Green’s Functions on Various Time Scales for the Time-Fractional Reaction-Diffusion Equation
Abstract
The time-fractional diffusion equation coupled with a first-order irreversible reaction is investigated by employing integral transforms. We derive Green’s functions for short and long times via approximations of the Mittag-Leffler function. The time value for which the crossover between short- and long-time asymptotic holds is presented in explicit form. Based on the developed Green’s functions, the exact analytic asymptotic solutions of the time-fractional reaction-diffusion equation are obtained. The applicability of the obtained solutions is demonstrated via quantification of the reaction-diffusion kinetics during heterogeneous catalytic chitin conversion to chitosan.
1. Introduction
Fractional calculus postulates derivatives and integrals of noninteger order. In recent years, fractional calculus has gained much interest because it deals with systems possessing long-tailed dynamics, memory effects, and nonlocality. The fractional-order tools are useful for the description of various processes and phenomena. The fractional-order tools are successively utilized instead of usual integer-order derivatives, e.g., in the Lotka-Volterra model [1], Kelvin-Voigt model [2], Debye relaxation model [3], heat conduction [4], anomalous diffusion modeling [5], Kuramoto-Sivashinsky and Korteweg-de Vries equations [6], and reactive transport modeling [7].
Fractional-order tools also play a central role in modeling transport phenomena. The non-Fickian transport may be mathematically represented in terms of the time-fractional, space-fractional, or space-time-fractional diffusion equation [8]. A transport process quantified in the frame of the fractional-order derivatives may be accompanied by a chemical reaction. This situation is typical in heterogeneous catalysis and adsorption processes. In this case, the fractional-order reaction-diffusion equation is used. For this equation, an additional complication is associated with the form of the reaction term. The latter may be either linear or nonlinear, both reversible and irreversible. The solution to a reaction-diffusion problem with nonlinear reaction kinetics may not be derived analytically [9]. In this paper, we treat the reaction-diffusion equation with a temporal fractional derivative and a linear reaction term. Seeking the analytic solutions to this equation is an extensive topic of the studies in the literature. Particularly, the analytic solutions to the problem were obtained using the homotopy perturbation method [10], the homotopy analysis transform method [11], a combination of homotopy analysis and Sumudu transform [12], a mixture of the homotopy perturbation method and the Yang transform [13], the Laplace transform coupled with the Adomian decomposition technique [14], the residual power series method [15], and a separation of variable technique [16]. The analytic solution to the problem may be also established via Green’s function approach. Green’s function for the time-fractional reaction-diffusion equation has been obtained in terms of the Fox H-function [17]. The integral representation of the relevant Green’s function has been reported [18].
The anomalous non-Fickian transport has been observed in various porous solids and fractured media [19–22]. The non-Fickian transport may be described by the time-fractional diffusion equation [23, 24]. Depending on the value of the anomalous diffusion exponent, the transport rate may be either superdiffusive (i.e., faster than standard Fickian) or subdiffusive (i.e., slower than standard Fickian) [25]. Since the transport stage is an obligatory step of a catalytic or sorption process, the effectiveness of such a process may be significantly improved by enhancing the transport rate, e.g., by keeping the superdiffusive regime of transport. To quantify the effect of anomalous transport on the rate of a process, a mathematical description of the anomalous reaction-diffusion process is required.
To achieve this goal, we introduce the asymptotic Green’s functions for short and long times for the time-fractional diffusion equation with a first-order irreversible reaction using the inverse spatial Fourier transforms of the different approximations of the Mittag-Leffler function. The derived asymptotic Green’s functions are further utilized to obtain the closed-form analytic solutions of the time-fractional reaction-diffusion equation at short and long times separately. The solutions are obtained for the diffusion problem on a semi-infinite rod with a reflecting boundary condition. The closed-form expression for the crossover time between the short- and long-time solutions is also derived. The applicability of the corresponding solutions is experimentally verified for a real reaction-diffusion process, e.g., diffusion-controlled conversion of chitin to chitosan.
2. Materials and Methods
2.1. Reaction-Diffusion Equation
Numerous definitions of the fractional derivative have been developed and successively applied to a description of different phenomena in various fields [27–32]. Some operators introduced as “fractional,” e.g., Caputo-Fabrizio [33], are also utilized for mathematical modeling of the relevant physical phenomena. The motivation to use Caputo’s definition is governed by the properties of this derivative [26]. Particularly, Caputo’s derivative from constant equals zero, as for a usual integer-order derivative. Boundary conditions for Caputo’s derivative are typically set in terms of integer-order derivatives. These properties are essential for modeling the reaction-diffusion process in a chemical reactor under steady-state conditions.
2.2. The Mittag-Leffler Function
2.3. Green’s Function Approach
Equation (10) provides the exact analytic solution of the reaction-diffusion equation. The solution may be established for a spatial position x = L in the range of 0 ≤ x = L < ∞.
3. Results and Discussions
3.1. Derivation of the Asymptotic Green’s Functions
3.2. Closed-Form Analytic Solutions
In the absence of the reaction term, i.e., k = 0, Equation (22) simplifies to the product of half of the initial concentration and the error function. This is a well-known solution of the standard diffusion equation in a semispace with the Dirichlet boundary conditions [38].
3.3. Implementation Results
Figure 1 demonstrates the asymptotic solutions at short and long times plotted using various time-fractional orders. The lower is α, the slower is the concentration decay of the diffusing species. This is typical for a subdiffusive process, i.e., the process characterized by α < 1. A subdiffusive process provides a lower diffusion rate compared to the standard Fickian diffusion. For 0.5 < α < 1, a weak subdiffusion is observed, whereas a strong subdiffusion holds for α in the range between 0 and 0.5 [25].
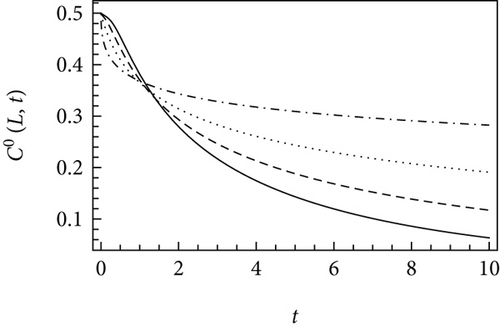
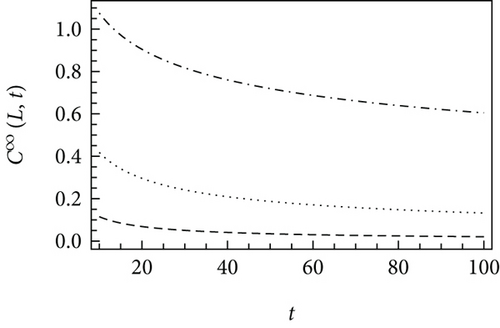
For the standard diffusion equation, the crossover between the short-time and long-time asymptotic solutions is in the range of approximately 0.5 × C0 [39]. In the case of the time-fractional diffusion, the crossover time strongly depends on the parameters of the time-fractional reaction-diffusion equation. Particularly, considering k = 0.1 s−α, K = 0.01 m2/sα, α = 0.8, and x = 0.1 m under Equation (18), the crossover time is t∗ = 10.3 s. For a catalytic reaction-diffusion system, consider more realistic parameters, e.g., k = 10−4 s-α, K = 10−6 m2/sα, α = 0.85, and a catalyst particle with a size of x = 0.002 m. This results in the calculated value of t∗ equal to 65.8 s.
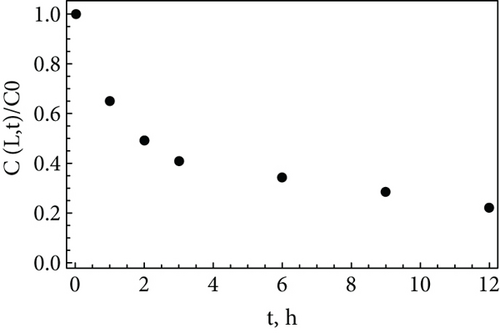
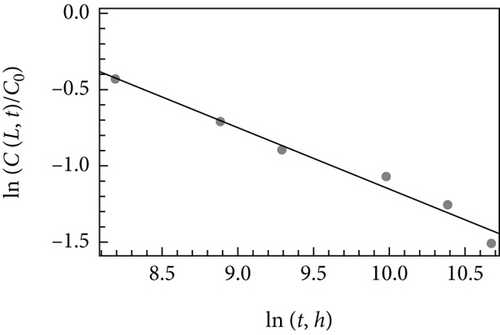
Figure 2(b) presents the experimental data fitted by Equation (23). There is a good correspondence between the experimental data and the theoretical solution. The fitted slope equals the fractional order (α = 0.40), as it follows from Equation (23). Considering L = 5 × 10−5 m and k = 2.3 × 10−4 m3/(mol × s) [40], the estimated value of K is 1.13 × 10−5 m2/sα. For these values, t∗ < 0; i.e., the long-time solution may be used in the whole experimental data range. The obtained results demonstrate that the solutions obtained via utilization of the asymptotic Green’s functions may be successively used for a phenomenological description of the living phenomena.
There exist many methods to derive the analytic solution for the time-fractional reaction-diffusion equation. However, the existing methods treat the concentration decay on the whole temporal scale with no possibility to crossover between the short and long times. It is worth noting that the behavior of the concentration decay may be different at various time scales [41, 42]. The power series methods typically admit the nonlinear reaction term, e.g., Fischer equation. Applying the integral transforms [43], as well as the separation of variables, gives the solutions in terms of integrals that cannot be derived analytically in closed form. The presented analytic approach is free of these drawbacks. It is developed for linear reaction kinetics.
4. Concluding Remarks
The asymptotic Greens’ functions for the time-fractional reaction-diffusion equation with linear reaction kinetics are developed using the integral transform approach. The Greens’ functions hold for short and long times. The asymptotic solutions of the time-fractional reaction-diffusion equation are derived and applied for a description of a real reaction-diffusion process.
Disclosure
This work was completed despite the unprovoked invasion of Ukraine by Russia, supported by Belarus.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors are thankful to the Armed Forces of Ukraine for serving our country and protecting our freedom. This research received funding from the National Research Foundation of Ukraine (NRFU) (grant 2020.02/0050). Funds originally granted by the NRFU were diverted, by a resolution of the Cabinet of Ministers of Ukraine, to defend Ukraine against the Russian invasion.
Open Research
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.




