Caputo Fractional Derivative for Analysis of COVID-19 and HIV/AIDS Transmission
Abstract
In this study, Caputo fractional derivative model of HIV and COVID-19 infections is analyzed. Moreover, the well-posedness of a model is verified to depict that the developed model is mathematically meaningful and biologically acceptable. Particularly, Mittag Leffler function is incorporated to show that total population size is bounded whereas fixed point theory is applied to show the existence and uniqueness of solution of the constructed Caputo fractional derivative model of HIV and COVID-19 infections. The study depicts that as the order of fractional derivative increase the size of the infected variable decrease as time increase. Additionally, memory effects correspond to order of derivative in the reduction of a number of populations infected both with HIV and COVID-19 infections. Numerical simulations are performed using MATLAB platform.
1. Introduction
COVID-19 wreaked havoc on both human life and the immense economic development. A severe economic crisis and the struggle for survival gripped the entire planet. Even the most economically developed nation lost faith and was unable to supply the urgently required resources asked by doctors at medical facilities [1]. The dynamic transmission of COVID-19 crises and attack on the upper respiratory organs cause the breathing system to quickly narrow, which has a significant negative impact [2]. Additionally, the prevalence of the virus has decreased overall as a result of the use of masks and the COVID-19 vaccine that reinforced through activation of memory effects by public health interventions [3]. Recently, in the worldwide it is registered that 692,576,573 cases, 6,903,976 deaths, and 664,687,106 recovered of COVID-19 [4]. On the other hand, the transmission of human immunodeficiency virus transmission through act of sexual practices, blood transfusion, and possible exposure to the virus is a burden in the worldwide [5]. Hence, different mathematical models have been developed to address the impact of diseases in the whole world [6–8]. For instance in engineering problems, the application of a regularized ψ–Hilfer fractional derivative is described by Jajarmi et al. [9] whereas Caputo fractional derivative is applied in the analysis of accelerated mass-spring system by Defterli et al. [10]. Recently, fractional derivative get a great attention due to indication of order of fractional derivative impact on memory effects to control the transmission and prevalence of infection in the population [11]. Caputo fractional derivative is applied in controlling the transmission dynamics of Nipah virus [12]. Also, some studies supported with mathematical modeling show that the occurrence of COVID-19 has significant impact on the HIV carriers [13, 14]. Moreover, the availability of the infections within the community inspires further investigation to consider the impact of memory effects through public health education intervention [15–18]. Also, the Caputo fractional derivative is incorporated to investigate banking data competition [19]. Recently, fractional derivative models are fitted to real data to describe the behavior of the real-world phenomena [20–26]. Therefore, in this study the memory effect is incorporated and a model with Caputo fractional derivative is developed and analyzed. The remaining portion of the paper is divided into the following sections: Section 2, which introduces preliminary ideas and the formulation of a mathematical model; Section 3, which analyzes the fractional model mathematically; Section 4, which uses numerical simulation; Section 5, which discusses the results; and Section 6, which contains the paper’s conclusion.
2. Mathematical Preliminary and Model Formulation
2.1. Mathematical Preliminary
In this section, revise basic definitions and theorems in fractional calculus that supports the main results of the current study.
Definition 2.1. [27] The order α fractional integral of Riemann–Liouville of a function f with is given by
Definition 2.2. [28] Let f be an element of the space of absolutely continuous function on [a, b], α > 0 and n − 1 < α ≤ n. The left and right Caputo fractional derivative of a function f(t) with order α is given by
Definition 2.3. [29] The Laplace transform of the left Caputo fractional derivative is defined as follows:
Definition 2.4. [29] The Laplace transform of the function tβ−1Eα,β(±μtα) is given by
Definition 2.5. [29] Let Eα,β(Z) be Mittage–Leffler function. Then
Lemma 1. (Generalized Mean Value Theorem) Suppose that f(t) ∈ C[a, b] and for 0 < α ≤ 1, then we have
Moreover, if , then there is a neighborhood N of t0 such that f(t) > f(a), ∀t ∈ N. Also, if , then there is a neighborhood N of t0 such that f(t) < f(a), ∀t ∈ N.
2.2. Model Formulation
- (i)
For this study a total population size is not constant;
- (ii)
Susceptible persons recruited at the rate τ;
- (iii)
A naturally death rate is μ;
- (iv)
Only COVID-19 infected individuals die as a result of infection at the rate φ;
- (v)
Only HIV/AIDS infected individuals die at the rate δ = ζ;
- (vi)
Death rate of coinfected individuals die due to infection at the rate ψ;
- (vii)
COVID-19 transmission rate due to direct contact is βc;
- (viii)
Transmission rate of HIV/AIDS due to unsafe exposure to virus is βh;
- (ix)
Recovery rate of only COVID-19 infected with temporary immunity is ω;
- (x)
Recovery rate of coinfected with temporary immunity is κ.
- (xi)
γ is immunity loss rate of COVID-19 recovered individuals R;
- (xii)
ξ is COVID-19 infection vaccination rate for only HIV infected individuals;
- (xiii)
ρ is COVID-19 infection vaccination rate for only susceptible individuals;
- (xiv)
η is immunity loss rate of COVID-19 infection recovered individuals Hr.
The total size of population under study is denoted by P(t) and defined as P(t) = S(t) + C(t) + R(t) + H(t) + Hc(t) + Hr(t) where S(t) is the size of susceptible population at time t, C(t) size of COVID-19 population at time t, R(t) size of only COVID-19 recovered population at time t, H(t) size of HIV/AIDS population at time t, Hc(t) size of coinfected population at time t, and Hr(t) size of COVID-19 recovered HIV population at time.
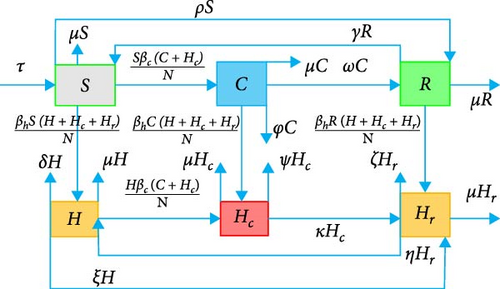
3. Fractional Model Analysis
3.1. Invariant Region
Theorem 1. The solutions of the model (1) are invariant in the region Ω proper subset of six-dimensional space over nonnegative real numbers such that
Proof. To show the boundedness of solution we employ the method employed by Qian et al. [31]. So, adding corresponding terms on left and right of qualities in model (1) we obtain
Implies,
Moreover, for computation purpose, we set the preceding expression to the form
Also, from the preceding expression, we obtain
Moreover, applying the Laplace transform on both sides of the preceding equation yields
According to Podlubny [29], applying Laplace transform definition in Caputo fractional derivative sense such that , the preceding equation reduces to the form
Also, for n = 1, 0 < α < 1, he preceding equation reduces to
Also, by summation property the preceding equation reduces to
Moreover, arranging the terms, the preceding equation can be written as follows:
Further, solving for , the foregoing equation reduces to
Also, applying inverse Laplace transform on both sides of the preceding equation, we obtain
Again applying property of inverse Laplace transform, the preceding equation gives
Also, using the relationship between inverse Laplace transform and Mittag Leffler function given by Qian et al. [31], , the preceding equation reduces to
Implies,
Also, the definition of Mittag Leffler function, , the preceding equation reduces to
Implies,
Hence, taking into consideration the Equation (3), we obtain:
Hence, as time t gets larger and larger the total population size is bounded between 0 and τα/μα.
3.2. Positivity Property
Theorem 2. The solution of the developed fractional model (1) is positive for all time t in the invariant region .
Proof. To show positivity of solutions, we employ the techniques applied by Baleanu et al. [12]. In similar fashion, the trajectory of solution of solution of model (1) along only one state-axis, where other state variables vanishes, gives
Similar to the procedures done for the boundedness computed in Section 3.1, the solution of the foregoing equation is solved, respectively, as follows:
Therefore, the preceding computed results show that the solution is positively invariant along the axis of state variables. Moreover, since the solution of the fractional model (1) is positive in the plane C − R − H − Hc − Hr plane, let t∗ > 0 such that S(t∗) = 0, C(t∗) > 0, R(t∗) > 0, H(t∗) > 0, Hc(t∗) > 0, Hr(t∗) > 0 and S(t) < S(t∗). On this plane,
Moreover, by Caputo fractional derivative mean value theorem sated and applied by Musa et al. [32], we have
Therefore, we get S(t) > S(t∗) which contradicts our earlier assumption for t∗. Hence, the state variable S(t) is nonnegative for all time t. Similarly, other state variables are nonnegative for all time t. Therefore, any solution of fractional derivative is nonnegative for all time t.
3.3. Existence and Uniqueness of Solution
Moreover, let .
Hence, the operator T is well-defined due to obvious continuity of H.
Theorem 4. Let , the function defined above satisfies
Proof. The first component of ϕ gives,
Let , then the preceding equation can be reduced to
Therefore,
In similar fashion the remaining can be shown. Consequently, we can conclude that
Theorem 5. Let the result of preceding theorem holds Ω = τα/Γ(α + 1). If ΩLk < 1, then there exists a unique solution of model (1) on (0, τα) which is uniformly Lyapunov stable.
Proof. The function is obviously continuous on its stated domain. Thus, the existence of the solution follows from the works done by Ahmad et al. [28].
For uniqueness, we apply Banach contraction mapping on operator T defined above. Next, we show T is both a self-map and a contraction. By definition, . Let us now define κ > ∥X0∥+Ωτα/1 − ΩLk and a closed convex set Bk = {X ∈ ζ : ∥X∥ζ ≤ k}. Thus, for self-map property it is enough to show that TBk ⊂ Bk. So let X ∈ Bk, then
It follows that the operator TX⊆Bk and T is indeed a self-map. Next, we prove that T is a contraction. Let X and satisfy the abbreviated dynamical system. The using the result of theorem above, we have
Hence, if ΩLk < 1 then T is contraction mapping and by Banach contraction mapping principle, T has a unique fixed point on [0, τα] which is a solution of model (1). Moreover, the uniformly Lyapunov stability of solutions follows as stated by Ahmad et al. [28].
3.4. Disease-Free Equilibrium
3.5. Endemic Equilibrium
Moreover, due to trick of the problem, we obtain the endemic equilibrium from numerical simulation.
3.6. Basic Reproduction Number
4. Numerical Simulations
4.1. Numerical Schemes for Caputo Fractional Derivative
The numerical solution of Caputo fractional derivative is performed using FDE12 [33]. Moreover, the initial population sizes are S(0) = 300, 000, C(0) = 300, R(0) = 0, H(0) = 5, 000, Hc = 0, Hr = 0. Moreover, parameter value is given in Table 1.
5. Results and Discussion
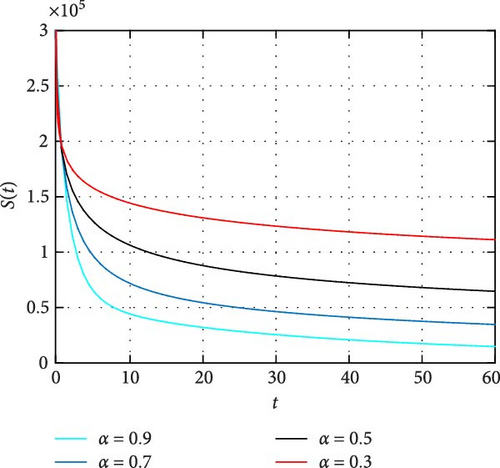
In Figure 2 the size of susceptible population decrease as time increase which shows the higher activation of human memory toward vaccination the less the population are susceptible. Moreover, the higher the order of fractional derivative the higher the mobilization of population toward vaccination. Figure 3 describes the dynamics of population size subject to the transmission dynamics of novel COVID-19 and memory effects through public health education. It is noticed that the intervention to activate memory of population significantly contributes to control the pandemic COVID-19. Moreover, the simulation shows that less memory effect contributes less in controlling of the infection whereas high involvement in the activation of human memory results in better way to control the infection.
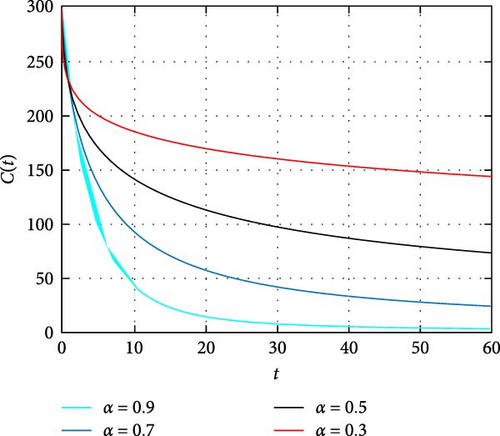
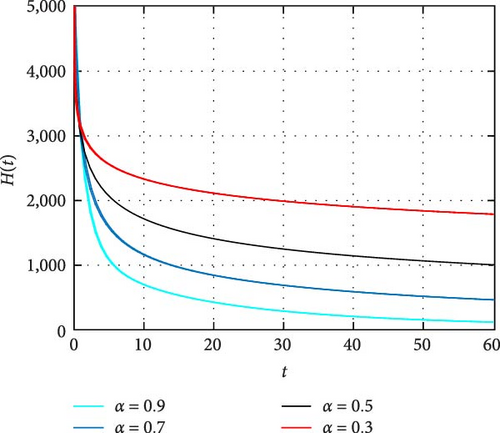
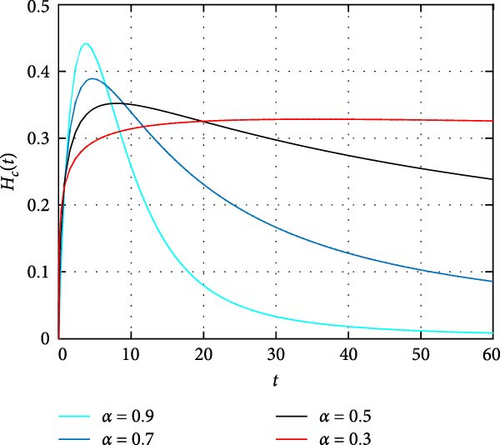
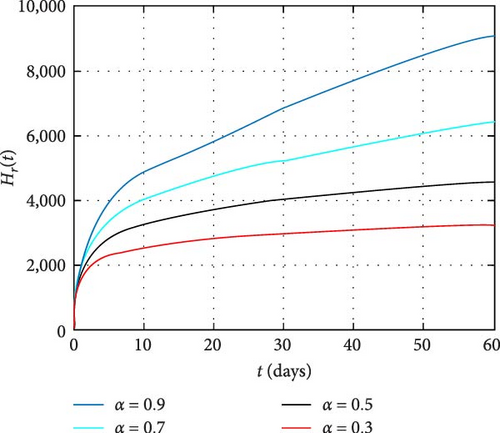
In Figure 4, dynamics of only HIV-infected population size is simulated. It is observed that the memory effect works well to control the transmission dynamics of HIV infection. Moreover, the simulation depicts there is comprise of population in the long run. Thus, effectively apply the memory effects before HIV infects many people. In Figure 5, the simulation of coinfected population size is depicted. It is shown that the number of individuals infected with both infections is high at the beginning and the memory effect is less effective. On the other hand, the memory effects work well for reducing coinfected individuals as time increase. In Figure 6, the dynamics of only HIV infected population with COVID-19 recovered status is simulated. It is observed that memory effect activation works continually for controlling COVID-19 for HIV infected population. Hence, a continuum memory effect helps HIV-infected population for recovery from pandemic COVID-19.
In Figure 7, the dynamics of population size is simulated. It is shown that the impact of COVID-19 can be controlled with effective memory effect whereas the HIV infection persists with population. In Figure 8, we observe that the more individuals are susceptible the more they get infected with COVID-19 infection whereas less the number of susceptible individuals the less the number of individuals infected with COVID-19 infection. In Figure 9, we observe that the more individuals are susceptible to HIV the more they get attacked with HIV infection whereas the less the number of susceptible individuals toward HIV the less the number of individuals exposed to the HIV infection. Figure 10 depicts the transferring of individuals to R(t) with immunity reduces the number of susceptible individuals.
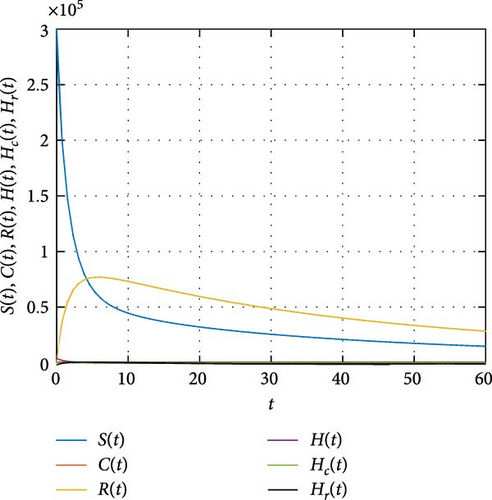
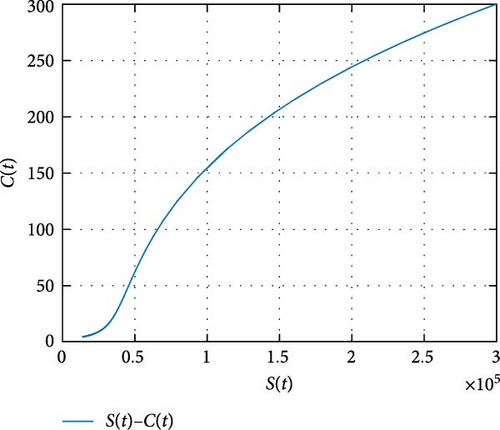
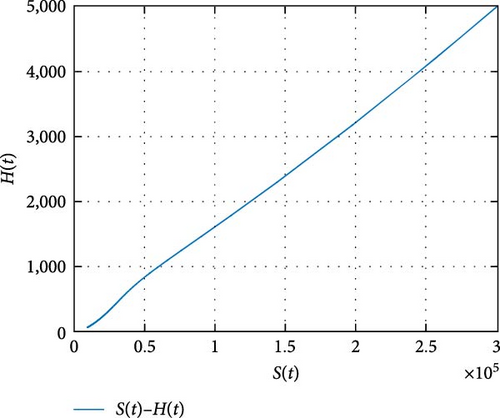
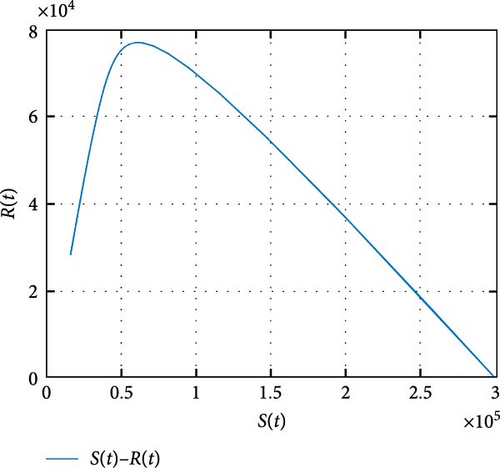
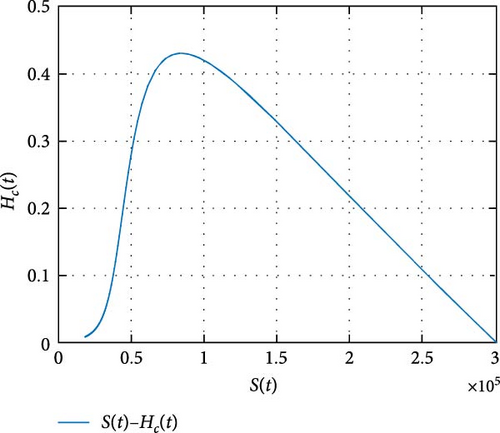
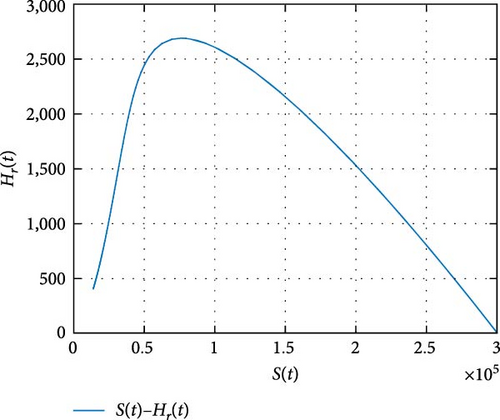
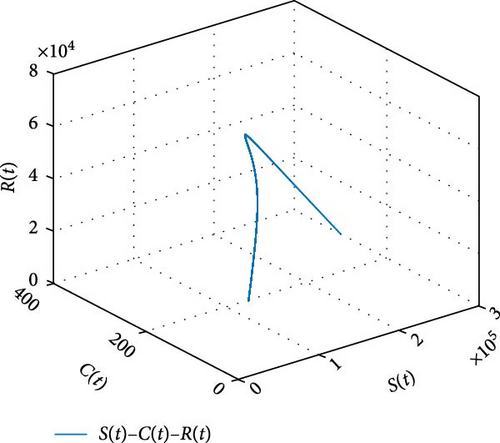
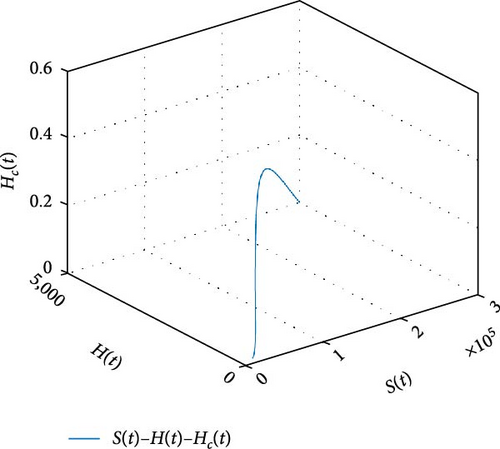
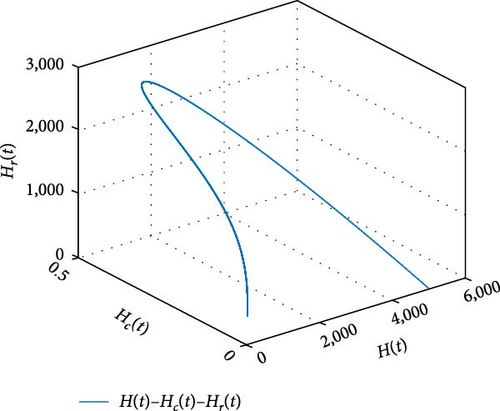
Figure 11 shows that the number of coinfected individuals decreases or increases along susceptible population size. Figure 12 describes that the number of HIV infection recovered individuals increases as susceptible decrease, but lately decrease as the number of susceptible individuals decreases. Figure 13 describes the dynamics of S(t), C(t), and R(t). The behavior of S(t) and C(t) alike as they decreases from the beginning whereas R(t) increase initially and decrease lately. Figure 14 describes the dynamics of S(t), H(t), and Hc(t). The behaviors of S(t) and H(t) are similarly get decrease from the beginning whereas Hc(t) increase initially and decrease lately. Figure 15 describes the dynamics of H(t), Hc(t), and Hr(t). The behaviors of Hc(t) and Hc(t) are similarly get increases from the beginning and decreases lately whereas S(t)decreases from the beginning.
6. Conclusion
In this study, fractional derivative is incorporated and the model of HIV and COVID-19 dynamics is developed and analyzed. Based on the simulations of HIV and COVID-19 dynamics, infections can be controlled for basic reproduction number is less than unity. The study depicts that HIV/AIDS and COVID-19 coinfection may increase, through direct interaction of coinfected individuals. Also, fractional derivative analysis shows increasing memory of population toward COVID-19 and HIV infections will increase the ability of population to control the infections. In the future study, one can extend our model to a new generalized fractional derivative and use real data to describe the biological phenomena. Numerical simulations are performed using MATLAB platform.
Conflicts of Interest
The author declares that there is no conflict of interest.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.




