Performance of Six Genetically Diverse Phylloxera Strains on 5C Teleki (V. berlandieri × V. riparia) Rootstock
Abstract
Background and Aims. Grapevine phylloxera, Daktulosphaira vitifoliae (Fitch), feeds on roots and leaves of Vitis spp. Susceptibility to phylloxera differs among rootstocks, such that Vitis spp. grafted onto resistant rootstocks can safeguard vineyards from phylloxera-induced damage in the long term. Diverse phylloxera genetic strains, however, vary in their ability to survive on different rootstocks. The 5C Teleki rootstock (V. berlandieri × V. riparia) is widely planted worldwide, but its resistance to phylloxera has not been characterised against the genetically diverse phylloxera strains present in Australia. Methods and Results. 5C Teleki roots and Vitis vinifera L. (positive control), either excised in Petri dishes or planted as whole plants in pots, were inoculated with eggs of six phylloxera strains (G1, G4, G19, G20, G30, and G38). On excised roots, G19, G20, G30, and G38 phylloxera survived to reproductive adults. The G1 and G4 phylloxera did not survive past the first instar stage. In potted vines, G4, G19, G20, G30, and G38 phylloxera strains induced nodosities on roots, but adults were only found on roots inoculated with G19 and G20 phylloxera strains. Conclusions. Results showed that 5C Teleki is resistant to the G1 phylloxera and susceptible to G19 strains. Performance of G4, G20, G30, and G38 differs depending on the assay used. 5C Teleki is likely tolerant of these strains. Significance of the study: the 5C Teleki rootstock is resistant to the G1 phylloxera strain but likely tolerant of others present in Australia. This implies that the rootstock can still host a population of phylloxera, and strict farm-gate hygiene should be employed to stop spread among vineyards and regions.
1. Introduction
Grapevine phylloxera (Daktulosphaira vitifoliae) (Fitch) is a significant pest of grapevines (Vitis spp.) worldwide. This sap-sucking pest devastated the European wine industry in the mid-1800s [1]. In Australia, phylloxera infestations led to the collapse of the wine industry in Geelong, the most important viticulture region until the early 1900s [2]. Native to North America, phylloxera co-evolved with the wild Vitis spp., which are resistant or tolerant to the root damage caused by this pest. Grafting European vine varieties onto American rootstocks is currently recognised as the best viticultural practice to safeguard vineyards from phylloxera-induced damage [3].
Phylloxera feeds on grapevine (Vitis spp.) leaves and roots. Feeding induces nodosities on young fibrous roots and tuberosities on older lignified roots. The nodosities and tuberosities act as food storage organs, and different life stages of phylloxera can be seen attached in clusters to these swellings [4]. The nodosities and tuberosities impede water uptake by the vines resulting in reduced leaf surface area for photosynthesis and leading to loss of vigour and production of small fruits [4–6]. These swellings subsequently split and expose roots to soil pathogens and infections, often leading to vine death [7, 8]. Research in the last decade has shown that the rate at which vines die is dependent on the genetics of different phylloxera strains attacking the vine [9, 10]. Unfortunately, Vitis vinifera L. vines are susceptible to most phylloxera biotypes and die within four to seven years following an infestation [11, 12].
Managing phylloxera with resistant rootstocks requires breeding of new rootstocks that are not only resistant to phylloxera but also meet the individual needs of growers and their land, including tolerance to lime, acceptability of scions, desired vine size, and grape varieties with compact fruits [3, 13]. Teleki rootstocks, such as 125AA Teleki-Kober, SO4 Teleki-Fuhr, 5BB Teleki-Kober, 5C Teleki, 8B Teleki, and 10A Teleki, have been favoured in Europe because of their resistance to phylloxera and are acknowledged worldwide as genetically rich propagation material [14]. The 5C Teleki rootstock became a popular option in the 1970s following the failure of the AXR #1 variety to withstand phylloxera infestations in the USA and Europe [15] although it is worth noting that 5C Teleki clones may differ when selected in different countries [16] and may have originally been selected from heterogenous material [17].
As rootstocks can vary in their resistance to specific phylloxera strains, it is crucial to assess the interaction between phylloxera strain and rootstock before widespread adoption of novel rootstocks [9, 18, 19]. Information on the performance of specific phylloxera strains on rootstock varieties is integral for developing tools such as the grapevine rootstock selector [20]. In Australia, ten commercial rootstocks have so far been evaluated for resistance, susceptibility, and tolerance against a selection of genetically diverse phylloxera genotypes [9]. The 5C Teleki rootstock is widely planted in Victoria and South Australia because of its tolerance to lime, moderate vigour, and suitability to grow in well-drained and fertile soils [3] (Howell 1987). Despite being widely grown, the ability of 5C Teleki to withstand phylloxera infestations is unknown but is expected to vary among phylloxera strains. Here, we assessed the susceptibility of 5C Teleki rootstock against six phylloxera strains (G1, G4, G19, G20, G30, and G38) by characterising their survival and development on both excised roots and in potted vines. This information could be used by growers when selecting new rootstock varieties, based on the presence of specific phylloxera strains in their region.
2. Materials and Methods
2.1. Insect Stock Cultures
Six genetically diverse phylloxera strains, G1, G4, G19, G20, G30, and G38, were used in experiments. For each phylloxera strain, insect cultures were established from eggs laid by a single adult [21]. G1 phylloxera strain was collected from the Yarra Valley in central Victoria, in the Maroondah Phylloxera Infested Zone (PIZ). G4 phylloxera strain was collected from the King Valley, G19 and G30 from Rutherglen, and G20 from the Buckland Valley, all in North East Victoria PIZ. Before the start of experiments, the identity of each phylloxera strain was confirmed using six nuclear DNA microsatellite markers as described by Umina et al. [22] and Agarwal et al. [23]. Phylloxera cultures were mass reared on excised roots of own-rooted V. vinifera “Chardonnay” under controlled conditions (25 ± 2°C) according to Kingston [24] under quarantine at Agriculture Victoria, Rutherglen.
2.2. Plant Material
Dormant vines of Riesling on 5C Teleki- and own-rooted Chardonnay on V. vinifera (positive control) rootstocks were provided by Yalumba Nursery in 2018 and planted in 80% general-purpose potting mix composed of composted pine bark, peat moss, and humic peat (Spotswood potting mixes and fertilisers, Yarra Glen, Victoria) mixed with 20% perlite in 20 cm diameter pots. Pots were kept in a shade house, and vines were fertilised with 3.5 g Osmocote™ (Scotts Osmocote®, Scotts Australia Pty Ltd) and 500 ml Thrive™ (Yates ©, DuluxGroup Australia) and watered for 2 min daily. The vines were kept in a shade house and drip irrigated for 2 min daily over six months to allow optimal root development before starting the experiments.
2.3. Excised Root Experiments
Each replicate consisted of a 5 cm length of root excised from a single plant of 5C Teleki. The excised root was positioned in a Petri dish (90 × 25 mm) lined with filter paper and inoculated with one of the six strains listed above (n = 10 replicates per phylloxera strain). For each replicate, we collected 10 eggs (between 1 and 5 days old) from stock cultures and placed them onto the root using a fine artist’s paintbrush (Sable spotter 300-R 2/0) moistened in molecular grade water. The Petri dish was then sealed with polyethylene film (GLAD ClingWrap Clorox Australia, Unley, South Australia), covered with aluminum foil, and incubated in a growth room at 25°C ± 2°C. The roots were watered and kept clean by wiping out visible fungal spores once every week using a fine paintbrush moistened with 80% ethanol. The same procedure was repeated for excised roots of own-rooted V. vinifera (Chardonnay) which acted as a positive control (n = 10 per phylloxera strain). No positive control was included for the G38 phylloxera strain due to a low rate of development resulting in few adults laying eggs within the study period.
We examined the roots 8, 18, 25, and 32 days after inoculation. On day 8, we recorded the number of first instars (hereafter referred to as crawlers); on day 18, 25, and 32, we recorded the number of intermediate instars and adults and the number of viable newly laid eggs, as well as the total number of live insects (i.e., total number of crawlers, intermediates, and adults). Intermediate stages were estimated based on the size [24] whilst adults were identified by clusters of eggs attached to or close to their abdomen. Newly laid eggs were determined as viable if they had a lemon/yellow colour and older eggs if they were a cream colour with characteristic red eye spots. Any eggs that were laid on days 18 and 25 were recorded, but then removed from the excised roots to discount a second generation.
- (i)
Survival: it is the number of adults that developed from the initial eggs inoculated on roots.
- (ii)
Net fecundity: it is the number of eggs produced/per adult/per day calculated at days 18, 25, and 32.
- (iii)
Gross fecundity: it is the total eggs laid over the lifetime of an adult calculated at day 32. This was summed up for the number of adults reached in each root for each of the three counting dates.
2.4. Potted Vines Experiments
Vines in pots were inoculated with phylloxera as per methods described in the study by Forneck et al. [21], Korosi et al. [25], and Clarke et al. [26]. Each replicate 5C Teleki vine (n = 8 per phylloxera strain) was inoculated with 20 eggs of a particular phylloxera genetic strain. First, eggs were collected using a moistened fine artist’s paintbrush and placed on filter paper. 5C Teleki vines were removed from pots so that the root system was exposed, allowing selection of fibrous (<1 mm diameter) and lignified roots (1 to 5 mm diameter). The filter paper containing the eggs was placed face down on the roots and immediately covered with a small amount of potting mix. A mesh netting (50 μm, Bugdorm Nylon Netting, Megaview Science CO, Ltd, Taiwan) was wrapped around the roots and potting mix containing the eggs and the ends of the cloth secured with a cable tie to enclose phylloxera, roots, and potting mix in a pocket (Figure S1). The vine was then repotted. A thin layer of sticky insect trap coating was applied around the base of each vine stem and the rim of each pot (TanglefootTM, the tanglefoot company, Grand Rapids, MI, USA) to avoid cross contamination between replicate vines. The vines were set up in the glasshouses at 22° ± 2°C and a relative humidity of 60–70% and fertilised with 100 ml of Charlie Carp fish fertiliser (1 : 10 dilution) (Charlie Carp LTD, Deniliquin, NSW, Australia) and drip irrigated for 2 min daily. A positive control, with own-rooted V. vinifera vines, was set up using the same method (n = 8 per phylloxera strain).
- (i)
Total adults combined for both the wingless and winged (alate) adults.
- (ii)
Total insects across all phylloxera developmental stages (eggs, first instars, intermediate instars, adults, and alates).
- (iii)
Number of nodosities on roots (for the potted vines used in this study, the roots did not have sufficiently developed lignified tissue to score tuberosities formation; for that reason, we chose to use nodosities on fibrous roots as an indicator of root damage following a phylloxera infestation).
2.5. Susceptibility Rating
The 5C Teleki vines were rated resistant when neither nodosities nor phylloxera were found living on roots; tolerant when nodosities and phylloxera et al. stages were found on roots at a level below that of V. vinifera; and susceptible when phylloxera reproduced in numbers is higher or equal to V. vinifera, with adults signifying the completion of an asexual life cycle [9].
2.6. Statistical Analysis
All statistical tests were performed in RStuido (version 4.1.1). We performed a series of generalised linear models (GLMs) and zero-inflated regression models to determine if variation in (i) survivorship (excised root assays), (ii) gross fecundity (excised root assays), (iii) net fecundity (excised root assays), (iv) the number of nodosities (potted vine assays), (v) total number of insects (potted vine assays), and (vi) the number of adults (potted vine assays) could be explained by phylloxera strain, rootstock (5C Teleki or V. vinifera), and number of days postinoculation (where appropriate) and their interactions. Interactions between genotype and rootstock were not included in our models for survivorship, and total number of insects due to a lack of power. GLMs were performed using the “MASS” package for nodosities, gross fecundity, and net fecundity, and zero-inflated regression models were performed using the “pscl” package for survivorship, total number of insects, and total number of adults. All models except for gross and net fecundity were fitted with a Poisson distribution. Gross and net fecundity were fitted with a negative binomial distribution. All models were assessed using likelihood ratio tests. Post hoc comparisons were performed using the “emmeans” package by comparing the performance of each phylloxera strain on 5C Teleki rootstock to their positive control (V. vinifera rootstock). Post hoc p values were adjusted for multiple comparisons using the Šidák method. G38 was not included in the analysis due to the lack of an adequate positive control.
3. Results
3.1. Excised Roots Experiments
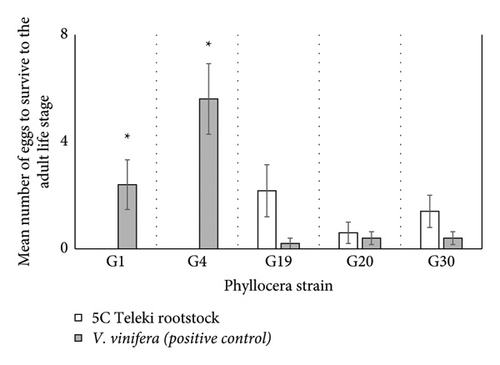
The model revealed a significant effect of phylloxera strain (df = 8; ꭓ2 = 23.74; p < 0.0001; Figure 1) and rootstock (df = 2; ꭓ2 = 43.05; p < 0.0001; Figure 1) on survivorship. Post hoc analysis revealed significantly more G1 and G4 adults survived on V. vinifera roots compared to 5C Teleki (G01: z-ratio −3.46 and p = 0.0005; G4: z-ratio = −5.290 and p < 0.0001; Figure 1). There was no significant difference in survivorship between the rootstocks for the G19, G20, or G30 strain (all p > 0.08). Indeed, G1 and G4 eggs did not survive past the first instar stage on 5C Teleki roots, whereas G19, G20, and G30 eggs all developed to reproductive adults (Figure 1). All phylloxera strains inoculated on excised own-rooted V. vinifera survived to the adult stage (Figure 1). The G38 eggs developed to reproductive adults (not included in the analysis).
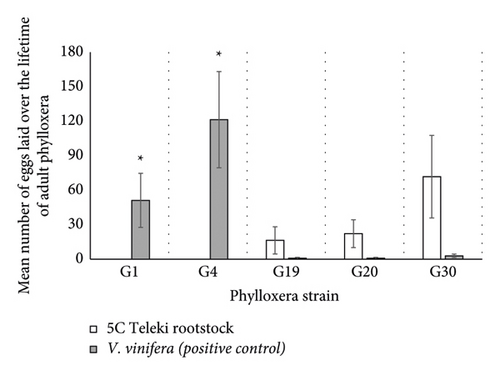
There was a significant effect of phylloxera strain (df = 8; ꭓ2 = 47.24; p < 0.0001; Figure 2) and rootstock (df = 2; ꭓ2 = 42.83; p < 0.0001; Figure 2) but not their interaction on gross fecundity. Importantly, G1 and G4 did not make it past the first instar; thus, their gross fecundity was zero. Post hoc analysis revealed that G1 and G4 strains experienced significantly higher gross fecundity on V. vinifera roots compared to 5C Teleki (G01: z-ratio −2.115 and p = 0.034;G4: z-ratio = −2.60 and p = 0.0092; Figure 2). Rootstock had no significant effect on gross fecundity on strains that were able to develop to the adult stage: G19, G20, or G30 strain (all p > 0.07).
There was no significant effect of phylloxera strain, rootstock, day postinoculation, or their interactions on net fecundity (all p > 0.1).
3.2. Potted Vine Experiments
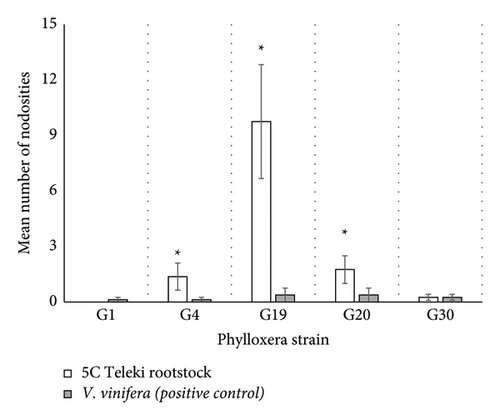
Our GLM revealed a significant interaction between phylloxera strain and rootstock on the number of nodosities induced (df = 4; ꭓ2 = 14.015; p = 0.0072; Figure 3). Post hoc analysis revealed that G4, G19, and G20 induced significantly more nodosities on 5C Teleki roots compared to the positive control (G4: z-ratio = 2.30 and p = 0.022; G19: z-ratio = 5.54 and p < 0.0001; G20: z-ratio = 2.42 and p = 0.015; Figure 3). There was no significant difference in the number of nodosities induced on either the 5C Teleki roots or V. vinifera roots for either the G1 or G30 strain. Hence, it is worth noting that G1 did not produce nodosities on 5C Teleki roots (Figure 3). G38 did induce nodosities on 5C Teleki roots but was not included in the analysis due to a lack of positive control.
There was a significant effect of phylloxera strain (df = 8; ꭓ2 = 1818.62; p < 0.0001; Figure 4(a)) and rootstock (df = 2; ꭓ2 = 28.36; p < 0.0001; Figure 4(a)) on the number of insects counted. Post hoc analysis revealed that significantly more insects were found on V. vinifera inoculated with G1, G4, G20, and G30 strains (G01: z-ratio = −4.12 m and p < 0.0001; G4: z-ratio = −3.54 and p = 0.0004; G20: z-ratio = −5.55 and p < 0.0001; G30: z-ratio = −2.80 and p = 0.0015; Figure 4(a)) whereas significantly more insects were found on 5C Teleki rootstocks inoculated with G19 (G19: z-ratio −299 and p = 0.0028; Figure 4(a)).
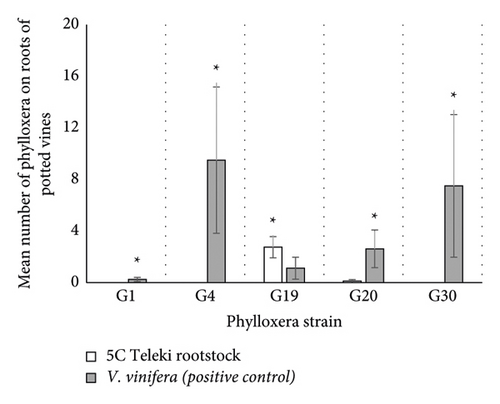
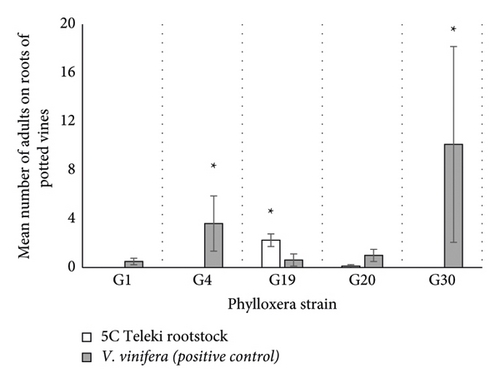
There was a significant effect of phylloxera strain (df = 8; ꭓ2 = 177.86; p < 0.0001; Figure 4(b)) and rootstock (df = 2; ꭓ2 = 19.15; p < 0.0001; Figure 4(b)), but not their interaction on the number of adults detected. Post hoc analysis revealed that significantly more adults were found on V. vinifera inoculated with the G4 and G30 strains (G4: z-ratio = −2.03 and p = 0.042; G30: z-ratio = −2.13 and p = 0.033; Figure 4(b)) whereas significantly more adults were found on 5C Teleki rootstocks inoculated with G19 (G19: z-ratio = 2.35 and p = 0.019; Figure 4(b)). No adults were recorded on 5C Teleki roots inoculated with G38, but G38 was not included in the analysis due to a lack of positive control.
3.3. Susceptibility Rating
On excised roots, 5C Teleki was rated susceptible to G19, G20, and G30 and resistant to G1 and G4 phylloxera (Table 1). In potted vines, 5C Teleki was rated susceptible to G19, tolerant to G4, G20, and G30, and resistant to G1 phylloxera (Table 1).
| Test | Phylloxera genetic strain | |||||
|---|---|---|---|---|---|---|
| G1 | G4 | G19 | G20 | G30 | ||
| 5C Teleki | Excised root | Resistant | Resistant | Susceptible | Susceptible | Susceptible |
| Potted vine | Resistant | Tolerant | Susceptible | Tolerant | Tolerant | |
- The rating is based on eight weeks of co-cultivation of the vines with phylloxera. Resistant: no survival to adult stage, tolerant: survival to adults but in lower numbers compared to V. vinifera, and susceptible: survival to adults in higher numbers compared to V. vinifera. A rating for the G38 phylloxera is not included as this strain was not tested against V. vinifera.
4. Discussion
Our results suggest that the 5C Teleki rootstock is resistant to G1 and shows some resistance to G4 using excised roots and potted vines experiments. The 5C Teleki rootstock was rated susceptible to G19 and either tolerant or susceptible to G20 and G30 depending on the assay. The number of eggs produced by G19, G20, and G30 on excised roots over just 32 days suggests that several generations could occur within the vine-growing season. This finding is significant because a single phylloxera adult contributes between 100 and 400 second-generation females per life cycle [27]. The asexual mode of reproduction typical of phylloxera allows for rapid growth in population due to the large quantities of eggs laid by a single female. Successful reproduction of specific genetic strains on 5C Teleki could support survival and population growth when conditions are favourable. High humidity and temperature and long day length have been cited in literature to favour phylloxera development and reproduction [28]. Although not included in the analysis due to the lack of positive control, G38 had low survival and gross fecundity on the excised root assays, and no insects or adults were recorded following the potted vine assays on 5C Teleki roots suggesting it is at least tolerant to this strain. The difference in the performance of each strain on the 5C Teleki rootstock suggests an interaction between rootstock and phylloxera strain. Rootstocks derived from Vitis species with American parentage have an evolutionary resistance to phylloxera, but our results clearly suggest that 5C Teleki is susceptible to at least some phylloxera strains. This is consistent with studies investigating the susceptibility of the 5BB Kober rootstock, which has similar parentage to 5C Teleki, showing that 5BB Kober is tolerant to G19, G20, and G30 and resistant to G1 and G4 phylloxera [9]. The G19, G20, and G30 phylloxera strains are genetically distinct to G1 and G4 [29], which may explain the differences in performances on 5C Teleki rootstocks. The G1 and G4 phylloxera strains are recognised as more virulent on the susceptible V. vinifera cultivar [22, 29]. The poor performance of G1 on 5C Teleki roots in both the excised and potted root assays suggests that 5C Teleki could be especially beneficial for regions in Victoria where this phylloxera strain dominates such as the Yarra Valley.
Nodosities induced on young fibrous roots play a significant role in providing food for developing phylloxera immature stages [21]. Nodosities supply a suitable environment for phylloxera development and protection from predators and are a source of nutrition [30, 31]. Nodosities are formed through the piercing of the parenchymal tissue by phylloxera, which stimulates the tissue to undergo hypertrophic cell division [32]. In our study, five of the phylloxera genetic strains tested induced a physiological response on the roots in the form of nodosities, yet only G19 and G20 completed an asexual generation on roots in the potted vine assays. This suggests that 5C Teleki rootstock provides an advantageous nutritional environment to sustain feeding for these genetic strains. This is consistent with previous studies. In Europe, De Benedictis et al. [15] found low, but significant, levels of phylloxera numbers on 5C Teleki roots and the initiation of nodosities on fibrous roots. Significant damage on 5C Teleki was also observed under field conditions with some phylloxera in Hungary [33, 34].
Nodosities can induce necrosis on vine roots, which has been shown to impact feeding by phylloxera and introduce fungal infections that can lead to vine deterioration [35, 36]. Previous studies have shown that necrotic nodosities can become an unsuitable feeding site for phylloxera as they are unable to supply the nutritional requirements to developing phylloxera stages [37]. Necrosis can be attributed to a potential hypersensitive reaction by vine roots that deter phylloxera from feeding [38] through a gene expression pathway that produces Auxin, which has been found in phylloxera saliva [39]. Auxin has been shown to cause necrosis on phylloxera resistant rootstocks, and there appears to be a differential gene expression pathway between genetically diverse phylloxera in Australia. Whether this is the case for 5C Teleki is unknown. In our study, necrosis was observed on roots of potted 5C Teleki vines inoculated with the G4, G19, G20, and G30 phylloxera (Supplementary Figure 2). This might explain the low number (if any) of adult and intermediate stages observed on potted 5C Teleki roots despite the completion of the asexual life cycle in our excised root assays. This difference highlights the importance of performing such assays under natural or seminatural conditions that allow the plant to have a physiological response to the inoculation.
In Australia, the performance of phylloxera on rootstocks provides the basis for rating rootstocks as resistant, tolerant, or susceptible. Performance ratings based on screening methods applied in this study are internationally recognised [12, 21, 30] and integrate excised root assays, potted plants (whole root system or roots-in-pockets), and small-scale field trials [11]. Each method has advantages in terms of replication and ease of use. Our results suggest that more than one screening method should be used. For example, the high number of eggs produced by the G20 and G30 strains on excised roots did not translate into high numbers of individuals in the potted vine assays, likely due to vine necrosis in the enclosed pockets; if we performed the excised root assays alone, we may have overestimated the susceptibility of 5C Teleki to these strains. Our results provide valuable information on the successful performance of certain phylloxera on 5C Teleki. A moderate population below ground, especially in the summer months when the reproductive efficiency is at its peak [40], could lead to quick spread into nearby susceptible rootstocks. Based on our findings, we may expect 5C Teleki roots to support moderate populations for some of the strains tested, specifically G19 and G20.
5. Conclusion
We have demonstrated that specific phylloxera genetic strains can survive and complete an asexual cycle on the 5C Teleki rootstock, which might imply a biosecurity risk. In the summer months, several generations of these strains, particularly G19 and G20, may be completed below ground on 5C Teleki plantings, and this could lead to high populations of the pest, causing first instars to move above ground. First instars could be transferred on machinery, footwear, or clothing between vineyards. This finding is particularly significant for regions where diverse genetic strains exist across multiple rootstocks where plantings of 5C Teleki predominate. Taken together, our results suggest that 5C Teleki is unsuitable for planting in regions with diverse genetic strains specifically G19 and G20. In contrast, our results suggest that the 5C Teleki rootstock is likely resistant to the G1 phylloxera strain and 5C Teleki could be especially beneficial for regions in Victoria, where this phylloxera strain dominates. Findings from this study provide information relative to rootstocks and phylloxera interactions, which is critical for decision-making during plantings in green or brown field and can be accessed through tools such as the Grapevine Rootstock Selector [20].
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
This research was cofunded by Wine Australia and Agriculture Victoria. The authors thank Dr Tony Dugdale for reviewing earlier versions of this manuscript. The authors appreciate the assistance of grape growers in north-east Victoria for allowing access to their vineyards to collect insects and vine roots used for maintaining stock phylloxera populations. The authors thank Ken Wilson who assisted in the setup of both the glasshouse and laboratory experiments. The Yalumba nursery, Nuriootpa, South Australia, is thanked for supplying 5C Teleki vines for the experiments.
Open Research
Data Availability
The data used to support the findings of this study are included within the supplementary information files.




