signals are ranked in the following order: hepatobiliary disorders (ROR 5.65 IC 29.15), metabolism and nutrition disorders (ROR 2.78 IC 28.12), blood and lymphatic system disorders (ROR 2.73 IC 28.12), investigations (ROR 2.19 IC 27.76), endocrine disorders (ROR 2.09 IC27.76), gastrointestinal disorders (ROR 1.87 IC 27.52), and so on. Conclusion. Despite its potential advantages, there is still limited clinical experience with vonoprazan, and the long-term safety and adverse effects of this drug are not fully understood. Further studies are needed to confirm its safety and efficacy in a larger patient population and to establish its role in the management of acid-related disorders. In the meantime, careful monitoring and patient education are recommended for those who are prescribed vonoprazan.
1. Introduction
Vonoprazan is a novel selective and noncompetitive oxidative-reduction state proton pump inhibitor (PPI), known as a potassium-competitive acid blocker (P-CAB) [1]. The metabolism of vonoprazan predominantly occurs through cytochrome P450 (CYP) 3A4, with additional contributions from CYP2B6, CYP2C19, CYP2D6, and SULT2A1, resulting in the formation of inactive metabolites [2]. Compared to traditional PPIs, vonoprazan has a faster onset of action, longer duration of action, and stronger acid-suppressive effect [3, 4]. As a potassium-competitive acid blocker, vonoprazan acts on a different site of the proton pump than traditional PPIs, making it a promising option for patients who are resistant or partially responsive to traditional PPI therapy. In addition, its fast onset and long duration of action may provide more effective relief for patients with gastroesophageal reflux disease (GERD) or other acid-related disorders. However, it just has been available in the market in Japan since February 2015 [1], so due to its novelty, the clinical application of vonoprazan is relatively limited, and its long-term safety and adverse effects still need to be further studied and confirmed.
The FDA adverse event reporting system (FAERS) is a database that contains information on adverse events and medication errors that have been reported to the US Food and Drug Administration (FDA). The database was created to support the FDA’s postmarketing safety surveillance program for all approved drug and therapeutic biologic products. FAERS collects adverse event reports from a variety of sources, including physician, pharmacist, consumer and health-professional. The reports contain information on the patient, the drug or biologic product involved, and the adverse event or medication error. The database also includes information on the outcome of the event, such as hospitalization or death. FAERS is an important tool for identifying potential safety issues with drugs and biologic products. The FDA uses the database to monitor the safety of products after they have been approved for use and to take appropriate regulatory action if necessary. Researchers and healthcare professionals can also use the database to conduct safety analyses and identify potential drug interactions or adverse events.
In this study, we conducted a pharmacovigilance analysis of vonoprazan using the FAERS data from 2015 to 2022, with the objective of offering guidance to clinicians and patients on the appropriate usage of vonoprazan.
2. Materials and Methods
The data for this study were the FAERS, conducted by the U.S. Food and Drug Administration (FDA), which aimed to surveil the safety of drug and therapeutic biologic products after postmarketing. This system collected demographic information, drug/biologic information, adverse event, outcomes for the event, and others are necessary for surveillance. All details of data are available on the FAERS website (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers).
In the period encompassing from 2015 to 2022, a total of 12,851,637 instances were documented, consisting of adverse event reports regarding vonoprazan, as reported by consumers and healthcare professionals. First, to prevent duplication of adverse event reports, as a single adverse event can be reported in multiple reports including follow-up reports for individual cases, we removed duplicate data based on priority rules, specifically, caseid < fda_dt < primaryid. Also, we deleted some special cases according to the text file that lists deleted files since 2019 quarter one for various reasons including combining cases. Second, in the drug file, we searched some terms in the list of drugname and prod_ai for vonoprazan such as “Vonoprazan,” “Vonoprazan fumarate,” “Takecab,” and so on. We have collected 3732 cases of adverse reactions related to the use of vonoprazan as a treatment approach. Third, we merged the three files of drug, demo, reac via primaryid. Finally, we got 2595 cases including 10,453 reports of vonoprazan adverse events (AEs). The reported data are standardized and classified using the preferred terms (PTs) and system organ classes (SOCs) from the Medical Dictionary for Regularly Activities (MedDRAs) [5]. The overall workflow of this study is shown in Figure 1.

2.1. Statistical Analysis
We used disproportionality method including calculation of the reporting odds ratio (ROR) and calculation of the information component (IC) to detect potential ADRs. 1 ADR signal was prompted when the evaluation criteria were met. The ROR approach is based on the assumption that the reporting of AEs follows a two-by-two contingency table. The IC method is based on the assumption that the use of a drug and the occurrence of an adverse event are independent. When a target drug is more likely to induce a target AE than all other drugs, it will typically get a higher score due to a higher disproportionality. The disproportionality method is based on the following two-by-two contingency table (Table 1), and the formulas for ROR and IC are listed as follows (Table 2). All analyses were conducted using R (https://www.R-project.org; version 4.2.2).
| Adverse event of interest | Other adverse event of interest | Total | |
|---|---|---|---|
| Drug of interest | a | b | (a + b) |
| Other drugs of interest | c | d | (c + d) |
| Total | (a + c) | (b + d) | (a + b + c + d) |
| Method | Formula | Criteria |
|---|---|---|
| ROR | ROR = (a/b)/(c/d) | a ≥ 3 |
| 95% CI = | 95% CI > 1 | |
| IC | IC = log2(a × (a + b + c + d))/((a + b)/(a + c)) | a ≥ 3 |
| IC025 = | IC025 > 0 | |
3. Ethical Statements
The data for this study were obtained solely from published FAERS data, eliminating the need for collecting raw data. As a result, Ethics Committee approval was deemed unnecessary. The data sources used in this study were previously approved by the local Ethics Committee and adhered to local laws. In addition, all participants provided informed consent by signing the necessary documentation.
4. Results
4.1. Basic Information of AE Report
Ultimately, we got 2595 AE reports of vonoprazan. The basic information of AE reports are shown in Table 3. There are 1392 patients with ARE who were men, accounting for 53.64%. The proportion of old patients (age ≥ 65 year) was highest in all groups. The number of annual reports has increased every year since 2015, with a maximum of 1077 cases in 2022. Most reported persons were medical professionals. 2538 of 2595 cases reported 4140 AE outcomes including 1639 (39.59%) hospitalization and 1543 (37.27%) other serious important medical events.
| n | (%) | |
|---|---|---|
| Gender | ||
| Male | 1392 | 53.64 |
| Female | 1028 | 39.61 |
| Missing | 175 | 6.74 |
| Total | 2595 | 100.00 |
| Age | ||
| <18 | 12 | 0.46 |
| 18–64 | 745 | 28.71 |
| ≥65 | 1592 | 61.35 |
| Missing | 246 | 9.48 |
| Total | 2595 | 100.00 |
| Reporting year | ||
| 2022 | 1077 | 41.50 |
| 2021 | 506 | 19.50 |
| 2020 | 217 | 8.36 |
| 2019 | 343 | 13.22 |
| 2018 | 247 | 9.52 |
| 2017 | 159 | 6.13 |
| 2016 | 45 | 1.73 |
| 2015 | 1 | 0.04 |
| Total | 2595 | 100.00 |
| Reported person | ||
| Physician | 1656 | 63.82 |
| Pharmacist | 418 | 16.11 |
| Consumer | 214 | 8.25 |
| Health-professional | 191 | 7.36 |
| Other health-professional | 93 | 3.58 |
| Missing | 23 | 0.89 |
| Total | 2595 | 100.00 |
| Outcome | ||
| Hospitalization | 1639 | 39.59 |
| Other serious important medical events | 1543 | 37.27 |
| Death | 558 | 13.48 |
| Life-threatening | 301 | 7.27 |
| Disability | 99 | 2.39 |
| Total | 4140 | 100.00 |
4.2. Adverse Reaction Frequency Analysis
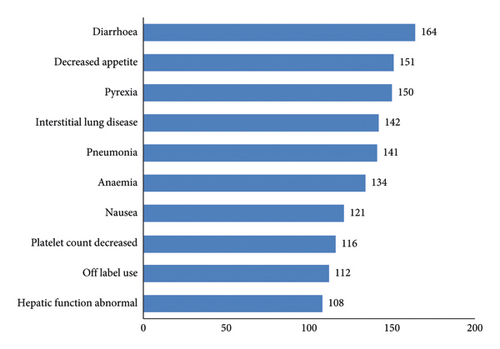
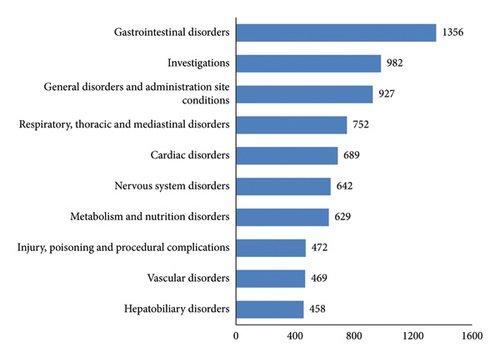
Of the 2,595 reports, 10,453 AEs were reported. The events that occur with a relatively high frequency are diarrhea, decreased appetite, pyrexia, interstitial lung disease, and pneumonia. AEs involved a total of 27 SOCs such as gastrointestinal disorders, nervous system disorders, vascular disorders, hepatobiliary disorders, and others (see Figures 2 and 3 for details).
4.3. PTs and SOCs Disproportionality Analysis
We performed disproportionality analysis both in PTs and SOCs. In PTs, the significant signals are ranked in the following order: plateletcrit increased (ROR 1447.3 IC 36.62), benign duodenal neoplasm (ROR 11157.84 IC 36.40), gallbladder volvulus (ROR 964.77 IC 36.20), myopathy endocrine (ROR 723.58 IC 35.88), pernio-like erythema (ROR 723.58 IC 35.88), septic coagulopathy (ROR 723.58 IC 35.88), and so on. In SOCs, the significant signals are ranked in the following order: hepatobiliary disorders (ROR 5.65 IC 29.15), metabolism and nutrition disorders (ROR 2.78 IC 28.12), blood and lymphatic system disorders (ROR 2.73 IC 28.12), investigations (ROR 2.19 IC 27.76), endocrine disorders (ROR 2.09 IC27.76), gastrointestinal disorders (ROR 1.87 IC 27.52), cardiac disorders (ROR 1.76 IC 27.47), renal and urinary disorders (ROR 1.73 IC 27.47), immune system disorders (ROR 1.47 IC 27.24), and respiratory, thoracic, and mediastinal disorders (ROR 1.25 IC 27.00) (see details in Tables 4 and 5), and we compared PTs with the adverse reactions in the drug package insert. Under the premise of removing PT according to the indication from professiona, adverse reactions not mentioned in the package insert were marked with an asterisk ( ∗).
| PT | ROR (95% CI) | IC (IC025) |
|---|---|---|
| Plateletcrit increased | 1447.3 (265.05–7902.79) | 36.62 (6.71) |
| Benign duodenal neoplasm ∗ | 1157.84 (224.6–5968.64) | 36.40 (7.06) |
| Gallbladder volvulus ∗ | 964.77 (100.34–9276.00) | 36.20 (3.77) |
| Myopathy endocrine ∗ | 723.58 (80.86–6474.59) | 35.88 (4.01) |
| Pernio-like erythema | 723.58 (80.86–6474.59) | 35.88 (4.01) |
| Septic coagulopathy ∗ | 723.58 (80.86–6474.59) | 35.88 (4.01) |
| Hypergastrinaemia ∗ | 579.47 (311.69–1077.31) | 35.62 (19.16) |
| Tertiary adrenal insufficiency ∗ | 482.39 (58.07–4007.30) | 35.40 (4.26) |
| Luteinising hormone deficiency ∗ | 413.47 (50.87–3361.05) | 35.20 (4.33) |
| Helicobacter sepsis ∗ | 361.79 (45.24–2892.99) | 35.03 (4.38) |
| Hypoferritinaemia ∗ | 361.79 (45.24–2892.99) | 35.03 (4.38) |
| Acrokeratosis paraneoplastica ∗ | 263.12 (33.97–2038.26) | 34.62 (4.47) |
| Infected vasculitis ∗ | 263.12 (33.97–2038.26) | 34.62 (4.47) |
| Antibiotic-associated colitis ∗ | 241.22 (57.00–1020.80) | 34.50 (8.15) |
| HER2 positive gastric cancer ∗ | 231.57 (54.84–977.79) | 34.45 (8.16) |
| Meningitis eosinophilic ∗ | 222.64 (29.12–1702.13) | 34.4 (04.50) |
| Pulmonary artery wall hypertrophy ∗ | 222.64 (29.12–1702.13) | 34.40 (4.50) |
| Atypical mycobacterial lymphadenitis ∗ | 206.74 (27.18–1572.35) | 34.30 (4.51) |
| Rathke’s cleft cyst ∗ | 203.17 (73.70–560.04) | 34.27 (12.43) |
| Duodenal polyp ∗ | 195.51 (106.14–360.14) | 34.22 (18.58) |
| SOC | ROR (95% CI) | IC (IC025) |
|---|---|---|
| Hepatobiliary disorders | 5.65 (5.14–6.2) | 29.15 (26.54) |
| Metabolism and nutrition disorders | 2.78 (2.57–3.02) | 28.12 (25.94) |
| Blood and lymphatic system disorders | 2.73 (2.48–3.01) | 28.12 (25.53) |
| Investigations | 2.19 (2.05–2.34) | 27.76 (26.00) |
| Endocrine disorders | 2.09 (1.79–2.45) | 27.76 (23.76) |
| Gastrointestinal disorders | 1.87 (1.77–1.98) | 27.52 (25.99) |
| Cardiac disorders | 1.76 (1.63–1.90) | 27.47 (25.43) |
| Renal and urinary disorders | 1.73 (1.57–1.91) | 27.47 (24.91) |
| Immune system disorders | 1.47 (1.34–1.62) | 27.24 (24.73) |
| Respiratory, thoracic and mediastinal disorders | 1.25 (1.16–1.35) | 27.00 (25.07) |
5. Discussion
Vonoprazan is a recently approved competitive potassium-competitive acid blocker (P-CAB) that works by competitively blocking the potassium-binding site of H+, K+-ATPase. Compared to traditional organic proton pump inhibitors (PPIs), vonoprazan has the advantages of fast onset, long-lasting acid suppression, minimal individual variation in acid suppression, and low susceptibility to the effects of diet. However, due to its novelty, there is a lack of clinical data, so this study discusses the potential adverse reactions of vonoprazan by mining the FAERS database for AE reports from 2015 to 2022.
5.1. Basic Information of AE Report
In the table of basic information of AE report, the male populace and the overall number of individuals exceed their female counterparts. This may be attributed to the fact that vonoprazan’s primary indication is for the treatment of gastroesophageal reflux disease, which is more prevalent among men and the elderly [6].
As time progresses, with vonoprazan gradually entering the market and more individuals utilizing it, the number of AEs has increased yearly. The majority of such reports are provided by healthcare professionals, thereby ensuring the reliability of the data. The main outcome of vonoprazan-induced adverse reactions is hospitalization.
5.2. Adverse Reaction Frequency Analysis
In the adverse reaction frequency analysis, the majority of reported cases pertain to the documented adverse effects in the medication’s package insert. For instance, diarrhoea, decreased appetite, pyrexia, and nausea fall under the category of gastrointestinal disorders; anaemia and platelet count decreased are classified as blood and lymphatic system disorders; and hepatic function abnormal is grouped under hepatobiliary disorders. This suggests that gastrointestinal reactions and blood and lymphatic system disorders, as well as hepatobiliary disorders, are among the more commonly observed adverse reactions associated with vonoprazan.
5.3. PTs and SOCs Disproportionality Analyses
The new PT signal we have excavated has been sorted according to ROR. Both plateletcrit increased and pernio-like erythema have been recorded in the drug manual’s adverse reactions section. In addition, the SOC signals we have discovered, namely, hepatobiliary disorders, blood and lymphatic system disorders, and gastrointestinal disorders, have also been mentioned in the manual, reminding us to be mindful of potential risks.
In the excavated signals, benign duodenal neoplasm (ROR1157.84 IC36.40), hypergastrinaemia (ROR579.47 IC 35.62), and duodenal polyp (ROR 195.51 IC34.22) are noteworthy. Currently, it is known that during conventional PPI therapy, the level of gastrin increases [7]. Therefore, it is necessary to consider the increased risk of elevated gastrin levels and gastric mucosal hyperplasia with vonoprazan, which is greater than with conventional PPIs [8]. Research studies have confirmed that PPI-related hypergastrinemia in mice may promote gastric cancer through gastrin receptors and PPI increases the risks of polyp [9, 10]. To our knowledge, there has been a reported case of central depressed-type gastric adenocarcinoma discovered during maintenance therapy for reflux esophagitis with P-CAB, as well as several reports of benign gastric polyps caused by vonoprazan, which gradually disappear after discontinuation [11–13]. In a study published in BMC Gastroenterology [14], the administration of vonoprazan led to an elevation in serum gastrin levels. The histopathological scrutiny of gastric mucosa revealed that hyperplasia of parietal, foveolar, and G cells was more prevalent with vonoprazan compared to lansoprazole during the 156th week of the maintenance phase. Nevertheless, we lack sufficient data concerning long-term adverse events, such as the risk of neuroendocrine tumors or gastric cancer resulting from heightened gastrin levels during vonoprazan therapy. The association between oral P-CABs and cancer development also remains ambiguous. However, the signal extraction from the FAERS database through this study implies that we ought to exercise prudence in relation to the risk of hypergastrinemia and its associated polyps and gastrointestinal malignancies that may be instigated by vonoprazan.
Vonoprazan may share a mechanism with other PPIs that reduces iron absorption, potentially causing hypoferritinemia [15]. PPIs such as vonoprazan act by blocking the proton pump that produces gastric acid in the stomach [15–18]. While this is beneficial for conditions such as GERD, it can also decrease the absorption of certain minerals and nutrients, including iron. Iron is best absorbed in an acidic environment, so when gastric acid production is reduced, iron absorption may also decrease. As a result, individuals taking vonoprazan or other PPIs who cannot obtain sufficient iron from their diet or supplements may be at an increased risk of developing hypoferritinemia and increased plateletcrit.
Although vonoprazan itself does not directly cause antibiotic-associated colitis, evidence suggests that the use of proton pump inhibitors (PPIs) such as vonoprazan may increase the risk of developing this disease. Antibiotic-associated colitis is a type of colonic inflammation caused by overgrowth of Clostridium difficile (C. difficile) following antibiotic treatment [19]. Antibiotics disrupt the normal balance of bacteria in the gut, allowing for overgrowth of C. difficile and subsequent infection. PPIs such as vonoprazan can alter the normal gut microbiota, which may increase the risk of developing C. difficile infection [20]. In addition, PPIs can lower the acidity of the stomach, which may increase the survival rate of C. difficile in the stomach and intestines [21]. Several studies have found an increased risk of C. difficile-associated diarrhea or colitis in patients taking PPIs (including vonoprazan) [20–22]. This risk is further increased if the patient is also taking antibiotics while using vonoprazan, which may lead to dysbiosis of the gut microbiome. There is limited information on the potential relationship between vonoprazan and some of the listed conditions such as myopathy endocrine, acrokeratosis paraneoplastica, infected vasculitis, meningitis eosinophilic, pulmonary artery wall hypertrophy, atypical mycobacterial lymphadenitis, and Rathke’s cleft cyst.
In light of the aforementioned, during the administration of vonoprazan, it is imperative to exercise vigilance regarding the potential occurrence of associated adverse reactions. Regularly engaging in gastroscopic examinations as well as relevant diagnostic procedures is paramount in order to forestall the manifestation of severe adverse effects.
5.4. Limitation
Several limitations are inherent in this study: the FAERS database relies solely on voluntary reporting, and as such, not all adverse events may be reported. This means that the database may not accurately reflect the true incidence of adverse events. In addition, the FAERS database does not take into account confounding factors that may impact the incidence of adverse events, such as patient demographics, comorbidities, and concomitant medications. As a result, it is essential to exercise caution when using FAERS and to evaluate potential ADR signals using clinical expertise.
6. Conclusion
Despite its potential advantages, there is still limited clinical experience with vonoprazan, and the long-term safety and adverse effects of this drug are not fully understood. Further studies are needed to confirm its safety and efficacy in a larger patient population and to establish its role in the management of acid-related disorders. In the meantime, careful monitoring and patient education are recommended for those who are prescribed vonoprazan.
Additional Points
This system collected demographic information, drug/biologic information, adverse event, outcomes for the event, and others are necessary for surveillance.
Conflicts of Interest
The authors declare that there are no conflicts of interest. The corresponding author was funded by the program during the course of this research. The aim of the program is to support outstanding scholars in their academic research and foster international cooperation.
Acknowledgments
The study was supported by the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine under the “2022-304 Key Discipline Construction of Integrative Clinical Medicine” project.
Open Research
Data Availability
All details of data are available on the FAERS website (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers).




