Clarifying the Pulmonary Arterial Morphology and Pulmonary Blood Supply in Patients with Tetralogy of Fallot and Pulmonary Atresia on Computed Tomography Angiography
Abstract
Aim. The present study sought to characterize the pulmonary arterial morphology and pulmonary blood supply in patients of tetralogy of Fallot and pulmonary atresia (TOF-PA) on CT angiography. Materials and Methods. We retrospectively reviewed our departmental database to identify patients with TOF-PA evaluated using CT angiography. The images were analysed to detect the presence of main and branch pulmonary arteries and pulmonary arterial confluence, presence of major aortopulmonary collateral arteries (MAPCAs), laterality and relation with pulmonary arterial morphology, and presence of patent arterial duct and associated intra- and extracardiac anomalies. Results. TOF-PA was identified in 177 patients (114 (64.4%) males; median age: 9 months). Pulmonary arteries were confluent in 142/177 (80.2%) patients. According to Somerville classification, type II pulmonary atresia was the most frequent pattern seen in 127/177 (71.8%). Based on McGoon’s ratio, pulmonary arteries were adequate for surgery in 123/177 (69.5%) patients. Patent arterial duct was present in 84/177 (47.5%) patients while MAPCAs were present in 124 (70.1%) patients, of which 72/124 (58.1%) patients had at least 1 essential MAPCA supplying either lung. According to Congenital Heart Surgeons’ Society classification, type B pulmonary arterial anatomy was the most prevalent, seen in 103/177 (57.6%) patients. Conclusion. TOF-PA is associated with marked morphologic variability in the pulmonary arterial arborization to supply the lungs. Cardiac CT angiography can accurately delineate the pulmonary arterial morphology, sources of pulmonary blood supply, and associated cardiovascular anomalies in patients with TOF-PA which aids in planning appropriate surgical management including decisions regarding the need for unifocalization of MAPCAs.
1. Introduction
Tetralogy of Fallot with pulmonary atresia (TOF-PA) is characterized by absence of luminal continuity between the right ventricle and pulmonary artery with the pulmonary bed supplied by various sources such as major aortopulmonary collateral arteries (MAPCAs) and patent arterial duct [1, 2]. There is associated marked morphologic variability in the pulmonary arterial arborization. Hence, comprehensive preoperative delineation of cardiovascular morphology including the pulmonary arterial morphology, sources of pulmonary blood supply, and associated cardiovascular anomalies is imperative for planning appropriate surgical management [3, 4].
Transthoracic echocardiography is usually the initial imaging modality in patients with TOF-PA. However, its role is limited in the accurate depiction of native pulmonary arteries, patent arterial duct, ventricular septal defects, and MAPCAs [5, 6]. Cardiac catheterization, although used frequently in the past decades, is invasive and can only provide indirect information distal to the atretic segment [7]. CT angiography, owing to its high spatial resolution and short scanning times, can accurately provide information on pulmonary arterial morphology, MAPCAs, and patent arterial duct and associated cardiovascular anomalies. Magnetic resonance angiography can also depict the pulmonary arterial anatomy, without use of ionizing radiation as in CT angiography. However, its use is limited by long scan times, frequent need for sedation or general anaesthesia in children, inability to delineate peripheral pulmonary arterial branches, and underestimation of luminal diameters secondary to turbulence in stenotic blood vessels [8–10].
Few studies in literature have previously evaluated the role of CT angiography in patients with TOF-PA [11]. The present study sought to comprehensively characterize the pulmonary arterial morphology, pulmonary blood supply, and associated intra- and extracardiac anomalies in patients with TOF-PA on cardiac CT angiography using a dual-source CT scanner.
2. Materials and Methods
This is a single-centre retrospective study performed at a tertiary care institute. The departmental electronic database was reviewed to identify all patients with TOF-PA evaluated using CT angiography. The retrospective evaluation of data was approved by the Institutional Ethics Committee, and the need for written informed consent was waived.
All included patients had undergone ECG-gated CT angiography on a dual-source CT scanner (Somatom FLASH or Somatom FORCE, Siemens Healthineers, Forchheim, Germany). Advanced dose modulation techniques like automatic tube current modulation (Care Dose; Siemens Healthineers, Forchheim, Germany) and low kilovoltage peak (80 kV) were used to minimize radiation exposure to the patients. Nonionic iodinated contrast was administered via a peripheral intravenous line (dose: 1.5–2.0 mL/kg; flow rates varying from 1.0 to 4.0 mL/second). No sedation was required in any case; however, the patients were immobilized using a binder during the scan.
Postprocessing techniques using multiplanar reconstructions, maximum intensity projections, and cinematic volume rendered reconstructions were employed during image analysis on a dedicated external workstation (Syngo.via; Siemens Healthineers, Forchheim, Germany).
A diagnosis of TOF-PA was made based on a sequential segmental approach used to diagnose and characterize congenital heart diseases. The CT angiography images were analysed to detect the presence/absence of the following: main and branch pulmonary arteries, pulmonary artery atresia/stenosis and its anatomic site, pulmonary arterial confluence, MAPCAs and their number, laterality and relation with pulmonary arterial morphology, patent arterial duct, dual supply, and other associated intra- and extracardiac anomalies.
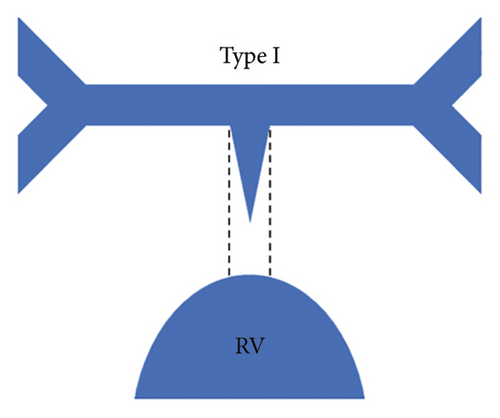
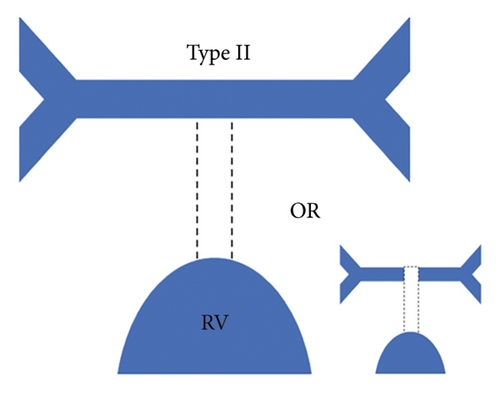
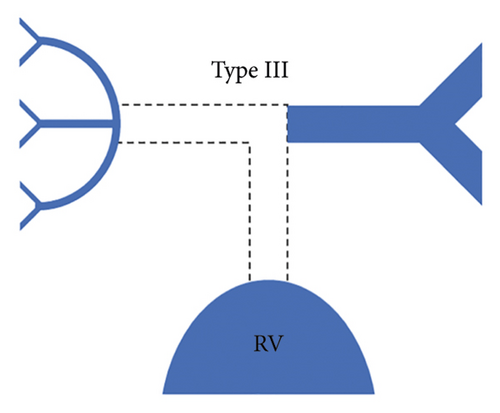
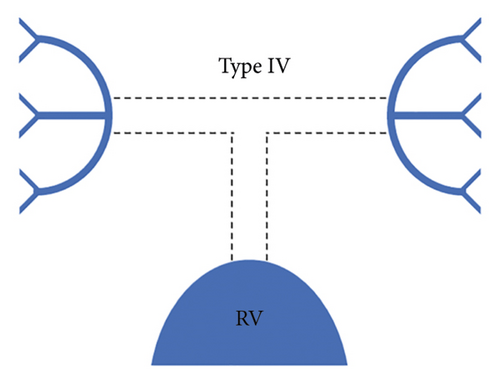
Each patient was classified using the Somerville classification as well as the Congenital Heart Surgeons’ Society (CHSS) classification. According to the Somerville classification, patients were classified into four types, namely, type I where the pulmonary trunk is present, type II where the pulmonary trunk is absent with both pulmonary arteries (confluent or nonconfluent) patent, type III where only one pulmonary artery is present, and type IV where intrapericardial pulmonary arteries are absent (Figure 1) [12].
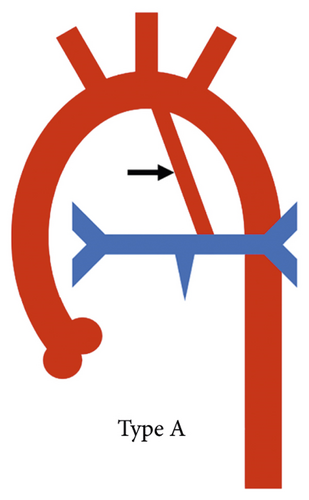
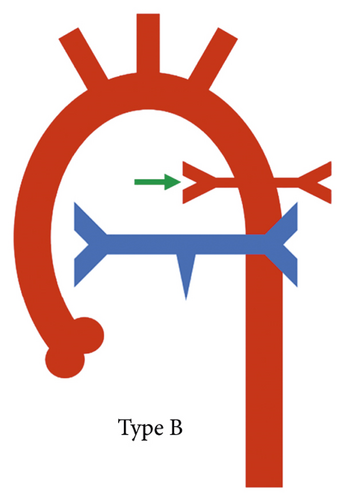
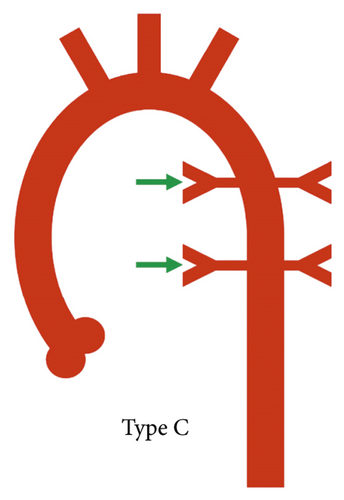
A practical classification has also been proposed by the Congenital Heart Surgeons’ Society based on the complexity of pulmonary blood supply which in turn indicates the complexity of surgical repair. In type A, native pulmonary arteries and patent arterial duct are present, in type B, both native pulmonary arteries and MAPCAs are present, and in type C, there are no native pulmonary arteries with blood supply exclusively through MAPCAs (Figure 2) [1].
McGoon’s ratio was calculated in patients with evidence of pulmonary hypoplasia or atresia according to the following equation: (LPA + RPA)/DTA where LPA = diameter of left pulmonary artery, RPA = diameter of right pulmonary artery, and DTA = diameter of descending thoracic aorta at the level of the diaphragmatic crus. Discrete pulmonary arterial stenosis was defined as ≥50% short segment narrowing of pulmonary trunk or either pulmonary artery. Diffuse pulmonary artery hypoplasia was defined based on z values (z values <−3) which were calculated using nomograms. A MAPCA was deemed redundant when it was seen supplying a segment of lung that was also receiving native pulmonary supply, while it was deemed essential when the MAPCA was the sole supply of a lung segment.
3. Results
The present study included 177 consecutive patients (114 (64.4%) males; median age: 9 months (range: 0.5–156 months; IQR, interquartile range: 4–24 months)) with a diagnosis of TOF-PA on CT angiography. All patients had normal visceroatrial arrangement with a left-sided heart.
3.1. Pulmonary Arterial Morphology
The central pulmonary arteries were confluent in 142/177 (80.2%) patients and nonconfluent in 35/177 (19.8%) patients. According to Somerville classification, type II pulmonary atresia (absent pulmonary trunk with both pulmonary arteries patent) was the most frequently observed pattern seen in 127/177 (71.8%) patients, whereas type III pulmonary atresia (only one pulmonary artery present) was the least commonly observed pattern in 10/177 (5.6%) patients. Type I pulmonary atresia (pulmonary trunk present) was seen in 19/177 (10.7%) patients while type IV pulmonary atresia (absent intrapericardial pulmonary arteries) was seen in 21/177 (11.9%) patients.
Median diameters of the right pulmonary artery (RPA) and left pulmonary artery (LPA) were 4.5 mm (IQR: 2.5–7 mm) and 4.5 mm (IQR: 2.975–6.5 mm). Median diameter of DTA at the level of diaphragmatic crus was 8 mm (IQR: 7–10 mm). Based on McGoon’s ratio, pulmonary arteries were adequate for surgery (McGoon’s ratio >0.8) in 123/177 (69.5%) patients while they were inadequate in 54/177 (30.5%) patients.
Pulmonary artery stenosis (RPA or LPA) was seen in 50/155 (32.25%) patients (RPA: 8/50 (8%) and LPA: 42/50 (84%)). The site of stenosis was ostial in 21/50 (42%) patients, mediastinal in 21/50 (42%) patients, both ostial and mediastinal in 7/50 (14%) patients, and hilar in 1/50 (2%) patient.
3.2. Patent Arterial Duct
A patent arterial duct was present in 83/177 (46.9%) patients (Figure 3). The duct was left-sided in 69/83 (83.1%) patients and right-sided in 13/83 (15.57%) patients. Bilateral patent arterial ducts reforming ipsilateral pulmonary arteries were seen in 1 (1.2%) patient.
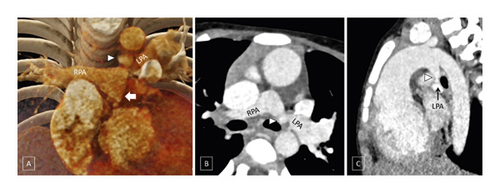
Stenosis of the patent arterial duct was observed in 70/83 (84.3%) patients. The most common site of insertion of the patent arterial duct was the mediastinal pulmonary artery (51/83 (61.4%) patients) followed by ostial pulmonary artery (29/84 (34.9%) patients). In the presence of patent arterial duct, pulmonary arteries were nonconfluent in only 9/83 (10.8%) patients compared to 26/94 (27.7%) patients in the absence of patent arterial duct (p = 0.0052). McGoon’s ratio was significantly higher in patients where patent arterial duct was present compared to those where it was absent (1.4 ± 0.6 vs. 0.9 ± 0.6; p < 0.0001).
In patients with a right-sided aortic arch, absence of patent arterial duct (35/53 (66%) patients) was the most common configuration followed by presence of a right-sided patent arterial duct (11/53 (20.8%) patients), a left-sided patent arterial duct (6/53 (11.3%) patients), and bilateral patent arterial ducts (1/53 (1.9%) patient). In patients with a left-sided aortic arch, an ipsilateral patent arterial duct (62/123 (50.4%) patients) was the most common configuration followed by absence of a patent arterial duct (59/123 (48%) patients) and presence of a right-sided patent arterial duct (2/123 (1.6%) patients). One patient with a double aortic arch had a left-sided patent arterial duct.
3.3. Major Aortopulmonary Collateral Arteries
MAPCAs were present in 124/177 (70.1%) patients (Figure 4). A total of 519 MAPCAs were observed in 176 patients, out of which 282 were supplying the right lung and 237 were supplying the left lung. In 1 patient, MAPCAs could not be evaluated optimally on either side due to excessive motion artifacts. Out of 519 MAPCAs, 192 (37%) showed presence of stenosis.
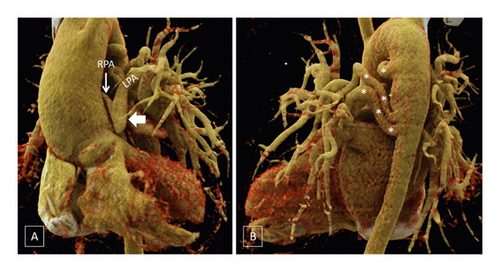
MAPCAs were present in only 35/83 (42.2%) patients in patients with patent arterial duct compared to 89/94 (94.7%) patients with absence of patent arterial duct (p < 0.0001). In the presence of both patent arterial duct and MAPCAs, majority of the MAPCAs were seen contralateral to the patent arterial duct.
In the right lung, all MAPCAs were essential in 49/177 (27.7%) patients while at least 1 MAPCA, but not all, was essential in another 12/177 (6.8%) patients. All MAPCAs were redundant in 55/177 (31.1%) patients while no MAPCA was seen in 57/177 (32.2%) patients. In the left lung, all MAPCAs were essential in 39/177 (22%) patients while at least 1 MAPCA, but not all, was essential in 14/177 (7.9%) patients. All MAPCAs were redundant in 44/177 (24.8%) patients while in 75/177 (42.4%) patients, no MAPCAs were demonstrated. Information regarding essentiality/redundancy was suboptimal in the right lung and left lung in 4 (2.3%) and 5 (2.8%) patients, respectively. Overall, in the 124 patients where MAPCAs were present, at least 1 essential MAPCA in either lung was observed in 72/124 (58.1%) patients while all MAPCAs were redundant in both lungs in 48/124 (38.7%) patients.
3.4. Source of Pulmonary Blood Flow
According to the CHSS classification, type B anatomy (native pulmonary arteries and MAPCAs present) was the most prevalent configuration, seen in 103/177 (57.6%) patients (Figure 5), whereas type C anatomy (no native pulmonary arteries with blood supply exclusively through MAPCAs) was least commonly observed, seen in 21/177 (11.9%) patients (Figure 6). Type A anatomy (native pulmonary arteries with patent arterial duct) was observed in 48/177 (27.1%) patients (Figure 3).
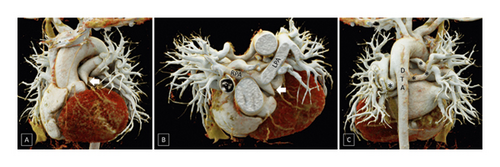
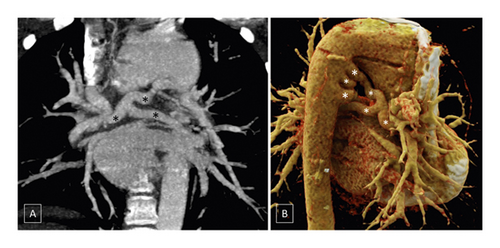
In simpler terms, the pulmonary blood supply was through a combination of native pulmonary arteries and MAPCAs in 67/177 (37.9%) patients while in 48/177 (27.1%) patients, the pulmonary blood supply was through a combination of native pulmonary arteries and patent arterial duct. In 35/177 (19.8%) patients, the lungs were supplied through combination of native pulmonary arteries, patent arterial duct, and MAPCAs while in 21/177 (11.9%) patients, MAPCAs were the sole source of pulmonary blood flow. In 3/177 (1.7%) patients, no MAPCAs or patent arterial duct could be demonstrated supplying the lung parenchyma, although small collaterals from the descending thoracic aorta could be visualized. In 3 (1.7%) patients, in addition to pulmonary atresia, there was anomalous origin of pulmonary artery from aorta (of which 1 patient also had MAPCAs on the contralateral side) or presence of aortopulmonary window which was the major source of pulmonary blood flow in these patients via the native pulmonary arteries.
3.5. Associated Cardiovascular Anomalies
In 23/177 (13%) patients, an ostium secundum atrial septal defect was observed. All patients had a subaortic ventricular septal defect (patients with TOF-PA) with an additional muscular ventricular septal defect seen in 7/177 (4%) patients.
Systemic venous abnormalities were present in 46/177 (25.9%) patients, the most common systemic venous abnormality being presence of bilateral superior caval vein seen in 22/177 (12.42%) patients followed by subaortic course of left brachiocephalic vein in 18/177 (10.17%) patients and retroinnominate course of left brachiocephalic vein in 3/177 (1.7%) patients. An “isolated” left superior caval vein was seen in 2/177 (1.1%) patients (having usual arrangement of organs with left-sided heart) with 1/177 (0.6%) patient having both double SVC and IVC. Pulmonary venous anomalies were seen in 3/177 (1.7%) patients with totally anomalous pulmonary venous drainage seen in 2/177 (1.1%) patients and partially anomalous pulmonary venous drainage in 1/177 (0.6%) patient.
A right-sided aortic arch was seen in 53/177 (29.9%) patients. Of these, mirror image branching was seen in 47/177 (26.55%) patients and an aberrant left subclavian artery (SCA) with a retroesophageal course was seen in 6/177 (3.4%) patients. An aberrant right subclavian artery with retroesophageal course was noted in 8/177 (4.5%). One (0.6%) patient had a double aortic arch forming a complete vascular ring.
Coronary arterial anomalies were seen in 18/177 (10.2%) patients with the commonest anomalies being presence of hypertrophied conal artery (5/177; 2.8%) and anterior interventricular artery arising from the right coronary sinus (3/177; 1.7%). Overall, a coronary artery crossing anterior to the right ventricular outflow tract was seen in 12/177 (6.8%) patients.
4. Discussion
TOF-PA is at the extreme end of the spectrum of the classical tetralogy of Fallot. Definitive management of TOF-PA patients depends upon various factors such as size of pulmonary arteries, type of obstruction in the right ventricular outflow tract, presence and properties of MAPCAs, and associated intra- and extracardiac malformations in the patient [13]. The blood flow in the pulmonary arteries through patent arterial duct or MAPCAs determines the growth and development of pulmonary arteries. In this study, we characterized the pulmonary arterial morphology, pulmonary blood supply, and associated intra- and extracardiac anomalies in patients of TOF-PA with the help of CT angiography.
4.1. Pulmonary Arterial Morphology
The assessment of pulmonary arterial morphology is the most important aspect in imaging of patients with TOF-PA. In the present study, nonconfluent pulmonary arteries were present in 19.8% patients while the pulmonary arteries were confluent in 80.2% patients. Similarly, Lin et al. found that 53/64 (82.8%) patients with TOF-PA had confluent pulmonary arteries [14]. In another study by Acherman et al., nonconfluent pulmonary arteries were present in 6/42 (14.2%) patients [5]. In our study, according to Somerville classification, type II pulmonary arterial anatomy was the most prevalent and was seen in 71.8% patients. As per the CHSS classification, type B pattern was the most common type seen in 57.6% patients. Liu et al. analysed a total of 116 patients with TOF-PA using multidetector CT angiography and correlated findings with invasive angiography and surgery [11]. They observed that both branch pulmonary arteries were absent in 29.3% patients as compared to 11.9% (type IV) patients in our study. In their study, they identified only the LPA and only the RPA in 3.4% and 1.7% patients, respectively, which is similar to the results of our study.
In the present study, median diameters of RPA and LPA were 4.5 mm each. The median McGoon’s ratio in our study was 1.1 (IQR: 0.7–1.5). According to McGoon’s ratio, adequate size of pulmonary arteries for definitive surgery (McGoon’s ratio >0.8) was present in 69.5% patients. In the study by Lin et al., the median diameters of the LPA and RPA as measured by CTA in 61 patients with intrapericardial pulmonary arteries were 4.83 and 5.19 mm, respectively. The median McGoon index in their study was 1.38 (interquartile range, 1.06–1.85). They also found that pulmonary arterial size was significantly larger in the patients with pulmonary trunk (type I, median 1.56), compared to those without (types II, III, and IV; median 1.25, p < 0.01) [14].
We observed pulmonary artery stenosis (RPA or LPA) in 32.25% patients, with LPA being most commonly affected by stenosis. Ostial and mediastinal stenoses were more common than hilar stenosis. In a study by Liu et al., branch pulmonary artery stenosis was observed in 18/116 (15.5%) patients with the left pulmonary artery being most commonly affected [11]. The authors also concluded that CTA was more accurate than cardiac catheterization and TTE in overall characterization of pulmonary artery anatomy, especially the branch pulmonary artery [11]. The hypoplastic branch pulmonary arteries are difficult to catheterize and can be mistakenly considered as absent during catheter angiography [15].
4.2. Patent Arterial Duct
In the present study, a patent arterial duct was present in 46.9% patients, which was most commonly left-sided. Acherman et al. showed the presence of patent arterial duct in 23/42 (57%) patients using echocardiography. The authors concluded that all patients with a patent arterial duct as the sole source of pulmonary blood supply had confluent pulmonary arteries, which was also seen in our study [5]. Liu et al. also found that 47/116 (40.5%) patients with TOF-PA had a patent arterial duct as noted at the time of surgery.
4.3. Major Aortopulmonary Collateral Arteries
MAPCAs are the primitive intersegmental arteries that have not involuted. In our study, MAPCAs were present in 70.1% patients and a total of 519 APCs were identified. In a study by Liu et al., a total of 326 MAPCAs were identified at surgery in 105 patients (90.5%). Of these, 135 (41.4%) supplied the left lung, 147 (45.1%) supplied the right lung, and 44 (13.5%) supplied both lungs. The authors stated that multidetector CT angiography could identify all surgically important MAPCAs. In the present study, of the 124 patients where MAPCAs were present, at least 1 essential MAPCA in either lung was observed in 72/124 (58.1%) patients. These patients would be candidates for unifocalization (connecting MAPCAs to native pulmonary arteries) before definitive repair.
Significant stenoses were present in 30% MAPCAs in their study versus 37% in our study [11]. The authors observed that CT angiography was 98% sensitive and 98.7% specific in identifying stenosis of MAPCAs. Presence of stenosis in the MAPCAs has a protective effect on the pulmonary vascular bed from development of pulmonary arterial hypertension.
4.4. Associated Cardiovascular Anomalies
Additional atrial and ventricular septal defects were seen in 13% and 4% patients, respectively. Systemic venous abnormalities were quite common and were seen in 25.9% patients. Another commonly associated anomaly was presence of right-sided aortic arch which was seen in 29.9% patients while 7.9% patients had presence of anomalous origin of a subclavian artery with retroesophageal course. Acherman et al. demonstrated a right-sided aortic arch in 17/42 (40%) patients with TOF-PA, with mirror image branching being the most common pattern. An aberrant subclavian artery was noted in 7.1% patients in their study [5].
4.5. Clinical Implications
Surgical management in patients with TOF-PA is primarily determined by the pulmonary arterial morphology and arborization pattern. In presence of relatively good-sized pulmonary arteries and no MAPCAs, palliative procedures like aortopulmonary shunt, with or without arterioplasty, can be performed in younger children and corrective procedures may be performed in older children [13]. Patients with short segment atresia may undergo intracardiac repair without need of a conduit. The adequacy of native pulmonary blood supply must be determined before corrective surgery. In case of “dual blood supply,” ligation or embolization of MAPCAs can be performed. However, in case of exclusive blood supply by multiple MAPCAs, unifocalization (connecting MAPCAs to native pulmonary arteries) is needed before definitive repair [16–18]. Additionally, preoperative recognition of coronary artery anomalies like coronary crossing the right ventricular outflow tract is an essential part of preoperative evaluation. The presence of systemic venous anomalies also modifies cannulation during cardiopulmonary bypass [14].
4.6. Limitations
This is the largest CT angiography-based study of patients with TOF-PA in the literature describing all the variations in pulmonary artery anatomy and associated anomalies. However, we acknowledge the following limitations. Firstly, it was a retrospective analysis of the data collected at our centre, and catheter angiography or surgical correlation to confirm the CT angiography findings was not available for all cases. However, an earlier study by Liu et al. had demonstrated excellent agreement between surgical and CT angiography findings and CT angiography was found to be better in comparison to catheter angiography and transthoracic echocardiography. Other studies have also demonstrated high diagnostic accuracy of CT angiography in comparison to invasive angiocardiography for this group of patients [14, 19, 20]. Secondly, in patients with TOF-PA, there is often a need for hemodynamic assessment which can only be provided by catheterization.
CT angiography also poses a risk of ionizing radiation. However, with the advent of advanced techniques like iterative reconstruction and ECG-based and anatomy-based tube current modulation, the radiation dose has markedly reduced [21]. A preliminary CT angiography can further obviate the need for invasive catheterization associated with an even higher radiation exposure.
5. Conclusion
TOF-PA is associated with marked morphologic variability in the pulmonary arterial arborization to supply the lungs. Cardiac CT angiography can accurately delineate the pulmonary arterial morphology, sources of pulmonary blood supply, and associated cardiovascular anomalies in patients with TOF-PA which aids in planning appropriate surgical management.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent
The Institutional Ethics Committee approved the retrospective evaluation of data and waived off the need for patient written informed consent.
Disclosure
Niraj Nirmal Pandey and Mumun Sinha are co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Niraj Nirmal Pandey and Mumun Sinha contributed equally to this study.
Open Research
Data Availability
Data are available on request due to privacy/ethical restrictions.