[Retracted] Bioactivation of Polyaniline for Biomedical Applications and Metal Oxide Composites
Abstract
In this work, the oxidative chemical synthesis of polyaniline (PANI) in the presence of glutamic acid (GA) is presented, using ammonium persulfate (APS) as the oxidizing agent. Syntheses were performed by varying the molar ratio of aniline:amino acid:oxidant. The products of the different reactions were characterized by SEM, TEM, and FTIR techniques. It was observed that the molar ratio of aniline:amino acid:oxidant used in the synthesis determines the composition and conformation of the resulting polymer and its morphological and electrochemical properties. Composite hydrogels were prepared by incorporating the drug-loaded PANI nanofibers in situ through polymerization and cross-linking of acrylamide. TEM images of the cross-section of the hydrogel revealed the formation of a three-dimensional system of the polyaniline nanofibers maintained by the insulating matrix of the polyacrylamide hydrogel. The in vitro release of the drug from the hydrogels composed of polyacrylamide/polyaniline against buffer solutions at different pH and temperature was studied, using orbital agitation. Finally, considering the potential of hydrogels composed of polyacrylamide/polyaniline for the controlled release of drugs, a study was conducted to evaluate their cytotoxicity against normal mouse subcutaneous tissue cells.
1. Introduction
Polyaniline has received much attention among conductive polymers, and extensive research has been done on it in both its native and functionalized forms. This is due to the ease with which polyaniline and its composites can be made. Polyaniline is traditionally synthesized by oxidative polymerization [1] from an aniline monomer or an anilinium salt using chemical synthesis and electrochemical synthesis [2]. Polyaniline is one of the most researched polymers due to its wide range of applications. The biocompatibility, biodegradability, and antiproliferative power of the polyaniline against pathogenic bacteria, as well as its conjugation capacity with biologically active molecules, have led to its use in enzymatic biosensors for triglyceride detection, support for enzyme immobilization, controlled drug release systems, tissue engineering, artificial muscles, and nerve growth and regeneration [3–5]. The reversible volumetric changes that polyaniline undergoes under controlled electrical pulses, on the other hand, have been used in the fabrication of actuators, as well as other biomedical applications [3–6]. A gel is a three-dimensional network of flexible chains made up of segments that are connected in a specific pattern and swollen by a liquid. If the solvating liquid is organic, it is called an organogel; if the solvating liquid is water, it is called a hydrogel. Hydrogels are made up of natural or synthetic polymers that intertwine to form a network that swells to form soft and elastic materials when exposed to water or biological fluids [7]. These systems increase their volume significantly while maintaining their shape until they reach physical-chemical equilibrium. Hydrogels that are sensitive to an electric field are gaining popularity due to their numerous applications. These materials have been used in the pharmaceutical industry for the controlled release of drugs. Sheng Tsai et al. [8] made electroconductive hydrogels out of polyvinyl alcohol cross-linked with diethyl acetamido-malonate and polyaniline as the conductive component. Electrical control was used to evaluate the hybrid system for cumulative ON-OFF indomethacin release. The efficiency of these devices was around 70% [8]. Hydrogels’ properties have allowed them to be used in biomedical applications such as biosensors, drug delivery systems, contact lenses, catheters, and wound dressings [9]. One of the most popular applications is matrices for immobilizing and stabilizing enzymes [8, 9]. Because of this, conductometric, amperometric, and optical transduction have been used in biorecognition systems. We can use it as a microbioreactor to capture and stabilize biological molecules and high control of biological reactions because of its high water content and structural distribution. Hydrogels have been used as bioactive films to hold various types of antibodies and to make DNA biochips in this way [9, 10]. Their ability to change volume and shape in response to solvent composition, temperature, pH, and electric field has led to their use in MEMS and artificial muscles. Its application in genetic engineering has been enabled by its high water content, elastic modules that are very similar to human tissues, and biocompatibility properties in vitro and in vivo [11].
Generally, electroactive films or polymeric membranes are used to release, exchange, or move specific molecules, usually ions. When a concentration gradient is maintained on each side of the membrane, cyclically increasing the concentration within the membrane, continuous electrical pumping is possible. When an electric field is established on opposite sides of the membrane, the oxidation states of the polymer are altered, resulting in release activation. Zhou et al. were the first to show that the oxidation state of anions influences their transport through polypyrrole films [12]. Tong-Sheng Tsai et al. [8] developed electroconductive hydrogels based on polyvinyl alcohol and polyaniline as an electroconductive polymer in 2011 for the controlled release of indomethacin via electrical stimulation. The current work examines the technique of incorporating glutamic acid into polyaniline structures manufactured by chemical means and the polymeric system’s capacity for regulated drug release.
2. Materials and Methods
2.1. Materials
Aniline L-glutamic acid (GA) (98%), the pH, and temperature of the release medium can be used to control the release of amoxicillin from hydrogels made of polyacrylamide and polyaniline.Other chemicals used in the current study were purchased from Sigma-Aldrich with AR grade 98-99% purity, and the reaction was continuously stirred. After 24 hours, the reaction mixture was filtered with deionized water until the filtrate's pH reached 6. And we can conclude that hydrogels composed of polyacrylamide polyaniline can be considered potential candidates for applications in electrically controlled release devices, such as iontophoretic patches, based on the ability to control drug release.
2.2. Methods
2.2.1. Chemical Synthesis of Polyaniline in the Presence of L-Glutamic Acid
To carry out the aniline polymerization, 2 g of L-glutamic acid (GA) were weighed and dissolved in 132 mL of deionized water, using magnetic stirring and a temperature of 60°C [13]. The GA solution was placed in a 3-necked flask and 5 mL of previously distilled aniline was added to it. Separately, 12.52 g of ammonium persulfate were dissolved with 70 mL of deionized water. The APS solution was placed in an addition funnel. The reaction system was closed, and a nitrogen flow was allowed to pass for 10 to 15 minutes. After the air was evacuated, the reaction system was sealed and the APS solution was dropped dropwise. Importantly, before and after adding the oxidant solution, the reaction flask was kept in an ice bath. The reaction was left under continuous stirring for 24 hours. Once 24 hours had elapsed, the reaction mixture was washed by filtration with deionized water until the filtrate reached a pH of 6. The product of this stage consisted of an aqueous suspension with a concentration of structures PANI fibrillar cells coated with GA. In Table 1, the syntheses performed according to molar ratios are listed.
| Synthesis | Molar ratio aniline:GA:APS |
|---|---|
| 1 | 1:0.13:1.0 |
| 2 | 1:0.25:1.0 |
| 3 | 1:0.38:1.0 |
| 4 | 1:0.50:1.0 |
| 5 | 1:0.25:0.6 |
| 6 | 1:0.25:1.4 |
FTIR spectra were obtained on GX Perkin-Elmer Spectrum equipment. The remaining suspension was used to carry out the characterizations by scanning electron microscopy (SEM) JEOL 5410LV model, transmission electron microscopy (TEM) JEOL JEM-2,100 F model, and UV-visible absorption spectra were acquired using a Perkin Elmer Lambda 20 spectrophotometer (USA), as well as for drug loading/release studies.
2.2.2. Study of the Aniline-Glutamic Acid Interaction
To evaluate the existence of interactions between the monomer and the amino acid before the aniline oxidation process, a titration curve of aniline with GA was obtained using UV-Vis absorption spectroscopy. An acetate buffer solution (0.2 M) was used to control the pH at 5, resembling the initial conditions of the chemical environment before the polymerization process. Aliquots of a GA solution (0.2738 M) were gradually added to 2.99 mL of the aniline solution in a buffer until a molar ratio of 1:0.25 was obtained.
2.2.3. Amoxicillin Loaded on Polyaniline Structures
The incorporation of the antibiotic was carried out by mixing 20 mL of the polyaniline suspension (0.0148 g/mL) with 10 mL of an aqueous solution of the drug (200 mg/L) for 24 hours, the optimal time from which does not increase the absorption of amoxicillin. The resulting mixture was transferred to dialysis tubes, which were placed in a beaker containing 500 mL of deionized water, to remove the drug not adsorbed on the polymer particles. Drug concentration was determined from the absorbance of the dialysis solution at 273 nm, using a calibration curve constructed by UV-Vis absorption spectroscopy from solutions of known concentration.
2.2.4. Release of Adsorbed Amoxicillin in Buffer Solutions
The dialysis tubes containing the polyaniline particles loaded with the antibiotic were immersed in 450 mL of buffer solutions of pH 5 (acetate (0.1 M)), 7 (phosphate (0.1 M)), and 9 (borate chloride (0.1 M)). The antibiotic release process was carried out in a Barnstead/Lab-Line/E-Class MAX 4000 (USA) model incubator at a controlled temperature of 37°C and orbital agitation of 80 rpm. Amoxicillin concentration in the release medium was monitored by taking 2 mL aliquots every 2 hours for 4 days. A similar buffer volume was added to the release medium to maintain immersion conditions.
2.2.5. Preparation of Hydrogels Composed of Polyacrylamide and Polyaniline Loaded with Amoxicillin for Release Studies in Buffer Solutions
The polyaniline/amoxicillin system was incorporated into a polyacrylamide hydrogel by adding a portion of the polyaniline colloidal suspension loaded with the antibiotic during the process of cross-linking and hydrogel formation. Aqueous solutions of acrylamide (AAm, monomer) and bisacrylamide (BisAAm, cross-linking monomer) were prepared at different weight-volume concentrations, as shown in Table 2. Fifteen milliliters of the AAm/BisAAm solution were taken and mixed with 10 ml of the polyaniline/amoxicillin suspension, by means of constant magnetic stirring at a temperature of 5°C. Once the previous mixture had been homogenized, 1 mL of an APS solution (initiator) with a concentration of 10% weight/volume was added, and stirring was continued, keeping the temperature at 5°C. After 3 minutes, 0.2 mL of a 99% solution of TEMED (catalyst) was added, and stirring was continued. After 2 minutes, the mixture was removed from the ice bath and kept at room temperature until the end of the polymerization process and gel formation. The hydrogels were identified as shown in Table 2. Under conditions identical to those described in the previous paragraph, a polyacrylamide hydrogel without incorporation of the polyaniline particles was prepared. This hydrogel was loaded with a dose of drug similar to that present in hydrogels containing the electroconductive polymer.
| Hidrogel | AAm/BisAAm solution/concentration (g/mL) | Polyaniline/amoxicillin suspension | Amoxicillin solution | APS solution | TEMED solution | ||||
|---|---|---|---|---|---|---|---|---|---|
| AAm | BisAAm | Volume (mL) | Volume (mL) | Volume (mL) | Concentration (%) | Volume (mL) | Concentration (%) | Volume (mL) | |
| A | 0.58 | 0.01 | 15 | 10 | 0 | 10 | 1 | 99 | 0.2 |
| B | 0.58 | 0.02 | 15 | 10 | 0 | 10 | 1 | 99 | 0.2 |
| C | 0.58 | 0.03 | 15 | 10 | 0 | 10 | 1 | 99 | 0.2 |
| D∗ | 0.58 | 0.02 | 15 | 0 | 10 | 10 | 1 | 99 | 0.2 |
- ∗AAm: acrylamide monomer and BisAAm: bisacrylamide (cross-linking monomer).
2.2.6. Cytotoxicity Study of Hydrogels Composed of Polyacrylamide and Polyaniline
For the toxicity studies of the composite hydrogels, the normal cell line L-929 (normal mouse subcutaneous tissue) was used, provided by the immunology laboratory of the University of Sonora. Cultures were maintained in DMEM supplemented with 5% fetal bovine serum and in a Fischer Scientific (USA) Isoterm incubator at 37°C, 5% CO2, and 80–90% relative humidity. In order to determine how the hydrogels affected the cells, a cell viability assay was performed. This test involved using the MTT method to incubate polyacrylamide or polyacrylamide/polyaniline hydrogels in a cell culture medium. The results of this experiment were then analyzed. At 37°C, three hydrogel cylinders of each composition, measuring 7 mm in diameter and 3 mm in thickness, were immersed in 10.2 mL of cell culture. After 24 hours, the hydrogels were removed, and the medium that contained the soluble compounds of the hydrogel was used for the viability assay. This medium is referred to as a “conditioned medium.” A cell suspension of 200,000 cells/mL was obtained starting with a culture in a logarithmic growth phase. Fifty microliters of the cell suspension were placed in a 96-well flat-bottom plate and incubated for 24 h for adherence. After this period, using the conditioned medium, a volume of 100 μL was completed in each well, reaching concentrations of the conditioned medium of 50, 25, 12.5, and 6,250% (v/v). In the case of the control experiment, the 100 μL was completed only with a standard culture medium. The plate was incubated for a period of 48 h. During this period, the effect of the stimulus on cell morphology was observed using an inverted Nikon Eclipse microscope. Once the incubation time had elapsed, 10 μL of MTT were added to each well and incubated for a period of 4 h. Subsequently, 100 μL of acidic isopropanol were added and allowed to stand for 10 minutes. Finally, the absorbance of the solution was read at 570 nm and 655 nm in an ELISA reader (BIO-RAD).
The statistical treatment of the experimental data was carried out through an ANOVA analysis of variance, using the Microsoft Excel XLSTAT complement and establishing a confidence interval of 95%. The existence of statistically significant differences (p < 0.05) between the mean determined for each type of sample against the value obtained in the control test using Dunnett’s test was verified.
3. Results and Discussion
3.1. Chemical Synthesis of Polyaniline in the Presence of L-Glutamic Acid
This section shows the results obtained from the different chemical-oxidative syntheses of polyaniline. Six types of synthesis were performed varying the aniline:amino acid and aniline:oxidizing agent molar ratios This study was carried out to establish the optimal reaction conditions that would favor obtaining polyaniline in its ES state, the incorporation of the amino acid into the polymeric system, and the formation of structures with a morphology with a high aspect ratio (quotient of the maximum dimension to the minimum of the particle).
3.2. Characterization Studies
3.2.1. Scanning Electron Microscopy (SEM)
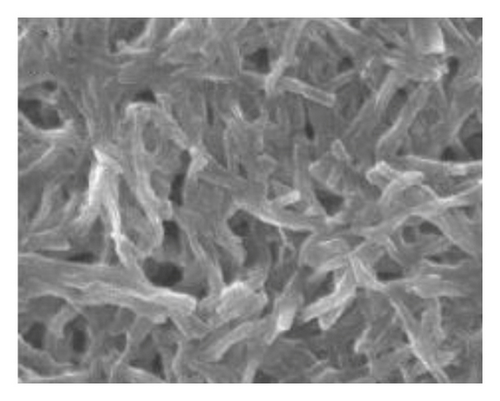
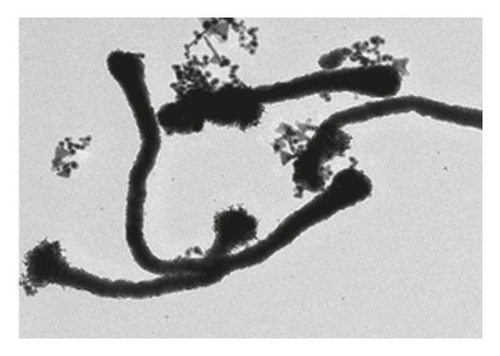
Figure 1 shows the SEM microscopy images of the reaction products of the different syntheses. It is observed on a micrometric scale that the oxidation product of aniline in the presence of GA has a cylindrical morphology with a high aspect ratio (length-diameter ratio). It is also appreciated that there are agglomerated particles, observing material where the individual structures are not distinguished. The diameter of the cylinders found for the different molar ratios ranges from 360 nm. It is observed that the morphology and the amount of agglomerated material of the structures have a strong dependence on the concentration of FA and APS.
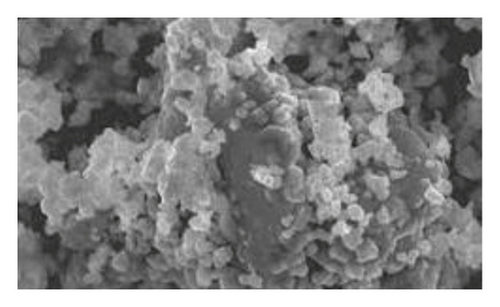
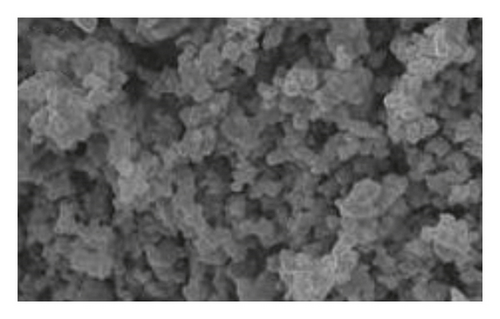
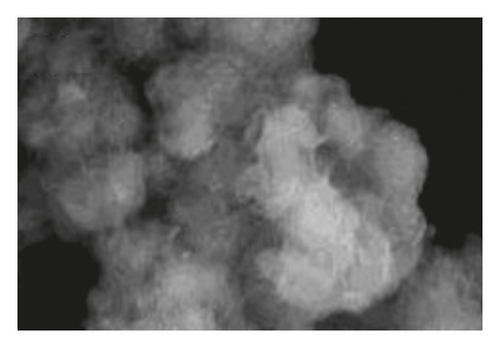
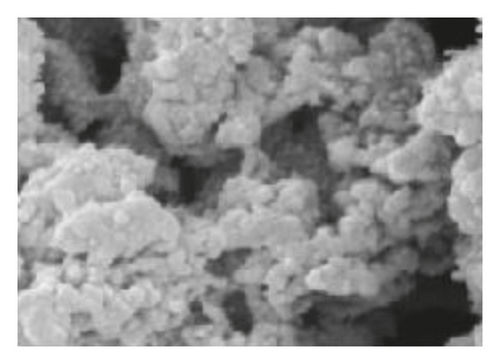

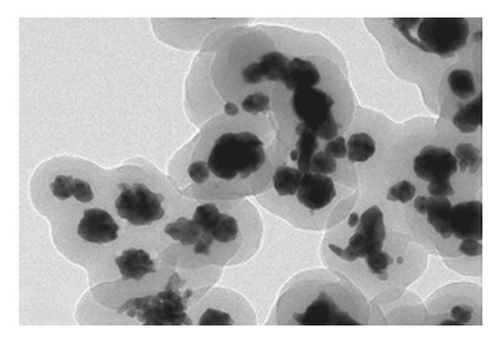
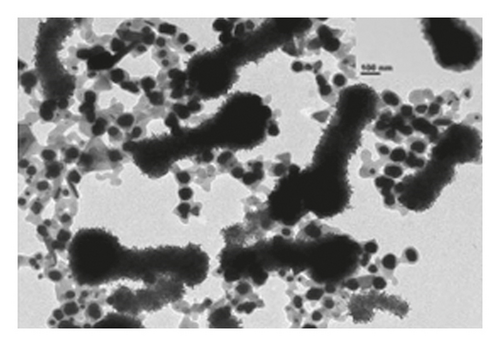
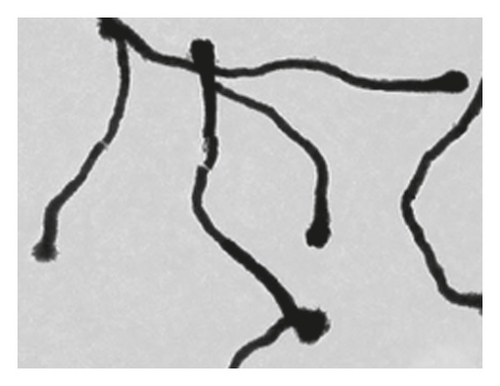
The TEM microscopy images shown in Figure 2, for the different molar ratios, preferentially produce primary structures with a cylindrical morphology, as had been observed by SEM microscopy at larger scales. It is important to highlight that for obtaining the TEM images, the diluted polyaniline suspensions were previously subjected to sonication to improve the visualization of the primary structures. It was observed that the individual cylindrical structures present a diameter from a nanometric scale (<100 nm) to micrometric scales (between 100 nm and 1 μm) and that the morphology of the submicron/nanostructures varies strongly with the concentration of FA and APS; that is, the shape of the structures can be controlled by changing the aniline:GA:APS molar ratio. For example, the primary structures of samples 1:0.13:1 and 1:0.25:1 showed a fibrillar morphology with a higher length-to-diameter ratio than those of other samples.

3.2.2. Infrared Spectroscopy (FTIR)
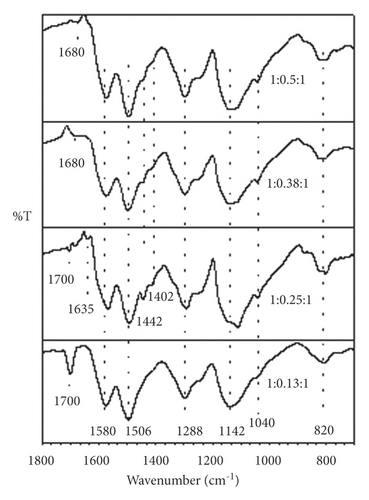
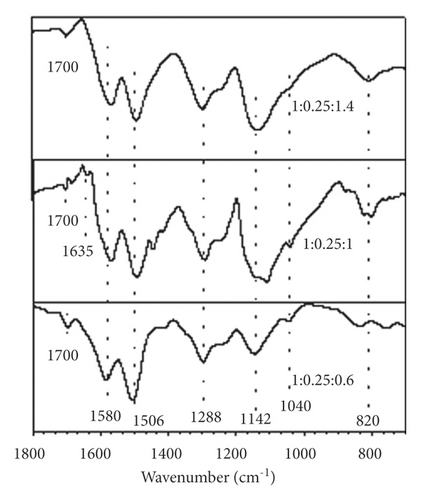
FTIR spectra corresponding to polyaniline synthesized at different molar ratios are shown in Figures 3(a) and 3(b). The samples show very similar spectra regardless of the aniline:GA and aniline:APS molar ratios used in the synthesis. In the spectrum region between 1,800 and 700 cm–1, main bands are observed with transmittance minima at 1,580 and 1,506 cm−1 that are attributed to the C–C stretching of the ring, in quinoid diimines benzenoid diamines, respectively [14]. Likewise, a peak at 1,288 cm−1 is detected, which is assigned to the vibration by stretching the C–N bonds between the quinoid and benzenoid units. Around 1,402 and 1,442 cm−1, there are two bands associated with the imine C–N bond strain and the intermediate order C–N bond strain between amine N and imine N. The bands with minima around 1,142 and 820 cm–1 are associated with the electronic absorption of the conjugate structure that forms the quinoid ring and the neighboring nitrogens and with the bending of the C–H bonds of the 1,4-disubstituted ring of polyaniline., respectively [15]. The appearance of these last signals indicates that the synthesized polymer predominantly presents a linear conformation, without structural defects, which is closely related to optimal values of electrical conductivity.
3.2.3. UV-Visible Absorption Spectroscopy
The characteristic bands of the ES state of polyaniline are identified in the UV-Vis absorption spectra. The spectra of the varied synthesis routes are very similar in general. Without taking into account the 1:0.25:1 sample, absorptions were attributed to π−π ∗ (λmax ∼348 nm), −π ∗ (λmax ∼430 nm), and π -polaron (λmax between 800 and 860 nm) transitions with well-localized bands in the remaining samples. These findings are in line with the conductive properties observed in all synthesized samples as well as those previously reported for other ES samples, such as polyaniline doped with camphor sulfonic acid [16]. The presence of an absorption maximum in the visible-near IR boundary region also suggests that the product of these syntheses presents its chains in a compact conformation. The fact that the polarons of each tetrameric unit are isolated from each other causes torsional defects between the aromatic rings, resulting in this type of conformation. As a result, the polaronic band appears to be well localized and has little energy dispersion. The localized band of the - polaron transition seen in the other samples is replaced by an elongated tail of free carriers in the near IR region in the 1:0.25:1 sample. This is typical of an extended chain conformation, in which torsion defects between aromatic rings are removed, favoring interaction between isolated adjacent polarons, resulting in increased polaronic band energy dispersion and delocalization.
3.3. Study of the Aniline-Glutamic Acid Interaction
Aniline was initially softened in an aqueous medium of GA prior to the oxidation process, and the pH of the solution that was produced by this step was approximately 5. An equimolar mixture of neutral aniline molecules, which are easily oxidized, and anilinium cations, which oxidize much more slowly, was present in this pH region. The pH of the medium drops rapidly to a range between 0.9 and 1.5 after the addition of APS and/or hydrogen sulfate salts [8]. GA exists primarily as a monovalent cation in this pH range, and it cannot form part of the polymer chain as a dopant ion in this state. It can, however, form supramolecular interactions with the polymer chain, while sulfate and hydrogen sulfate ions derived from APS reduction stabilize the charged polymer chain and act as dopant ions. After completion of the reaction and purification of the polymer, the pH of the resulting polyaniline suspension was around 5. In this condition, the GA takes the form of a monovalent anion, which allows it to stabilize the charge of the polyaniline chain as the counterion. This conclusion is based on the results of electrical conductivity FTIR, where the presence of the amino acid and sulfur-containing species was demonstrated, once the polymerization process had finished.
3.4. Release of Adsorbed Drug versus Buffer Solutions
At 25 and 37°C with continual mechanical stirring, amoxicillin release profiles from the polyaniline/amoxicillin systems encapsulated in cellophane membranes were compared to buffer solutions. At 37°C, the cumulative drug release quantities at pH 5, 7, and 9 were 0.667 g (82.9%), 0.615 g (75.9%), and 0.791 g (98.0%), respectively, after 48 h of exposure to these pH values. It is hypothesized that an ion exchange process between the buffer ionic species and the counterions of the polyaniline salt system is responsible for the drug’s release in both neutral and acidic environments (pH = 7 and 5). Amoxicillin is a zwitterion in this pH range; hence, it cannot interact with the polymeric structure electrostatically.
3.5. Hydrogels Composed of Polyacrylamide and Drug-Loaded Polyaniline
Composite polyacrylamide/polyaniline/amoxicillin hydrogels were prepared using the drug-loaded 1:0.25:1 polyaniline sample. Qualitatively, at the macroscopic level, the hydrogels presented a soft consistency, maintaining their geometry once extracted from the mold, which allowed their easy handling in drug characterization and release studies.
3.5.1. Morphological Characterization
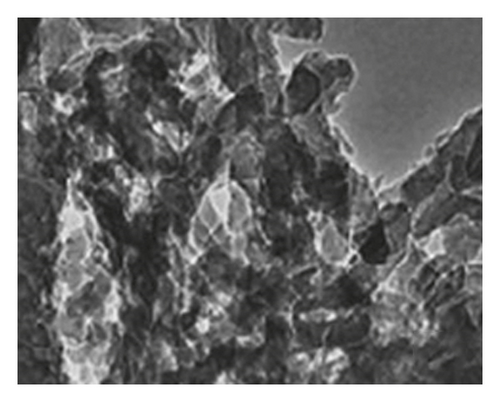
Figure 4 presents micrographs obtained from the cross-section of hydrogel B. The images reveal the presence of polyaniline submicro/nanostructures, as observed after its synthesis by SEM (Figure 1(b)) and TEM (Figure 2(b)) within the hydrogel matrix. It is observed that the structures maintain their fibrillar morphology after the drug loading process and its incorporation into the hydrogel, conserving a high length-diameter aspect ratio and being distributed homogeneously, in random directions, and without significant agglomeration. A few SEM and TEM images in the literature show irregular granules of the electroconductive polymer filling the micrometric voids of the hydrogel, which is worth pointing out [17]. A three-dimensional network of polyaniline nanofibers supported by an insulating hydrogel matrix can be formed using the method described in this study.
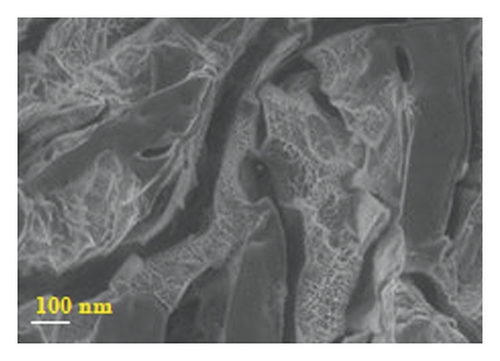
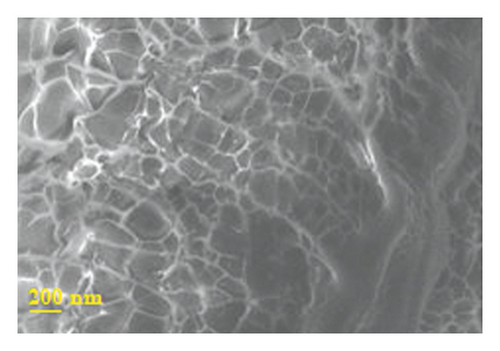
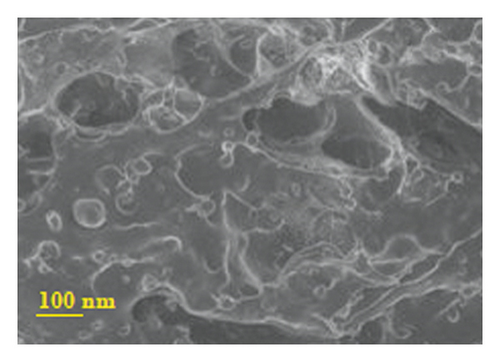
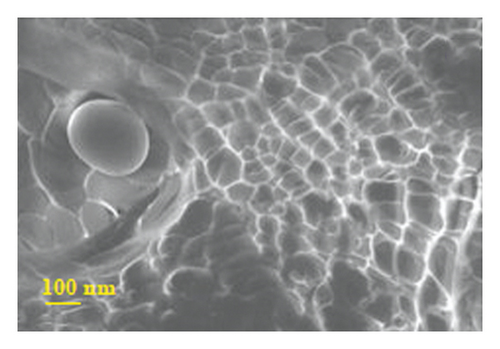
3.5.2. In Vitro Release of Amoxicillin from Drug-Loaded Polyacrylamide-Polyaniline Composite Hydrogel Using Orbital Shaking
The release kinetics of amoxicillin adsorbed on polyaniline structures from polyacrylamide/polyaniline/amoxicillin composite hydrogels was studied as a function of the degree of cross-linking of the hydrogel (AAm:BisAAm mass ratio), the pH of the release medium, and the temperature for which the three formulations described. Additionally, the effect on the release kinetics of the form of incorporation of the drug into the hydrogel was analyzed, for which the formulation of the polyacrylamide gel without particles was used of polyaniline, loaded only with the antibiotic.
Results observed that the amount of antibiotic released varies with respect to the pH of the release medium, regardless of the degree of cross-linking of the hydrogel. At a higher pH value, the release kinetics is faster, and the % release is higher at equilibrium. This behavior is explained by taking into account the nature of the electroconductive polymer. The more significant release in the basic medium is mainly attributed to the undoping of polyaniline, which releases FA molecules surrounding the polyaniline structures and functions as counterions [17]. Consequently, the release of FAs is induced by amoxicillin molecules. The drug release in neutral and acid medium is explained by an ionic exchange process between the polyaniline doping species and the buffer anions, a process that according to the experimental results is less favored in acid medium. Hydrogel A, which contains a lower concentration of BisAAm, showed a different behavior than expected. By having a less cross-linking agent, it was expected that the polymeric network would be less compact than the rest of the concentrations, and therefore, the release would be favored, in agreement with the swelling results for this type of hydrogel. However, the experimental result showed that at this ratio, the release kinetics is slower and the % release at equilibrium is lower than for hydrogel B, a result that was corroborated by repeating the experimental procedure. The influence of temperature on the antibiotic release kinetics was analyzed in hydrogel B, since the highest equilibrium % release was achieved in this formulation. It is concluded that the adsorption of the drug on the polyaniline structures and its subsequent incorporation into the hydrogel favors its application in a prolonged drug release system; however, a more exhaustive analysis should be carried out on the kinetics that controls the release of bioactive molecules in a temperature-controlled environment. The “ON/OFF” type release pattern of the drug from the electroconductive system due to the effect of the electrical stimulus can be associated with the movement of the charged drug molecules by the impulse of the electric field. In the polyaniline/amoxicillin system, the first effect was ruled out because amoxicillin at neutral pH has no net charge. Therefore, drug release was attributed to the electrochemical reduction of polyaniline, which causes changes in the charge density of the polymer and contraction of its volume, which synergistically leads to the release of amoxicillin noncovalently bound submicron/polyaniline nanostructures.
3.5.3. Cellular Cytotoxicity Study
The recent results have indicated that polymer purification substantially improves biocompatibility, eliminating residues or by-products responsible for cytotoxicity [18]. Considering the potentiality shown by hydrogels composed of polyacrylamide/polyaniline for the controlled release of drugs, a study was carried out to evaluate the cytotoxicity of the polyacrylamide and polyacrylamide/polyaniline systems, analyzing their effect on cell proliferation using a cell viability assay. Figure 5 demonstrates the feasibility of normal mouse subcutaneous culture cells visible to different concentrations of the medium in contact with the hydrogels (conditioned medium).
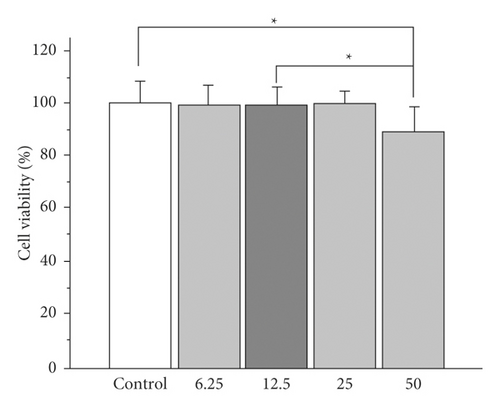
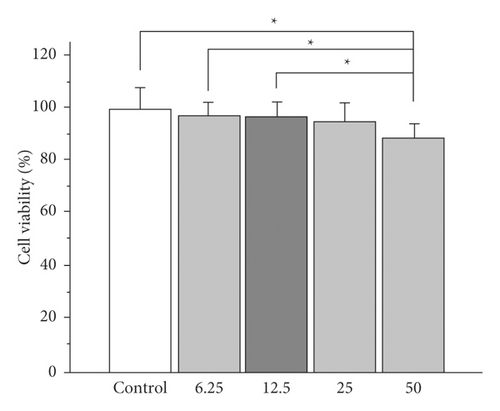
In both systems, viability greater than 85% is observed for all cases, with a statistically significant reduction in cell viability being observed only for the highest concentration of conditioned medium (50%) compared to the control sample (cells exposed to medium) standard crop. However, cell viability remains above 80% even in this case.
In particular, for polyacrylamide/polyaniline hydrogels, viability declines with increasing concentration of the conditioned medium; however, no significant difference is observed at the same concentration between both gels. This fact suggests a minimal toxic effect of polyaniline nanostructures on cells. The decrease in viability observed when 50% of the medium is conditioned can be attributed to unpolymerized acrylamide residues, a monomer whose toxicity has been widely documented [18].
In general, viability of 80% or higher is indicative of the absence of cytotoxicity in accordance with the ISO 10993–5 standard. The results found by this assay show high cell viability when the cells have been exposed to culture media conditioned with polyacrylamide or polyacrylamide/polyaniline hydrogels, which is indicative of minimal cytotoxicity of these systems. These in vitro cytotoxicity results also suggest that a washing step for the hydrogels may be desirable before being exposed to continuous contact with cells to minimize acrylamide residues. Cell viability was 100% when cells were exposed to a conventional culture medium (the control) as well as polyacrylamide/polyaniline gel-conditioned conditions. Conditions medium with polyacrylamide and polyacrylamide/polyaniline hydrogels (50% concentration) exposed cells display a similar shape to control cells, with no signs of cell injury. These findings are in line with those in Figure 5, which depicts the vitality of cells.
4. Conclusions
Ammonium persulfate can be employed as an oxidant to create one-dimensional nanostructured polyaniline systems with high length/diameter ratios. Glutamic acid is used as a dopant in polyaniline structures, along with sulfur-containing anions produced by ammonium persulfate reduction. The resultant products’ composition, electrical, morphological, and optical properties vary depending on the molar ratio of aniline:amino acid:oxidant used in the synthesis. The release of amoxicillin from polyaniline-based fibrillar structures may be modulated as the pH of the release medium varies, allowing for accurate dosage. By integrating amoxicillin-loaded polyaniline particles in situ during acrylamide polymerization and cross-linking, amoxicillin-loaded polyaniline particles can be efficiently encased in a polyacrylamide hydrogel. When amoxicillin is just in the bulk of the hydrogel, it is more strongly held on the polyaniline particles. When compared to a hydrogel without polyaniline fibers, the composite hydrogel has a slower drug release kinetics, allowing it to be used in prolonged-release systems. The pH and temperature of the release medium can be used to control the release of amoxicillin from hydrogels made of polyacrylamide and polyaniline. We can conclude that hydrogels composed of polyacrylamide/polyaniline can be considered potential candidates for applications in electrically controlled release devices, such as iontophoretic patches, based on the ability to control drug release using low-intensity voltages and the lack of cytotoxicity found for this material in in vitro cell viability studies. While conventional iontophoretic devices are based on the electromigration of ionized pharmaceuticals, the release from the polyacrylamide/polyaniline system is regulated by the redox characteristics of the electroconductive polymer, which broadens the spectrum of possibilities to molecules in a neutral state.
Conflicts of Interest
The author declares that there are no conflicts of interest regarding the publication of this paper.
Open Research
Data Availability
The data underlying the results presented in the study are available within the manuscript.




