Efficacy and Safety of Trastuzumab Combined with Pertuzumab for Human Epidermal Growth Factor Receptor-2-Positive Breast Cancer: A Meta-Analysis Study of Randomized Control Trials
Abstract
Objective. This systematic review was aimed to evaluate the efficacy and safety of trastuzumab in combination with pertuzumab for human epidermal growth factor receptor-2 (HER2)-positive breast cancer (BC). Methods. A comprehensive search of PubMed, Web of Science, Embase, China National Knowledge Internet, and Wanfang databases was conducted. All randomized controlled trials on trastuzumab in combination with pertuzumab for HER2-positive BC from the time of database construction to October 2022 were included. A meta-analysis of the included literature was performed using STATA 17.0 software. Results. A total of 8 studies were included, involving a total of 7628 patients, including 3814 patients in the treatment group and 3814 patients in the control groups. The results of the meta-analysis showed that the median progression-free survival was much shorter in the treatment group (trastuzumab combined with pertuzumab) than in the control group (placebo) (OR = 0.656, 95% CI (0.581, 0.741), P < 0.001) and that patients in the treatment group experienced significantly more cases of diarrhea than those in the control group (OR = 2.429, 95% CI (2.065, 2.856), P < 0.001) while experiencing significantly less cases of constipation (OR = 0.641, 95% CI (0.473, 0.869), P = 0.004). Notably, the incidence of nausea and vomiting did not differ significantly between the two groups. In addition, there was no significant difference between the two groups in the incidence of systemic adverse effects such as neutropenia, fatigue, myalgia, and cardiac disease (P > 0.05). However, the treatment group had a much higher incidence of rash than the control group (OR = 1.915, 95% CI (1.505, 2.437), P < 0.001). The risk of serious adverse reactions was markedly higher in patients in the treatment group than that in the control group (OR = 1.342, 95% CI (1.206, 1.494), P < 0.001). Conclusion. The combination of trastuzumab and pertuzumab was not effective in improving the intermediate progression-free survival of patients. However, adverse effects, including diarrhea and rash, are the limiting factors for the current promotion of this combination method.
1. Introduction
Breast cancer is the most common cancer in women and the most common cause of cancer deaths worldwide. Statistics from the International Agency for Research on Cancer 2020 study show that there are approximately 19.3 million new cancer cases and 10 million cancer-related deaths worldwide each year. With an estimated 2.3 million new cases each year, breast cancer (11.7%) has surpassed lung cancer (11.4%) as the most common cancer among women [1]. Breast cancer development has been discovered to be closely correlated with the overamplification or expression of human epidermal growth factor receptor-2 (HER2-protein) transcribed from the ERBB2 gene on human chromosome 17q [2]. HER2-positive subgroups account for 15–20% of all breast cancers. Although such breast cancer subtypes respond well to targeted therapies that inhibit the HER2 receptor, patients obtain different clinical outcomes [3].
HER2-targeted therapies, such as the use of trastuzumab, pertuzumab, lapatinib, or lenvatinib, are effective in preventing cancer stem cells from driving tumor growth [4]. Trastuzumab, an anti-HER2 monoclonal antibody, is currently the standard drug for adjuvant and metastatic treatment of breast cancer in clinical practice. However, this drug therapy will create high costs and even target cell resistance (15%) with long-term use [5, 6]. Therefore, research into new therapeutic alternatives to enhance the efficacy of trastuzumab or to address the drug resistance of target cells continues to be a top priority for the treatment of HER2-positive breast cancer. Pertuzumab is a humanized monoclonal antibody with a complementary mechanism of action to trastuzumab. To be specific, pertuzumab inhibits heterodimerization of HER2 as well as HER family receptors by binding to the dimerization structural domain, while trastuzumab inhibits HER2 dimerization by binding near the transmembrane structural domain [7]. The therapeutic effectiveness of trastuzumab combined with pertuzumab in the management of HER2-positive breast cancer has not, however, been thoroughly examined. Consequently, the aim of this study was to systematically evaluate the available randomized controlled trials (RCTs) by means of meta-analysis. The efficacy and adverse effects of trastuzumab in combination with pertuzumab in the treatment of HER2-positive breast cancer were reviewed, and the effectiveness and safety of dual HER2 blockade regimens were evaluated. It is anticipated that this study will provide a theoretical basis for the selection of subsequent clinical regimens and the development of disease guidelines.
2. Materials and Methods
This systematic review followed the Preferred Reporting Item for Systematic Review and Meta-Analysis (PRISMA) guideline.
2.1. Sources and Search Strategy
According to the analysis process in the Cochrane Handbook, data were screened from PubMed, Web of Science, Embase, China National Knowledge Internet, and Wanfang databases. The keywords were set as “trastuzumab,” “pertuzumab,” and “HER2-positive” “breast cancer” for a comprehensive systematic literature search. The Chinese database was searched using the corresponding Chinese terms. References of the retrieved records as well as gray literature were searched manually. The literature was selected regardless of age, region, or language from the time of database construction to October 2022.
2.2. Literature Screening
The inclusion criteria for the literature are as follows: (1) subjects of the included studies: women patients at the age of >18 years who had been diagnosed with HER2-positive breast cancer based on diagnostic criteria of breast cancer [8]; (2) interventions in the included studies: the control group treated with trastuzumab (CID, 163341910; CAS, 1018448-65-1) alone or trastuzumab combined with chemotherapy such as docetaxel, while the treatment group treated with trastuzumab combined with pertuzumab (CID, 472422729; CAS, 380610-27-5) (combined or not combined with chemotherapy); (3) outcomes indicators: primary outcomes including progression-free survival, gastrointestinal adverse reactions, and systemic adverse reactions and secondary outcomes including the rate of serious adverse reactions and death from adverse reactions (at least include any of the above indicators); and (4) the included studies were randomized controlled trials that avoided multiple biases.
The exclusion criteria are as follows: (1) other publication types, such as independent abstracts, case reports, preclinical studies, reviews, meta-analyses, editorials, conference reports, books, and letters; (2) animal studies; and (3) studies with overlapping data, missing or insufficient data, and similar data.
2.3. Data Extraction
Following the inclusion and exclusion criteria, two researchers conducted a search and initial conformity assessment based on titles and abstracts. After removing duplicate records and irrelevant records, both researchers evaluated all abstracts and identified potentially eligible records. Those deemed eligible were analyzed in full text. Disagreements were resolved through a discussion between the two researchers. If the resolution fails, a third researcher will intervene to judge and finally resolve the disagreement. During this study, all references were stored in Endnote X9 (Thomson ResearchSoft, USA). Extracted data included authors’ names, year of article publication, sample size of each group, interventions, study types, and outcome indicators.
2.4. Quality Assessment
The quality of the included studies was evaluated by using the Cochrane “risk of bias tool” [9] by two researchers independently. Seven domains were scored for the risk of bias, and each item was rated as low, unclear, or high risk of bias.
2.5. Statistical Analysis
All analyses were performed using STATA 17.0 software (StataCorp, China). I2 and χ2 tests were used to test the heterogeneity of the included studies. Random-effects models (I2 > 50% or P < 0.1) or fixed-effects models (I2 < 50% or P > 0.1) were applied for meta-analysis and statistical summary. For continuous variables, the standardized mean difference (SMD) and 95% confidence interval (CI) summary statistics represented the summarized results, while for categorical variables, the relative hazard ratio (OR) and 95% CI were used as measures. Sensitivity analysis was performed to confirm the stability of the overall effect. Beggar’s funnel plot and Egger’s test were employed to assess publication bias. P < 0.05 was considered a statistically significant difference.
3. Results
3.1. Screening Results
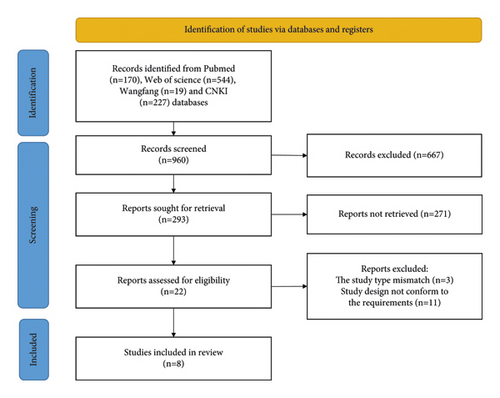
After screening the database, a total of 960 studies were retrieved. Finally, 8 studies were included in the meta-analysis after excluding ineligible literature based on the title, abstract, and full text [7, 10–16]. The literature screening process is shown in Figure 1. The included studies included 5 foreign-language studies, and 3 Chinese-language ones were published from 2015 to 2022. These studies involved a total of 7628 patients, including 3814 patients in the treatment group and 3814 patients in the control group. The characteristics of each included study are shown in Table 1. The risk of bias summary and the risk of bias graph are shown in Figures 2(a) and 2(b). All studies had a low risk of random sequence generation (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias.
| No | Author (year) | Age (tre/con) | Cases (female/male) | Intervention | Study type | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | |||||
| 1 | J.X. Tang, 2022 | 41.50 ± 8.45; 42.00 ± 8.50 | 30/0 | 30/0 | Trastuzumab + pertuzumab + docetaxel | Trastuzumab + docetaxel | RCT | ⑥⑦⑧ |
| 2 | L. Chen, 2022 | 52.18 ± 14.97; 51.91 ± 14.32 | 42/0 | 42/0 | Trastuzumab + pertuzumab | Trastuzumab | RCT | ④⑤⑦⑩⑫ |
| 3 | S.Y. MA, 2021 | 43.92 ± 21.77; 45.78 ± 20.86 | 23 | 23 | Trastuzumab + pertuzumab + basic treatment | Trastuzumab + basic treatment | RCT | ②④⑤⑧⑨ |
| 4 | Swain, Sandra M, 2020 | — | 407/1 | 396/0 | Trastuzumab + pertuzumab + docetaxel | Placebo + trastuzumab + docetaxel | RCT | ②③④⑤⑥⑦⑧⑨ |
| 5 | von Minckwitz, Gunter, 2017 | 51.7 ± 10.9; 51.4 ± 10.7 | 2397/3 | 2396/8 | Trastuzumab + pertuzumab + docetaxel | Placebo + trastuzumab + docetaxel | RCT | ②③⑪⑫⑬ |
| 6 | Gianni, Luca, 2016 | — | 107/0 | 107/0 | Trastuzumab + pertuzumab + docetaxel | Trastuzumab + docetaxel | RCT | ①②③④⑤⑥⑦⑧⑩⑪⑫⑬ |
| 7 | Fleeman, Nigel, 2015 | — | 402/0 | 406/0 | Trastuzumab + pertuzumab + docetaxel | Placebo + trastuzumab + docetaxel | RCT | ①⑩⑪⑫⑬ |
| 8 | Swain, Sandra M, 2015 | — | 402/0 | 406/0 | Trastuzumab + pertuzumab + docetaxel | Placebo + trastuzumab + docetaxel | RCT | ①②③④⑤⑥⑦⑧⑨ |
- RCT, randomized controlled trial; ①, progression-free survival, PFS; ②, diarrhea; ③, neutropenia; ④, nausea; ⑤, fatigue; ⑥, rash; ⑦, vomiting; ⑧, myalgia; ⑨, constipation; ⑩, any adverse event; ⑪, serious adverse event; ⑫, all cardiac disorders; ⑬, adverse event leading to death.
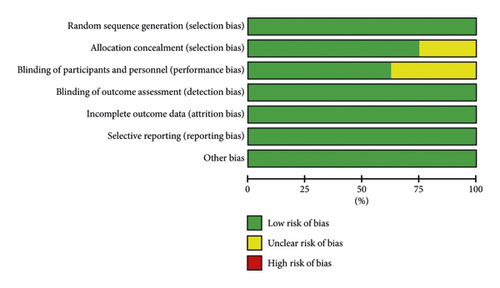
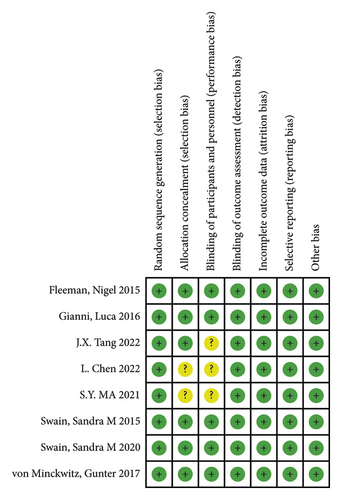
3.2. Meta-Analysis of Primary Outcomes
3.2.1. Progression-Free Survival
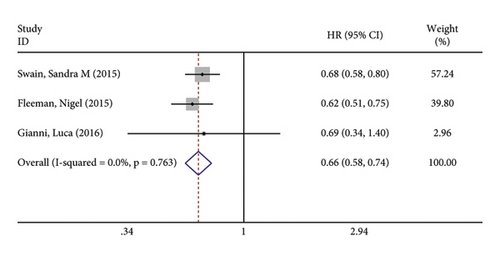
Three studies reported progression-free survival of patients after treatment. There was no significant heterogeneity in the included studies (I2 = 0.0%; P = 0.763). The pooled results using fixed-effects models found that patients in the treatment group had significantly shorter progression-free survival than in the control group (OR = 0.656, 95% CI (0.581, 0.741), P < 0.001) (Figure 3(a)). Furthermore, sensitivity analysis revealed (Figure 3(b)) that the results of the total effect did not change too significantly even when the studies were excluded one by one, and then, the left studies received analysis of the total effect, which indicates that the results are robust and reliable.

3.2.2. Gastrointestinal Adverse Effects
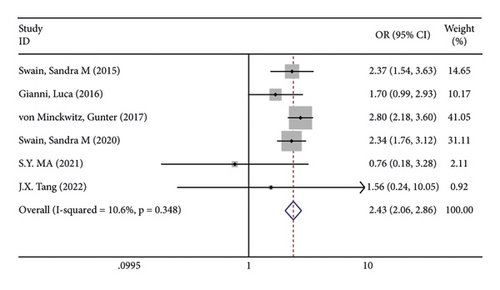
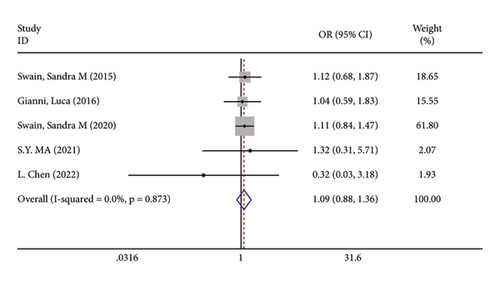
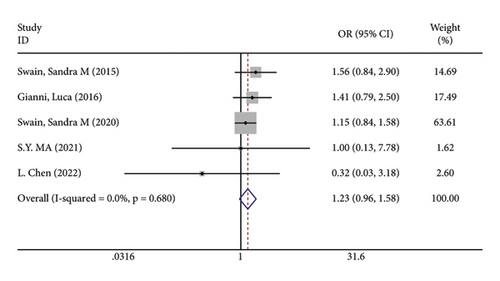
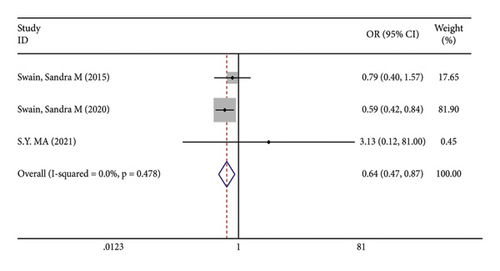
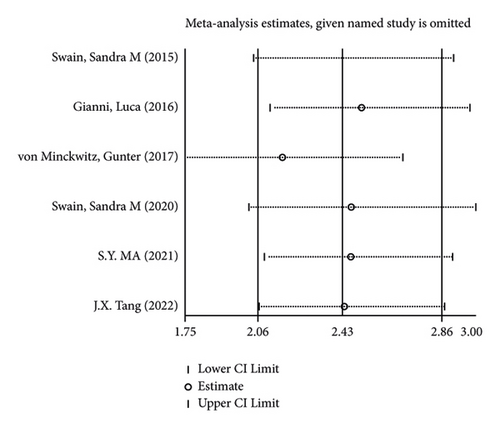
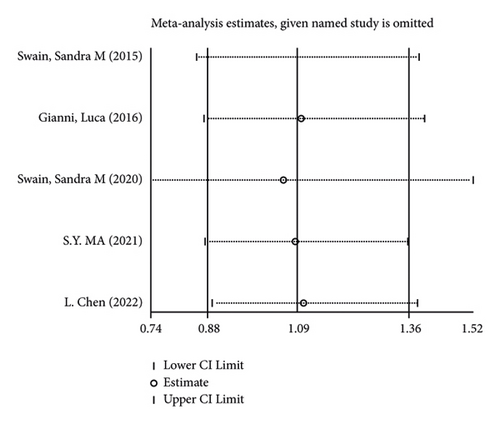
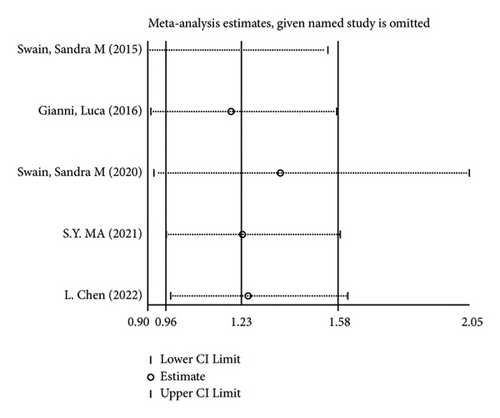
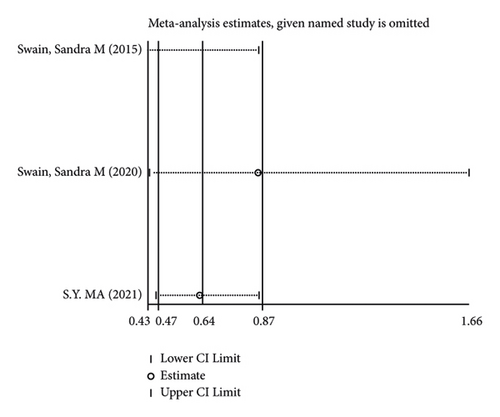
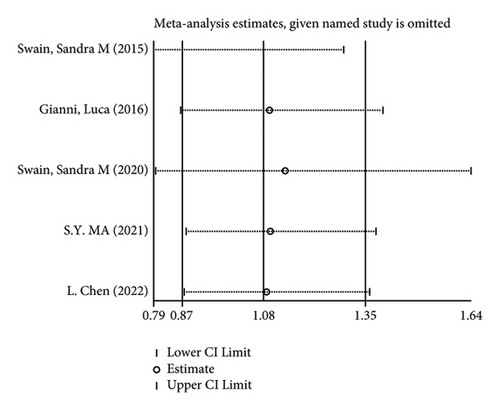
There were 5 studies mentioned diarrhea in breast cancer patients. According to the results of the fixed-effects model (I2 = 10.6%; P = 0.348) analysis, the incidence of diarrhea was obviously higher in the treatment group than in the control group (OR = 2.429, 95% CI (2.065, 2.856), P < 0.001) (Figure 4(a)). The comparison performed on nausea and vomiting in the two groups of patients of the five studies revealed no significant heterogeneity among these studies (nausea: I2 = 0.0%, P = 0.873; vomiting: I2 = 0.0%, P = 0.680). We summed up the effect sizes using fixed-effects models and discovered that there was no significant difference in the incidence of nausea (OR = 1.094, 95% CI (0.878, 1.364), P = 0.423) and vomiting (OR = 1.232, 95% CI (0.961, 1.579), P = 0.099) between the two groups of patients (Figures 4(b) and 4(c)). Among the five studies, three reported constipation in the patients without heterogeneity (I2 = 0.0%; P = 0.478). Compared to the control group, the treatment group exhibited a notable decrease in the incidence of constipation (OR = 0.641, 95% CI (0.473, 0.869), P = 0.004), as demonstrated by the statistics of fixed-effects models (Figure 4(d)).
As for the sensitivity analysis (Figures 5(a)–5(d)), the analysis of the total effect carried out following excluding each study one by one displayed no significant change in the results, indicating the robust reliability of the results.
3.2.3. Systemic Adverse Reactions
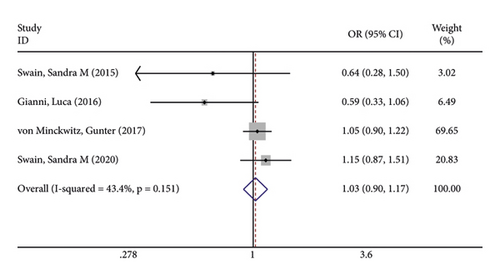
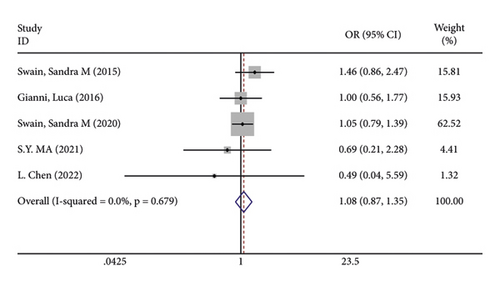
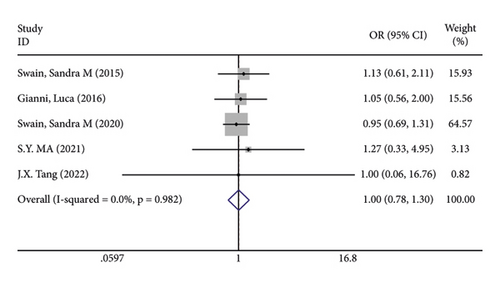
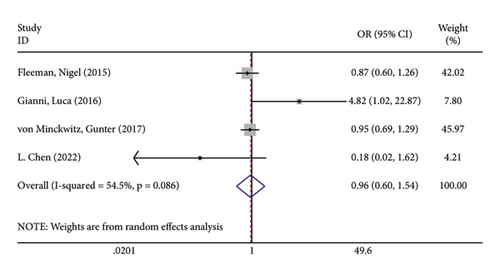
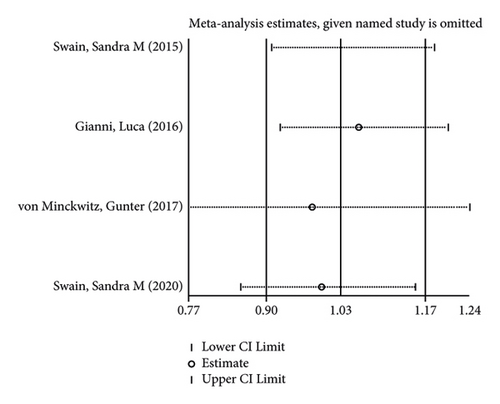
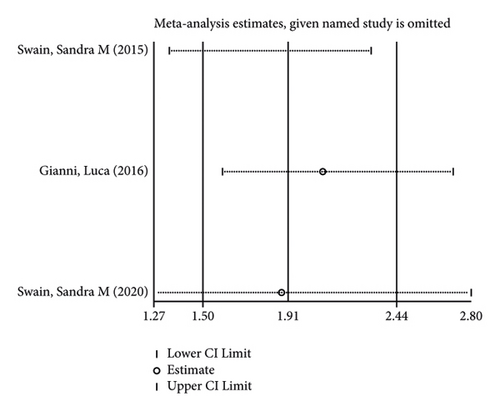
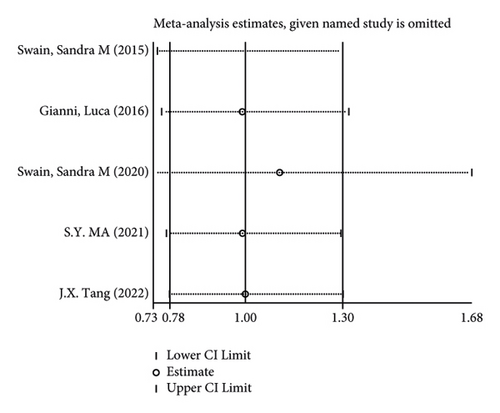
In terms of systemic adverse reactions, four studies described neutropenic leukopenia in patients with breast cancer and no significant heterogeneity was found in the four studies (I2 = 43.4%; P = 0.151). The results of the fixed-effects model analysis uncovered (Figure 6(a)) that there was no obvious difference in the incidence of posttreatment neutropenia between the two groups of patients (OR = 1.026, 95% CI (0.901, 1.168), P = 0.703). Five studies pointed out the risk of developing fatigue. The analysis results using fixed-effects models (I2 = 0.0%; P = 0.679) revealed no statistically difference in the incidence of fatigue between the two groups of patients (OR = 1.083, 95% CI (0.866, 1.354), P = 0.487) (Figure 6(b)). Three studies indicated rash in the patients with no heterogeneity (I2 = 39.2%; P = 0.193). Using fixed-effects models, the three studies were analyzed, and the incidence of rash was discovered much higher in the treatment group than in the control group (OR = 1.915, 95% CI (1.505, 2.437), P < 0.001) (Figure 6(c)). Five papers talked about the symptoms of myalgia. Of these studies, no heterogeneity existed (I2 = 0.0%; P = 0.982). After being pooled by fixed-effects models, the data displayed no significant difference between the two groups (OR = 1.005, 95% CI (0.779, 1.296), P = 0.971) (Figure 6(d)). Apart from that, there are four studies that documented cardiac disorders in the breast cancer patients. Similarly, there was no significant heterogeneity among them (I2 = 54.5%; P = 0.086). The random-effects model analysis also showed no significant difference between the two groups (OR = 0.965, 95% CI (0.604, 1.540), P = 0.880) (Figure 6(e)).

Furthermore, its sensitivity analysis revealed (Figures 7(a)–7(e)) that after excluding each study individually, none of the studies were found to have a significant effect on the overall effect. This indicates that the results of this study are relatively robust and reliable.
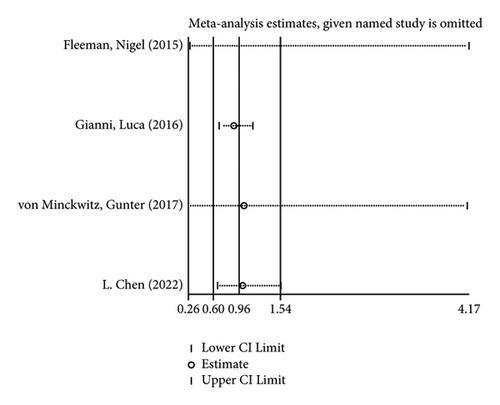
3.3. Incidence of Adverse Reactions
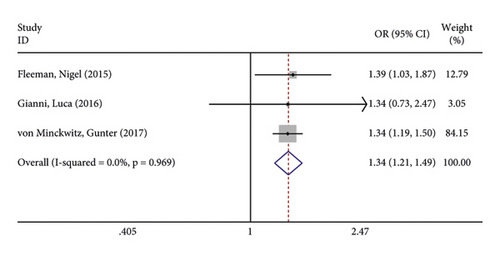
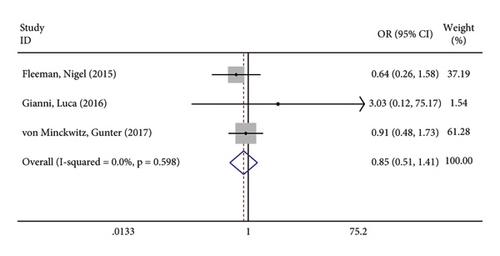
The incidence of serious adverse reactions was recorded in 3 studies showing no heterogeneity (I2 = 0.0%; P = 0.969). The results of the fixed-effects model analysis showed that the incidence of serious adverse reactions was markedly higher in the treatment group than in the control group (OR = 1.342, 95% CI (1.206, 1.494), P < 0.001) (Figure 8(a)). In addition, 3 articles documented adverse events leading to death, and there was no heterogeneity among the three studies (I2 = 0.0%; P = 0.598). The results of meta-analysis disclosed that no difference in adverse reaction was lethality exhibited between the two groups of patients (OR = 0.845, 95% CI (0.507, 1.409), P = 0.519) (Figure 8(b)).
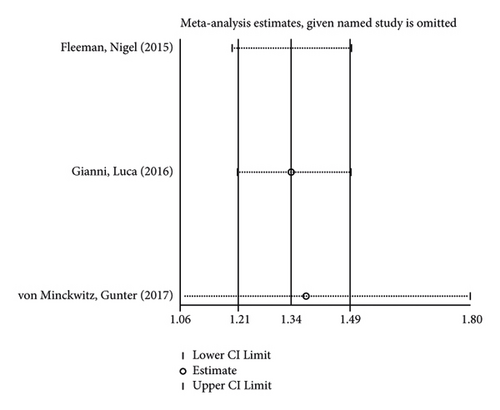
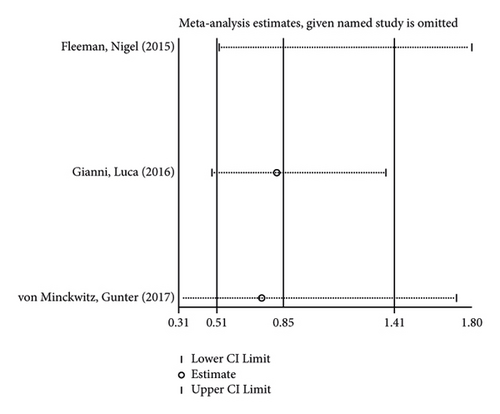
In the like manner, its sensitivity analysis revealed (Figures 8(c) and 8(d)) that the results for the incidence of adverse reactions and lethality of the reactions in both groups were relatively robust and reliable.
4. Discussion
Approximately 15–20% of patients with breast cancer overexpress HER2 [17], and most of whom have a poor prognosis with significantly shorter progression-free and overall survival, lower quality of survival, and a variety of adverse actions [18]. The combination of trastuzumab and pertuzumab is frequently utilized in clinical practice and has established itself as the gold standard of care for patients with unresectable HER2-positive breast cancer with metastatic or local recurrence, with the dual inhibitory effects of HER2 signaling through different mechanisms [14]. Reports on the efficacy and safety of the combination, however, show clinical heterogeneity. This study thus reviewed the efficacy and adverse effects of trastuzumab combined with pertuzumab in the treatment of HER2-positive breast cancer.
A database search resulted in the inclusion of eight studies for meta-analysis in this study. We found that patients receiving the combination treatment had quite shorter median progression-free survival than in the control group. This may be due to the fact that the drug combination frequently results in adverse reactions, which may be an important cause of disability and death in patients. Unfortunately, the combination treatment was associated with a greater risk of diarrhea, although gastrointestinal symptoms such as nausea, vomiting, and constipation did not differ between single and combination treatments. One study stated that diarrhea was common in patients receiving trastuzumab-based combination with pertuzumab in the setting of metastatic or early-stage breast cancer [19]. It can be explained by EGFR downregulation and/or blockade, which results in chloride hypersecretion and secretory diarrhea by reversing the acute inhibitory effect of epidermal growth factor on chloride secretion [20]. However, most diarrheas were mild, and their frequency decreased during the course of the therapy cycles.
Subsequently, we found that the combination of trastuzumab and pertuzumab induced rash. HER2, present in keratin-forming cells, is an epidermal growth factor receptor overexpressed in breast cancer cells [21]. In breast cancer patients, the combination therapy can result in epidermal differentiation failure and the development of rash [22]. The current meta-analysis showed no increase in cardiotoxicity after the combination treatment. The HER2-PI3K pathway, one of the most variable pathways in cancer, is essential for maintaining the physiological function of the heart, especially when cardiac dysfunction is already present [23]. But no clinical trial data demonstrated the correlation between cardiotoxicity and feedback mechanisms in the pathway [24]. In this study, the incidence of adverse reactions was lower in the treatment group than in the control group, while the risk of serious adverse reactions was higher than in the control group. Overall, there are still limitations in the promotion of this combination treatment.
Limitations of this study are displayed as follows: Although the included median progression-free survival is a good predictor of survival, the effect of long-term survival outcomes may be worse than those in the survival period [25]. On the other hand, as less than 10 studies were included for some indicators, the validity of the final conclusions was compromised by some publication bias. To improve the conclusions, more randomized controlled research should be looked for and included.
5. Conclusion
To put it in a nutshell, the combination of trastuzumab and pertuzumab may represent a substantial advance for the treatment of HER2-positive breast cancer. However, adverse effects, including diarrhea and rash, are limiting factors for the current promotion. Before the combination treatment can be of real value, more research is required to recognize and manage its side effects.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
QDG contributed to conception and design. QDG was involved in drafting the manuscript and revising it critically for important intellectual content. QDG and XFD made substantial contributions to acquisition of data. QDG and XFD were responsible for analysis and interpretation of data. All the authors have read and approved the final manuscript. All the authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




