Co-pyrolysis of Hardwood Combined with Industrial Pressed Oil Cake and Agricultural Residues for Enhanced Bio-Oil Production
Abstract
Lignocellulosic biomass is the potential raw material for the production of biofuels through pyrolysis. It is an effective technique for converting biomass to biofuels. However, biofuel from agricultural residues and woody-based feedstocks shows poor fuel properties due to higher oxygen content. Co-pyrolysis is a promising process to produce high-quality bio-oil by two or more different materials. Forestry, industrial, and agricultural outcomes are the ideal co-feedstocks for improved bio-oil quality. In this study, individual and co-pyrolysis of hardwood, pressed mustard oil cake and corncob were conducted at a temperature of 500°C. Before conducting pyrolysis experiments, thermogravimetric analysis was conducted to evaluate thermal degradation behavior. Through individual pyrolysis, corncob yielded a maximum bio-oil of 43.9 wt%. On the other hand co-pyrolysis on binary blends of hardwood and corncob produced maximum bio-oil of 46.2 wt%. Compared to individual pyrolysis, the binary blend produced more bio-oil, suggesting a synergistic effect between hardwood and corncob. The decreased bio-oil yield of 40.1 wt% during co-pyrolysis of ternary blends suggests negative synergistic effects prejudiced by the volatiles available in the biomass mixture. The improved quantitative synergistic results in the co-pyrolysis process give crucial information for the development of feed-flexible, higher bio-oil production and clean operating systems. The characterization studies on bio-oil by Fourier transform-infrared spectroscopy (FTIR), gas chromatography–mass spectrometry (GC-MS), and 1H NMR spectroscopy have shown that the bio-oil is a combination of aliphatic and oxygenated compounds. The analysis of the heating value shows that the bio-oil can be utilized as a fuel for heating applications.
1. Introduction
The rapid development of industrialization, the growth of the population, and the shortage of fossil fuels encouraged researchers to find novel alternative energy sources. According to a study conducted in 2009, the availability of coal is decreasing fast and will reach zero in 2112, and it will be the only fossil resource after 2042 [1]. At present, numerous efforts are ongoing to identify environmentally friendly alternative energy sources. Out of many other alternative energy sources, research on biomass energy has played a significant role. More than half of the research on renewable energy technologies over the past two decades has concentrated on bioenergy (56%) and solar energy (26%) [2]. The abundant availability of biomass throughout the world can support researchers by creating higher research opportunities. The global available biomass per year was estimated to be 220 billion tonnes [3]. The availability of total biomass resources in a country varies depending on weather conditions and agricultural activities. But compared to solar and wind, it is not a seasonable one. In many countries, biomass is widely used for generating biofuels and chemicals.
In India, biomass is considered an important energy source in view of the benefits it offers. Biomass provides for more than 32% of global primary energy consumption, and about 70% of the world’s population relies on it for their energy needs. It is estimated that more than 750 million metric tonnes of waste biomass are generated per year and a surplus of 230 million metric tonnes of biomass is produced from agricultural processes [4]. Bioenergy is a strong alternative for addressing energy demand as well as emission challenges due to carbon neutrality [5]. In this context, thermochemical conversion has been considered a promising option in recent years due to its efficient usage [6]. Pyrolysis is one of the most popular thermochemical conversion processes and has attracted much attention because of the production of high value-added elements and its effective utilization [7]. Biomass is a lignocellulosic substance that is composed mainly of cellulose, hemicellulose, and lignin. In pyrolysis, at elevated temperatures, the macromolecules of the biomass will decompose in an inert atmosphere, leading to the generation of liquid oil, char, and gas [8]. Lignin is the most stable component which does not degrade at low temperature. In some cases, it decomposed when the temperature reached 700°C [9]. Cellulose and hemicellulose are the two polysaccharides which decompose between temperatures of 300 and 400°C. Pyrolysis of higher lignin biomass materials involves endothermic reactions; on the other hand, pyrolysis of lower lignin biomass materials involves an exothermic reaction [10]. Furthermore, it is evident that lignin is the primary determinant for decomposition and yield. According to Di Blasi and Branca [11], cellulose and hemicellulose are the causes of the formation of pyrolysis oil, whereas lignin promotes char and gas products. Due to increased energy density and easier transportation, pyrolysis oil has received a lot of interest among these products. The quantity of biofuel and its properties is generally influenced by feedstock characteristics and the method of pyrolysis [12]. At the same time, major parameters of the pyrolysis process, including reactor temperature, heating rate, residence time, size of the feedstock, and flow rate of the sweeping gas, also influence the yield quantity as well as quality.
Co-pyrolysis is the alternative technique of using two or more different feedstocks to improve the quality of bio-oils. Several studies have focused on employing various types of feedstocks for the co-pyrolysis process and have achieved good results [13]. The interaction between the raw materials and synergistic effects is influencing the yield percentage of the bio-oil [14]. Sowmya Dhanalakshmi et al. [15] utilized palm shell and lemongrass for the co-pyrolysis process and produced 47.10 wt% of pyrolysis oil. Hope et al. [16] conducted a co-pyrolysis process by blending three different biomasses such as sugarcane bagasse, poppy capsule pulp, and rice husk. Through individual pyrolysis, sugarcane bagasse pyrolysis yielded a maximum bio-oil of 27.4%. But co-pyrolysis of these biomass materials yielded more bio-oil than individual pyrolysis due to synergistic interactions. Wood and agricultural residues are the common feedstocks used for pyrolysis process. In many studies, wood and wood wastes are combined with many feedstocks to produce biofuels. Chen et al. [17] combined wood wastes with municipal sewage sludge for the production of biochar at 800°C. Echresh Zadeh et al. [18] utilized hardwood and softwood for bio-oil production. The obtained oil through this study was 30.2 wt% and 24.4 wt%. Co-pyrolysis of coal, rice straw, and wood was conducted by Krerkkaiwan et al. [13] in order to analyze the synergetic effect. During the reaction, the synergistic effect was obtained by transferring OH and H radicals. Samanya et al. [19] conducted co-pyrolysis experiments on a combination of sewage sludge, hardwood, rapeseed, and straw. In this study, the biomass fuel types had a significant impact on the co-pyrolysis process and bio-oil yield.
The goal of this research is to utilize hardwood, pressed mustard oil cake, and corncob straw for the co-pyrolysis process. These three feedstocks are obtained from forestry, industrial, and agricultural processes. To the best of our knowledge, there were no studies concentrated on the combination of woody, industrial, and agricultural wastes. The experiments are conducted on individual, binary, and ternary blends with the aid of a fixed bed reactor. The work is also aimed to find the interaction of the selected feedstock on the co-pyrolysis process and characterization of the bio-oil products.
2. Materials and Methods
2.1. Materials
The feedstocks used for this study are hardwood, pressed mustard oil cake, and corncob which belong to forestry residues, industrial by-products, and agricultural outcomes. The wooden chips are collected from a nearby saw mill; de-oiled cakes are collected from oil industries; and corncob are collected from nearby agricultural fields in Coimbatore, India. All three types of feedstocks were collected in a separate bag and stored in the laboratory. Before conducting the experiments, the samples were dried in open sunlight for more than a week. The dried samples are then milled and sieved into <0.5 mm in diameter. The sieved samples are further heated to dry for 1 hr at ±100°C to reduce moisture prior to the experiments. The tests were carried out by following ASTM standards. The CHNS analyzer (Elementar Vario EL-III) was employed to find its component analysis.
2.2. Reactor Set Up
The reactor employed for this investigation is a batch type fixed bed type. It mainly consists of a reactor, a condenser, and an oil and gas collecting system. The reactor has a 100 mm diameter and 150 mm length. The reactor is well insulated and heated by using an electrical heater. The temperature of the reactor is controlled by a PID controller and measured using K type thermocouples located at two points. The exit of the reactor is connected to the condenser unit. The surplus of ice water (5°C) is supplied to the condenser. During experimentation, the connection was ensured with no tar deposition.
2.3. Experimental Procedure
For each experiment, 60 grams of samples was prepared and loaded into the reactor. For individual pyrolysis, 60 grams of samples was loaded directly into the reactor core. For the co-pyrolysis process, binary blends were prepared by mixing two samples at a ratio of 1: 1 (30 + 30 grams). For ternary blends, the samples were blended at a 1 : 1 : 1 ratio (20 + 20 + 20 grams). The reactor was heated to 500°C with a heating rate of 10°C and kept for 30 min to complete volatilization. A total of 7 experimental runs were conducted using individual, binary, and ternary blends under the same experimental conditions. Table 1 displays the current experimental conditions used for this study. The experiments were conducted until no vapor was visually obtained from the reactor. The condensed oil is saved in a separate beaker and weighed. The char is collected separately and weighed after the reactor is cooled to an atmospheric temperature. The mas of the gas fractions were calculated by mass difference. In order to check the repeatability of the experimental yields, the experiments were conducted three times under the same operating conditions, and the average yield value was taken into consideration.
| Pyrolysis type | Feedstock |
|---|---|
| Individual pyrolysis | Hardwood |
| Mustard oil cake | |
| Corncob | |
| Co-pyrolysis | Hardwood + mustard oil cake |
| Hardwood + corncob | |
| Mustard oil cake + corncob | |
| Hardwood + mustard oil cake + corncob | |
2.4. Characterization Study and Product Analysis
The thermogravimetric study was conducted using the TGA-701 analyzer. This analysis was performed to examine the thermal behavior of the selected three biomass materials by measuring their degradation rates with respect to temperature and time. The analysis was conducted under a nitrogen environment by feeding 5 mg of sample into the furnace. The flow rate of the N2 was maintained at 50 mL/min. In this study, the feedstocks are heated from atmospheric temperature to 600°C at the constant heating rate of 10°C/min. The data obtained from this study was also used for an extended kinetic and thermodynamic study [20]. The elemental composition of the samples and oil products was measured with the help of the Elementar Vario EL-III. The heating value of the oils is found by a Parr-6772 bomb calorimetric thermometer. The FT-IR analysis of the oil was carried out using a BRUKER TENSOR 27 FTIR spectrometer. The spectra were recorded over a range of 400–4000 cm-1. For this analysis, the oil sample was diluted in KBr plate. The organic elements present in the oil were analyzed by Thermo MS DSQ II spectroscopy. For the analysis, helium gas was used as the carrier gas, and the column flow rate was fixed at 1 mL/min. The separation was done on a DB-35 column. The isothermal program of the oven was set to 50°C at the initial condition and raised to 250°C with standard increment of 5°C/min. The detailed operating condition of the GC is given in Table 2. The NIST library of mass spectra was used to identify the compounds. The 1H NMR analysis of the water free bio-oil was recorded with the help of the Bruker Ultrashield-400 and a high-performance digital FT NMR spectrometer.
| Instrument | GC-MS | |
|---|---|---|
| Make | Thermo MS DSQ II | |
| GC condition | ||
| Column | DB-35 | |
| Dimension | 30 m × 0.25 mm × 0.25 μm | |
| Injection mode | Split type | |
| Spit ratio | 10 | |
| Injection temperature | 200°C | |
| Flow control mode | Linear velocity | |
| Column flow | 10 ml/min | |
| Carrier gas | Helium | |
| Column oven temperature | 70°C | |
| Column oven temperature progress | ||
| Rate | Temperature in °C | Hold time in m |
| — | 70 | 5 |
| 10 | 250 | 7 |
| MS conditions | ||
| Ion source temperature | 200°C | |
| Interface temperature | 250°C | |
| Scan range | 50–650 m/z |
3. Results and Discussion
3.1. Materials Characterization
Table 3 illustrates the findings of the proximate and ultimate analysis of the selected samples, and Table 4 shows the properties of blended feedstocks. The properties in Table 4 are calculated by arithmetic mean value. From Table 3, it is observed that the weight percentage of carbon and hydrogen in corncob is high compared to the other two materials. At the same time, sulfur and nitrogen contents were identified as low when compared to hardwood and mustard oil cake. The hydrogen content of mustard oil cake is higher than hardwood and corncob. Compared to corncob and hardwood, the nitrogen content of mustard oil cake is higher. Proximate analysis is a realistic and more helpful way to determine the efficiency of any biomass material for biofuel production [21]. Volatile matter and fixed carbon are the two important indicators for pyrolysis biofuel. The higher volatile matter in the sample enhances the production of bio-oil and gas fractions. Whereas the presence of higher fixed carbon provides most biochar, it boosts the carbon conversion rate [22]. The moisture in the biomass restricts heat transfer to the core of the material and decreases the overall efficiency of the process [23]. The moisture content in mustard seed cake is identified as 10.76 wt% which is slightly more than hardwood and corncob. When the ash content of the materials was considered, it was found that mustard oil cake contained a higher ash percentage (6.9 wt%). A higher ash percentage in the sample always decreases the yield of biofuel.
| Parameters | Hardwood | Mustard oil cake | Corncob |
|---|---|---|---|
| Proximate analysis (wt%) | |||
| Volatile matter | 70.15 | 66.32 | 70.85 |
| Fixed carbon | 15.30 | 16.02 | 16.90 |
| Moisture content | 6.3 | 10.76 | 8.75 |
| Ash | 5.4 | 6.9 | 3.5 |
| Ultimate analysis (wt%) | |||
| C | 46.21 | 41.21 | 40.15 |
| H | 6.4 | 7.20 | 5.95 |
| N | 2.1 | 6.41 | 1.21 |
| S | 0.3 | 0.9 | 0.2 |
| O (by difference) | 45.0 | 45.18 | 52.49 |
| Volatile matter | Moisture content | Ash | |
|---|---|---|---|
| Hardwood + mustard oil cake | 68.23 | 8.53 | 6.15 |
| Hardwood + corncob | 70.5 | 7.52 | 4.45 |
| Mustard oil cake + corncob | 68.58 | 5.2 | 5.2 |
| Hardwood + mustard oil cake + corncob | 69.1 | 8.63 | 5.26 |
3.2. Thermogravimetric Analysis
3.2.1. Thermogravimetric Analysis of Individual Biomass
The TGA and DTG analysis of three selected individual biomass and their mixtures are carried out under a nitrogen environment as shown in Figures 1 and 2. Around 5% to 8% of mass loss was obtained due to the evaporation of moisture between 30°C and 140°C. Sudden mass loss for hardwood, mustard oil cake, and corncob appeared at 380°C, 320°C and 300°C, respectively, which represents the release of volatiles. The volatiles during pyrolysis for all the biomass samples were released in three steps. The initial step is the evaporation of moisture content, whereas the second phase is the release of volatiles due to the breakdown of molecules. The major destruction of mass was occurred for mustard oil cake, hardwood, and corncob at 360, 375, and 380°C, respectively. From the analysis, it was confirmed that all three biomass materials were completely pyrolyzed at 470°C. After reaching this temperature, the constant weight loss represents the burning of char particles until 650°C [24]. The unburnt char obtained at the end of the study was accounted to 22% for hardwood, 18% for mustard oil cake, and 17% for corncob. The presence of higher volatiles and lower ash in the corncob contributed to the rapid breakdown compared to the other two biomass materials. This is also the same as the outcomes of the study conducted by Munir et al. [25]. The peak at 250 to 350°C in the DTG analysis represents the decomposition of cellulose and hemicellulose. Compared to lignin, cellulose and hemicelluloses start to decompose at lower temperatures [26]. Extractives and decomposition of cellulose and hemicelluloses followed by lignin breakdown or char formation are the common processes in biomass pyrolysis. From thermogravimetric study, it can be understood that the maximum conversion and reaction is found for all biomass materials between 250°C and 470°C. Overall, the selected materials for this study have the potential to be converted into biofuels or energy.
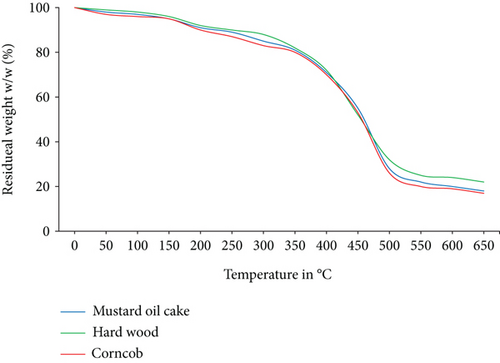
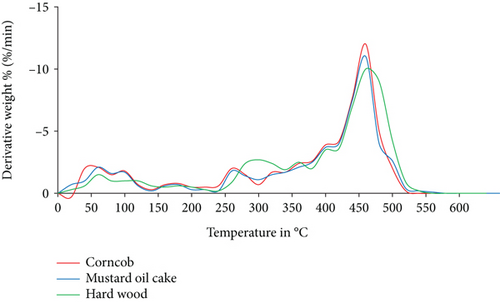
3.2.2. Thermogravimetric Analysis of Binary and Ternary Biomass Blends
The TGA and DTG analysis of the binary and ternary biomass blends is shown in Figures 3 and 4. Similar to the individual biomass materials, the binary and ternary blends were decomposed at temperature ranges from 150°C to 470°C. For all the blends, the maximum mass loss occurred at a temperature between 350°C and 470°C. Compared to other blends, the decomposition of the ternary blend starts late at a temperature of 370°C. The higher amount of volatile matter in the blend of hardwood and corncob contributed to the rapid breakdown compared to the other two binary and ternary blends. The char particles obtained at the end of the analysis were accounted 20% to 24% for binary blends and 16% for ternary blends. From the analysis, it was confirmed that all the blends were completely pyrolyzed at 475°C.
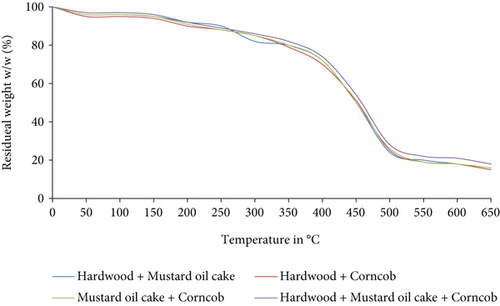
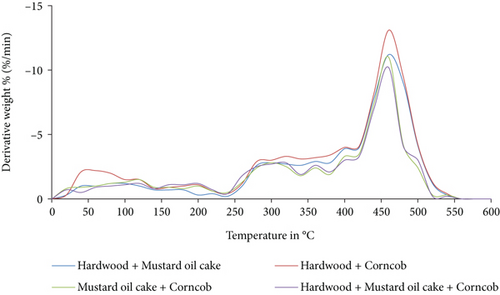
3.3. Product Yields
3.3.1. Individual Pyrolysis Characteristics
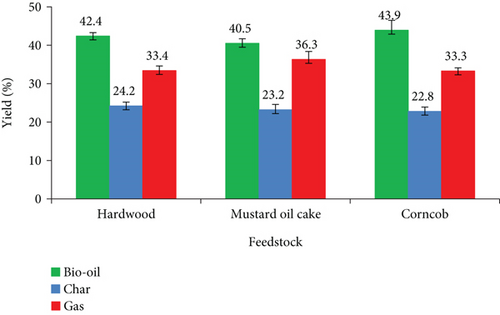
The product yields of hardwood, mustard oil cake, and corncob pyrolysis are shown in Figure 5. The temperature of the reactor has a substantial impact on both conversion and bio-oil production. Due to lower heat transfer phenomena, lower temperatures say below 350°C are always preferred for the production of higher char. When the temperature exceeds 600°C, most of the reaction products are noncondensable gases. Higher gas products at higher temperatures are due to the secondary cracking of the pyrolysis vapors. Prepyrolysis is the initial stage of material degradation, which occurs between 120°C and 200°C. The removal of moisture, the bond breaking, and the formation of free radicals occur during this stage. Pyrolysis of cellulose begins below 100°C and is characterized by a decrease in the degree of polymerization. During heating, the hemicellulose decomposes faster than cellulose at a temperature between 200°C and 250°C compared to cellulose between 240°C and 350°C [27]. The decomposition of cellulose and hemicellulose is the main reason for the formation of the maximum bio-oil yield. Generally, more char yield can be obtained by decomposing the feed particle at a lower temperature, which happens at heteroatoms inside the structure [28]. The extensive disintegration of biomass material at high temperatures induces high molecular dislocation and produces a variety of chemical components. According to the literature [29–31], massive conversion of biomass to bio-oil and its fragments occurs when the reaction temperature is kept between 400 and 500°C. The experiments in this phase are conducted under the same operating conditions of 500°C with a heating rate of 20°C/min. In the experiments, corncob produced a higher bio-oil of 43.9 wt%, whereas hardwood and mustard oil cake yielded 42.4 wt% and 40.5 wt%, respectively. The higher volatile matter present in the corncob may be the reason for yielding maximum bio-oil. According to Asadullah et al. [32], volatile matters are transformed into bio-oil as they condensate, and feedstock substantially improves the yield of bio-oil. The increased bio-oil production from corncob could be attributable to the presence of higher cellulose and hemicellulose. They are highly volatile and help in the formation of bio-oil [33]. The lower bio-oil from hardwood and mustard oil cake attributed to the presence of lower volatile matters compared to corncob. In addition to that, there is a strong relationship between bio-oil production and ash in the biomass materials. Ash in the biomass is generally favored for char production [34]. Ash is a noncombustible material and remains in solid form. The higher percentage of ash in mustard oil cake produced the most of char during pyrolysis. Hardwood, mustard oil cake, and corncob produced maximum char yields of 24.2 wt%, 23.2 wt%, and 22.8 wt%, respectively. It is also confirmed that hardwood produces a maximum char of 24.2 wt% since it has higher ash content. The amount of gas produced from mustard oil cake is 36.3 wt%. The production of noncondensable gas from hardwood and corncob is almost the same.
3.3.2. Co-pyrolysis Characteristics with Binary Blends
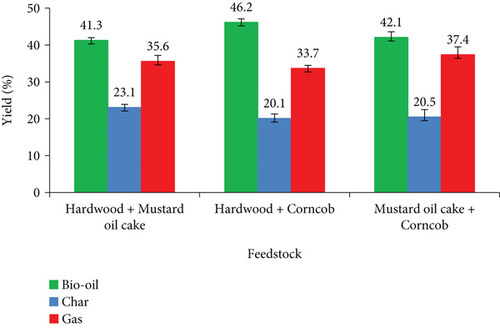
Figure 6 shows the product yields during co-pyrolysis utilizing binary blends. During the co-pyrolysis process, a maximum bio-oil yield of 46.2 wt% is acquired from the combination of hardwood and corncob. For this combination, there is no synergistic effect identified for gas yields. The amount of gas produced in this combination is equal to the arithmetic mean of the gas produced from individual pyrolysis. There is a negative synergistic effect on char yield with increased bio-oil yield. When hardwood is pyrolyzed individually, it produces 42.4 wt% of bio-oil, while corncob produces 43.9 wt% of bio-oil. But the combination of these two materials yielded 46.2 wt% of bio-oil, which is more than the arithmetic mean value of bio-oils obtained from individual feedstocks. Co-pyrolysis of hardwood with mustard oil cake and corncob with mustard oil cake produced 41.3 and 46.2 wt% of bio-oil, respectively. The production of bio-oil from hardwood and corncob combined with mustard oil cake is lower due to the lower volatile content in the combined feedstocks. It is clearly identified in Table 4. The higher ash content with the combination of hardwood and mustard oil cake produced more char products than other combinations, which is reliable from previous studies [35, 36]. During the co-pyrolysis reaction, radical interactions can have synergistic effects. It is based on the composition of the biomass material, temperature, heating rate, and hydrogen exchange [37]. Among them, blending feedstock is a crucial one that can have a substantial impact on synergistic effects, and it can be modified complicatedly [38].
3.3.3. Co-pyrolysis Characteristics with Ternary Blends
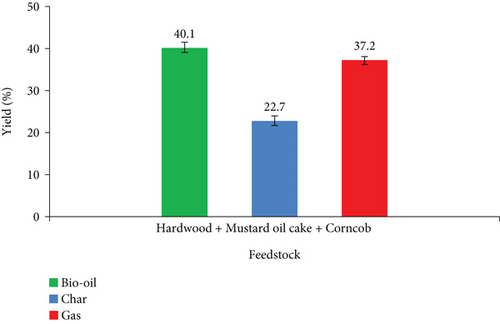
Figure 7 illustrates the product yields during co-pyrolysis utilizing a ternary blend. In this phase, the production of bio-oil is decreased compared to binary blends. There is a negative synergistic effect on bio-oil yield with increased char and gas production. When hardwood is pyrolyzed individually, it produces 42.4 wt% of bio-oil, mustard oil cake produces 40.5 wt% of bio-oil, and corncob produced 43.9 wt% of bio-oil. But the combination of these three materials yielded 40.1 wt% of bio-oil, which is lower than the arithmetic mean value of bio-oils obtained from individual feedstocks. According to Zhu et al. [39], the negative synergistic effects were prejudiced by the volatiles available in the biomass mixture. The mechanism of the synergistic interaction between biomass materials is unclear [40]. In ternary blends, the total availability of volatile contents is 69.1 wt% which is less than the binary mixture of hardwood and corncob, producing lower bio-oil. In this phase, positive synergy was identified in gas production. When hardwood is pyrolyzed, 33.4 wt% of gas is released, mustard oil cake produces 36.3 wt% of gas, and corncob produces 33.3 wt% of gas. But the combination of these three materials yielded 37.4 wt% of gas which is 9.04% higher than the arithmetic mean value. This may be owing to the catalytic effect of ash which promotes secondary reactions. As a result, the produced bio-oil was further degraded into gas products [41]. Previously, Gong et al. [42] and Zhang et al. [43] showed a positive synergistic effect on gas products with decreased oil and char yield during the co-pyrolysis process.
3.4. Calorific Value Analysis
Table 5 shows the results of the higher heating value of all bio-oils. The bio-oil obtained from hardwood and corncob blends shows a higher heating value of 26.50 MJ/kg. The observed higher heating value led to a synergistic effect. The lower colorific value of the bio-oil obtained from mustard oil cake is due to the presence of higher oxygen content with lower volatile content [44]. Bio-oil with heating value of more than 20 MJ/kg is recommended for use as a low-energy fuel. For agricultural biomass, which is actually good enough, the lower heating value can be improved by various chemical processes.
| Source | Heating value |
|---|---|
| Hardwood | 23.42 |
| Mustard oil cake | 15.30 |
| Corncob | 22.82 |
| Hardwood + mustard oil cake | 20.25 |
| Hardwood + corncob | 26.50 |
| Mustard oil cake + corncob | 19.65 |
| Hardwood + mustard oil cake + corncob | 17.10 |
3.5. Chemical Analysis
The chemical characterization study to find the presence of functional groups and chemical elements was done by FTIR, GC-MS, and 1H NMR analysis. The bio-oil acquired from co-pyrolysis of hardwood and corncob was used for this analysis since it yielded the maximum bio-oil in this study.
3.5.1. FTIR Analysis
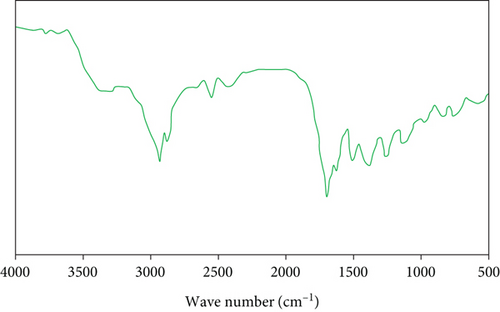
Figure 8 shows the FTIR spectra of the bio-oil. As expected, the spectra show the occurrence of alcohol, phenolic compounds, and carboxylic acid components. In the spectra, O-H stretching vibrations between 3200 cm-1 and 3450 cm-1 indicate the existence of polymeric hydroxyl compounds and alcohols. The aliphatic and aromatic C–H stretching vibrations appeared between 2800 and 3000 cm-1. The carbonyl groups were also seen in the bio-oil between 1650 and 1750 cm-1. Alkenes and aromatic chemicals are found in the sample between 1600 and 1650 cm-1. The bending vibration of the aliphatic C–H groups also appears between 1350 and 1450 cm-1. The C–O stretching and O–H bending vibrations appeared between 1200 and 1250 cm-1.
3.5.2. GC-MS Analysis
Table 6 shows the presence of various chemical elements identified through GC-MS. These elements are identified with respect to retention time related to peak area. More than 35% of phenols and their derivatives are identified in this analysis. The degradation of lignin in the feedstock may be responsible for the presence of these phenolic elements. The bio-oil was found to be made up of several functional groups, including aliphatic, aromatic, ketone, ester, phenol, and fatty acids. Several processes, such as dehydration, decarboxylation, and decarboxylation of the biomass constituents, are responsible for the variety of chemical compounds [45, 46]. The major identified compounds are phenol 2,5-dimethyl-, phenol 2,6-dimethoxy-, phenol 2-methoxy-4-vinylphenol, and 2-isopropyl-2,5-dihydrofuran with an area percentage of 11.40, 10.91, 8.22 5.41, and 5.36, respectively. Phenols and their derivatives are widely used in the cosmetic and food industries. Some of the elements identified are combustible and can be used as biodiesel for energy and power production. The obtained chemical elements are in good agreement with previously published reports on wood-based biomass pyrolysis [47, 48].
| RT/min | Compound name | Molecular name | % area |
|---|---|---|---|
| 5.31 | 2-Furanmethanol | C5H6O2 | 2.11 |
| 6.26 | 2-Ethylhexyl benzoate | C15H22O2 | 0.96 |
| 7.83 | Trans-2-furanmethanol | C5H6O2 | 4.52 |
| 10.25 | d-Glucoheptose | C7H14O7 | 0.44 |
| 15.26 | Phenol, 2,5-dimethyl- | C8H10O | 11.40 |
| 17.56 | Trans-propenylsyringol | C11H14O3 | 1.05 |
| 19.22 | Benzene | C6H6 | 1.74 |
| 19.99 | 5-Hydroxymethyl-2-furaldehyde | C6H6O3 | 4.30 |
| 21.11 | Furfural | C5H4O2 | 4.63 |
| 21.52 | Cyclodecasiloxane, eicosamethyl- | C20H60O10Si10 | 2.01 |
| 22.58 | Phenol, 2,6-dimethoxy- | C8H10O3 | 10.91 |
| 23.40 | Methanol | CH3OH | 4.55 |
| 24.63 | d-Mannose | C6H12O6 | 0.77 |
| 25.09 | Phenol | C6H6O | 8.22 |
| 26.24 | 2-(2,4,6-Trimethylphenyl)butylamine | C13H21N | 1.70 |
| 29.78 | Linalyl 2-methylpropanoate | C14H24O2 | 0.96 |
| 30.15 | 2,3,5-Trimethoxytoluene | C10H14O3 | 1.82 |
| 31.12 | Di-(2-ethylhexyl)phthalate | C24H38O4 | 3.60 |
| 31.45 | 2-Methoxy-4-vinylphenol | C9H10O2 | 5.41 |
| 31.84 | Butanoic acid | C24H34O6 | 1.72 |
| 32.94 | Cyclopentanol | C5H10O | 3.33 |
| 34.88 | 2H-Pyran, 2-(2 heptadecynyloxy)tetrahydro- | C22H40O2 | 3.75 |
| 35.45 | 1,2-benzendiol | C6H6O | 1.99 |
| 36.18 | 2-Isopropyl-2,5-dihydrofuran | C7H12O | 5.36 |
| 36.41 | Oleic acid | C18H34O2 | 3.12 |
| 37.70 | 4,5,6,7-Tetrahydrophthalimidine | C8H11NO | 0.22 |
| 37.98 | Stigmasterol | C29H48O | 0.56 |
| 38.41 | 2,20-Dioxospirilloxanthin | C42H56O4 | 3.07 |
| 39.14 | Octadecenoic acid | C18H36O2 | 2.04 |
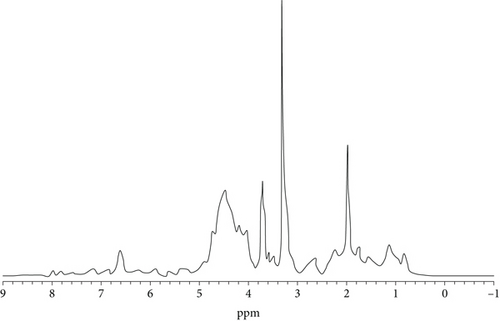
3.5.3. 1H NMR Analysis
The structural determination of chemical compounds requires 1H NMR studies. The 1H NMR provides information about the proton environment. Figure 9 shows the 1H NMR spectra of the bio-oil obtained from hardwood and corncob. The presence of alkoxy, ketone, olefinic, aromatic, and alcohol in the bio-oil is revealed by this analysis at 0.5–2.5 ppm, 3–3.6 ppm, 4.2–4.9 ppm, and 6.7–6.9 ppm. The obtained results are also consistent with Kumar et al. [49], Yorgun and Yıldız [50], and Soni and Karmee [51].
4. Conclusion
In this work, the co-pyrolysis of industrial and agricultural biomass with wood wastes was examined to evaluate the synergistic effects between the materials. Pressed mustard oil cake and corncob along with hardwood were investigated by preparing binary and ternary blends as lignocellulosic representatives for individual and co-pyrolysis processes. The co-pyrolysis of hardwood with corncob enhances the conversion efficiency during pyrolysis. The highest degree of synergy was perceived with hardwood with corncob followed by mustard oil cake and corncob. The decrement in bio-oil production with ternary blends suggested negative synergistic effects opinionated by the lower volatiles in the mixture. Co-pyrolysis is a recommended approach for sustainable energy production with improved carbon conversion and volatile yield. The outcomes of the study suggested to produce bio-oil with improved quality using different lignocellulosic biomass that exhibited interaction.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.




