Optimization of Biodiesel Production Parameters from Cucurbita maxima Waste Oil Using Microwave Assisted via Box-Behnken Design Approach
Abstract
The production of biodiesel from vegetables or fruits waste oils has high potential as renewable energy. The Cucurbita maxima wastes are massive source of oils, which are believed to indicate the possible sources of renewable energy whose biodiesel can be produced. Hence, the study explores the potential of the Cucurbita maxima wastes, for the production of biodiesel. In this study, the Soxhlet extraction method was used to extract Cucurbita maxima waste oil using an organic solvent. Through Box-Behnken design (BBD), the effects of methanol to oil molar ratio (6–10), catalyst concentration (2–6%), and reaction time (45–75 min) on the transesterification efficiency of methyl esters were investigated. The oil contents of Cucurbita maxima waste was found to be 44.6 ± 0.21%. This oil was characterized, and after obtaining the pure characterized oil, biodiesel was produced using microwave assisted by the transesterification process. The optimum conversion efficiency of the Cucurbita maxima waste oil to fatty acid methyl ether was 97.76%, at the optimal parameters, methanol to oil ratio (8.4 : 1), catalyst concentration (3.14%), and reaction time (57.12 min). The results revealed that all parameters have a significant effect on the yield of biodiesel (p < 0.0001). The physicochemical properties reveal that the Cucurbita maxima waste oil could be applied as a potential source of material for methyl ester production. The fatty acid profile of the oil indicated that it was mainly composed of unsaturated fatty acid, which ensures good flow properties of the fuel. The results of these studies showed the prospective of Cucurbita maxima wastes as a new potential feedstock for biodiesel production.
1. Introduction
Globally, the energy requirement is elaborating progressively because of population growth and industrial development. Thus, raising energy demand and fossil fuel prices along with the concern of the environment motivated the investigators to look for alternative fuel energy [1, 2]. Biodiesel is considered as an alternative fuel having properties such as nontoxic, renewable energy, sulfur-free, clean-burning, ester-based, and low emission as compared to fossil fuels [2]. It is fatty acid methyl ester, which is derived from animal fats and vegetable oils [3]. Production of biodiesel is often accomplished through a transesterification reaction of triglycerides in animal fats and vegetable oils with monoalkyl alcohols using different catalysts [4]. Mostly, biodiesel can be produced using edible and nonedible sources; however, owing to the consumable source, which is used as food, a new boulevard of producing biodiesel through noneatable sources as possible feedstock is important for renewable energy production [5].
New low-cost oil of crops wastes is required to produce economical oils suitable for the production of biodiesel [6]. One of the potential alternative sources of oil used for biodiesel production is Cucurbita maxima waste [7]. The Cucurbita maxima, one of the largest popular vegetable plants consumed worldwide [8], has been recently realized as an alternative source of feedstocks for biodiesel production. Cucurbita maxima are natural source of oils [9] and antioxidants such as carotenoids and tocopherols [10] used for human health and an excellent source of unsaturated fatty acids mainly oleic and linoleic acid [11]. Cucurbita maximum is a creeper largely found very frequently on the roofs of houses and the fences of houses and cultivated throughout most warm regions of the world [7]. Cucurbita maximum is cultivated throughout most warm regions of the world. The fruits of Cucurbita maxima are extensive weighing 8−10 kg, sometimes up to 20 kg, and the medium-sized fruit “weighs 2−3 kg and has orange color flesh [12]. Due to its large production for consumable purposes, Cucurbita maxima waste is generally considered as agroindustrial waste [7, 8]. Waste from the Cucurbita maxima-processing industry indicates an interesting source for recovering industrial residue into renewable energy while removing this waste to assist protect the environment in the circumstance of sustainable development [12]. In this consideration, it is a good idea to use Cucurbita maxima waste oils since these materials can be used as a continuous supply to produce useful products that will help to fulfil the ever-enhancing world energy demands [7]. In addition, this approach will help to reduce environmental problems caused by the illegal deposition of waste materials [3, 7, 10]. To the best of the author’s knowledge, Cucurbita maxima wastes are an unutilized source of energy, and few studies have been published on biodiesel production from Cucurbita maxima wastes.
Different investigators have reported their researches for the production of biodiesel from edible and nonedible oil employing the conventional heating method [13]. The conventional heating method has the problem of low yield, inefficient heating, and the excess amount of raw materials and high operating [2]. Apart from these techniques, different process intensification used for biodiesel production is membrane reactor, steam distillation, [14], microwave, and ultrasonic [15]. Among these, microwave irradiation-assisted greatly influences the yield of biodiesel production. This method reduces the reaction time, thereby allowing for fast and evenly dispersed internal heating, accelerates the molecular degree of heating of the catalyst, oil, and alcohol mixtures, and ultimately improves the separation step [16, 17].
Optimization of process parameters is an effective way for experimental tests and data analysis to attain the best yield with optimum variables [18, 19]. Response surface methodology- (RSM-) based Box-Behnken design supplies the effect of all variables to be contributed for biodiesel production with all information of critical variables over the dictated range of experimental parameters and modelling the process mathematically, saving cost and time by decreasing the number of experiments [15, 16]. Therefore, this study was aimed at producing and optimizing methyl ester from Cucurbita maxima wastes using microwave-assisted transesterification via the Box-Behnken design approach. In addition, the effects of methanol to oil ratio, potassium hydroxide (KOH) catalyst concentration and reaction time, and the physicochemical property of oil and biodiesel were investigated.
2. Materials and Methodology
2.1. Plant Material and Chemical Preparation
The Cucurbita maxima waste used for this study was collected from Holeta Agricultural Centre, Ethiopia, for all the experiments. Then, after collection, the samples were packed in bags and transported to the Chemical Engineering Laboratory, Jimma Institute of Technology, Jimma University, Ethiopia. The collected Cucurbita maxima waste was washed and sun-dried for six days to obtain constant weight. The dried Cucurbita maxima waste was ground into powdered form by using a mortar and pestle to maximize the surface area for efficiency of oil extraction and was sieved through a 45 mesh carbon steel sieve to obtain a similar particle size. The powdered samples were then covered in glass bottles and stored at 4 °C for the next analyses. All chemicals and reagents of analytical grade including ethanol (purity; 99.9%), n-hexane (purity; 99.9%), methanol (purity; 98.9%), petroleum ether, lanthanum nitrate, ceric ammonium nitrate, potassium hydroxide (purity; 98%), acetic acid, and sodium hydroxide (purity; 98%) were purchased from the chemical product suppliers (C. Fun General Trading Plc., Addis Ababa, Ethiopia).
2.2. Extraction of Oil from Cucurbita maxima Wastes
In this study, Cucurbita maxima waste oil was extracted according to the methodology suggested by Nayak and Vyas [19] with some modifications. The Cucurbita maxima waste powder (20 g) was mixed with organic solvent 60 mL of n-hexane in conical flasks and placed in Soxhlet (Lab-Line Instruments, Inc., Melrose Park, Illinois, France), which was initially dipped into n-hexane so that the solvent diffused completely into the sample. After 10 min, the thimble was subjected to the system that contained n-hexane at 50°C for 6 h [20]. The experiments were done repeatedly, and at the end of the extraction process, the oil was filtered using a vacuum filter and kept for 48 h to settle any suspended particles, and the oils dissolved in n-hexane were recovered using a rotary evaporator at 40 °C. Then, the solvent-free oils were left in an airtight container, and it was subjected to characterize as per standard testing method to ascertain their suitability for the production of biodiesel. The obtained oil was calculated based on the weight of Cucurbita maxima waste powder, and the recovery was expressed as percentages of the oil yield.
2.3. Physicochemical Characteristics of Cucurbita maxima Waste Oil
The physicochemical properties of the extracted oil (density moisture content, ash content, kinematic viscosity, saponification value, acid value, iodine value, and peroxide value, and free fatty acid (FFA)) were determined according to ASTM standard since the identification of the physiochemical characteristics of the oil is very critical on the yield of biodiesel produced [21].
2.3.1. Biodiesel Production: Transesterification Reaction
The microwave irradiation-assisted alkaline-catalyzed transesterification of Cucurbita maxima waste oil was carried out using an Anton Paar Monowave 401 HP microwave reactor and an Autosampler MAR36. The Anton Paar Monowave 401 microwave reactor with Autosampler MAR 364 has infrared sensors for temperature control, pressure sensors to monitor the reaction in the closed vessel, and a built-in magnetic stirrer to ensure that the reaction mixture is agitated properly [15]. The microwave reactor automatically recorded the temperature and pressure profiles during the trials. The microwave reactor’s heating profile consists of different steps [22], the closed vessel is heated to a specified temperature, the set temperature is maintained for a certain period, and the closed vessel is cooled to a set temperature to stop the chemical reaction. The power output was controlled during the reaction based on the temperature set for the experiment. During the microwave-assisted transesterification process, the power output was continuously monitored to avoid the reaction from turning exothermic. At the end of the reaction, the product was collected and allowed to settle for a day. The general biodiesel production process is shown in Figure 1.
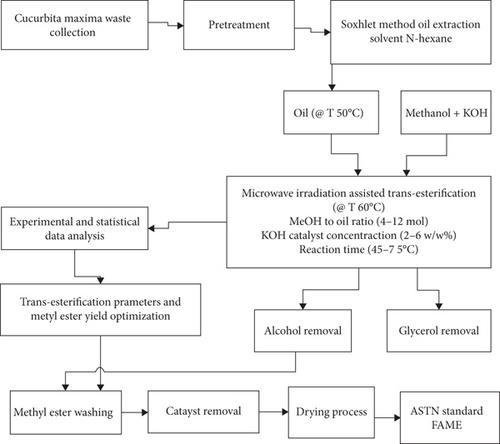
2.4. Experimental Design and Statistical Data Analysis
An experimental design used in biodiesel production for the series of parameters was generated by response surface methodology. Box-Behnken design [18] and the range of parameters are shown in Table 1 with coded levels. The process parameters selected were methanol to oil molar ratio, X1, in the range of 6–10; catalyst concentration, X2, in the range of 2–6%; and reaction time, X3, in the range of 45–75 min.
| Parameters | Symbol | Coded levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Catalyst concentration (w/w%) | X1 | 2 | 4 | 6 |
| Methanol to oil molar ratio (mol/mol) | X2 | 6 | 8 | 10 |
| Reaction time (min) | X3 | 45 | 60 | 75 |
2.5. Statistical Data Analysis
2.6. Biodiesel Characterization
The physicochemical property of Cucurbita maxima waste oil biodiesel was carried out at the optimum conditions. Kinematic viscosity, density, acid value, iodine value, and peroxide value were determined according to the standard methods described [23], for physicochemical property of fuel analysis as shown in Table 2.
| Properties | Standard methods |
|---|---|
| Moisture content (%) | AOCS Cd 2C-30 (1996) |
| Kinematic viscosity (mm2/s) @ 40°C | AOCS Dd 8-35 method |
| Density (g/cm3) | ASTM D6751 method |
| Acid value (mg KOH/g) | ASTM) D6751 method |
| Iodine value (g I2/100 ml of oil) | EN14214 method |
| Peroxide value (meq/kg) | AOCS Dc 17b-35(1998) method |
2.6.1. Fourier Transform Infrared (FTIR) Spectroscopy Functional Group Analysis
An FTIR spectrum was developed in the wavenumber between 400 and 4000 cm-1 [1]. The infrared radiation was propagated through the sample to get its corresponding spectrum that was averaged out from several data achievements. All spectra were normalized and baseline-adjusted with the requirement of FTIR software.
2.6.2. Gas Chromatography Fatty Acid Composition Analysis
Gas chromatography detection was accomplished utilizing a gas chromatograph (GC-2016, Shamash, USA) fitted with a flame ionization sensor and capillary column (Omega wax, 25 m 0.2 m, and 0.2 m). The sensor and injector were set at room temperature and 27°C, respectively. The oven program (300°C–10 min, 10°C/min to 27°C, fixed at constant 27°C–10 min) with helium as carrier gas at 4 cm3/min of flow rate [16]. The sample (30 mg) was dissolved in 120 mL of high purity n-hexane and then converted to a 5 mL autotaster. The sample was tested by applying the Thermo Trance 1420-GC with a split injector, flame ionization sensor, and Tri-Clue autotaster.
3. Result and Discussion
3.1. Characterization of Cucurbita maxima Waste Oil
The oil contents, ash content, moisture content, and chemical properties of the oil, namely, the iodine value, saponification value, peroxide value, and acid value, were determined by standard procedures described by ASTMD methods as shown in Table 3. Most of the biodiesels have been commercially produced from vegetable oils. Generally, humans have consumed these oils as food. In this context, this case has contributed to the conflict of fuel against food supply all over the world [21]. Hence, nonconsumable wastes of consumable feedstocks can be judged in biodiesel production; particularly, novel raw materials are very significant since the cost of biodiesel is highly counted on the expense of feedstock [24, 25]. Interestingly, the oil content of Cucurbita maxima waste was found to be at 44.6 ± 0.21% by the Soxhlet extraction method. This result was confirmed to the oil (45%) produced from pumpkin (Cucurbita pepo L.) seed by Schinas et al. [7], using methanol and sodium hydroxide (NaOH) as a catalyst for biodiesel production.
| Components | Result | ASTM standard | Test methods |
|---|---|---|---|
| Ash content (%) | 2.02 ± 0.31 | 5.6−7.7 | ASTM D645 |
| Moisture content (%) | 5.3 ± 1.10 | 8 ± 1.3 | ASTM-D574 |
| Acid value (mg KOH/g) | 0.51 ± 0.40 | 3.5−4.0 | ASTM-D767 |
| Saponification value (mg KOH/g oil) | 183 ± 0.21 | 97.8−196 | ASTM-D982 |
| Iodine value ((g I2/100 ml oil) | 102 ± 0.32 | 79.5−110 | ASTM-D235 |
| Free fatty acid (mg KOH/g) | 0.81 ± 1.01 | 0.35−1.6 | ASTM D415 |
| Kinematic viscosity (mm2/s @ 40 °C) | 34.6 ± 0.11 | 36.5 | ASTM D-445 |
The physicochemical properties of Cucurbita maxima waste oil were shown in Table 2. These results were confirmed with ASTM standard value [26] and proved that the produced biodiesel from Cucurbita maxima waste oil can be ignitable without any problem to use for biodiesel production [17], whereas a large amount of these components induce self-generated hydrolysis reaction and deteriorates the oil, which enhances heat departure due to evaporation [27]. Other properties such as acid value, saponification value, iodine value, free fatty acid, and kinematic viscosity of Cucurbita maxima waste oil were also tested and shown in Table 2. It is important to note that the tested results correspond to those obtained for soybean oil [5], sugar beet agroindustrial waste [28], and waste frying oil [29]. Interestingly, these results indicated that Cucurbita maxima waste oil fits the standard requirement of the oil as a potential source of energy for biodiesel production [7, 17]. The result shown for saponification value of the oil was 183 ± 0.21 mg KOH/g. This shows that alkali would be needed to enable it to neutralize the useable free fatty acid released by the oil [30].
3.2. Experimental Data Analysis
In this study, microwave irradiation-assisted was used during the transesterification process to reduce the processing time compared to the conventional technique [15, 31]. This work was also conducted to study the influence of transesterification parameter amount of catalyst concentration (X1), methanol-oil-molar ratio (X2), and process time (X3) on the yield of biodiesel produced from Cucurbita maxima waste oil using the Box-Behnken design approach. Experimental and predicted results of the biodiesel conversion are indicated in Table 4. The considerable variation in the conversion of biodiesel under different transesterification reaction parameters could be seen. The yield of biodiesel conversion obtained from Cucurbita maxima waste oil was found to be in the range from 87.4 to 97.2%. The yield of biodiesel conversion in this study was corresponding to the yield of biodiesel (97.49% to 99.38%) investigated by Chung [18] from Jatropha curcas (J. curcas) oil at optimal conditions of methanol to oil ratio (10.74), catalyst concentration (1.26 wt%), and flow rate of transesterification (1.62 mL/min). The yield obtained in this study is relatively somewhat lower than those reported in the literature [18]. This may be because of the type of oil used as a high-saturated fatty acid composition compared to other types of oils [32]. Despite the comparatively lower yield, however, it could be noted that apart from the process parameters reported by Chung [18], the optimal conditions of methanol to oil ratio and catalyst concentration in this study are both towards the lower.
| Run no. | Independent variables | Experimental predicted | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Value | Value | |
| % | mol | min | wt% | wt% | |
| 1 | 2 | 10 | 60 | 89.7 | 89.93 |
| 2 | 6 | 10 | 60 | 91.4 | 91.60 |
| 3 | 2 | 6 | 60 | 87.6 | 87.40 |
| 4 | 2 | 8 | 45 | 87.4 | 87.39 |
| 5 | 2 | 8 | 75 | 90.8 | 90.79 |
| 6 | 6 | 8 | 45 | 89.7 | 89.71 |
| 7 | 4 | 10 | 75 | 96.2 | 95.99 |
| 8 | 4 | 8 | 60 | 96.7 | 96.87 |
| 9 | 4 | 8 | 60 | 97.2 | 97.10 |
| 10 | 4 | 10 | 45 | 90.8 | 90.59 |
| 11 | 4 | 8 | 60 | 96.9 | 96.87 |
| 12 | 6 | 6 | 60 | 91.5 | 91.28 |
| 13 | 4 | 6 | 75 | 92.8 | 93.01 |
| 14 | 6 | 8 | 75 | 94.0 | 94.01 |
| 15 | 4 | 6 | 45 | 89.5 | 89.71 |
The effect of transesterification parameters on the yield of biodiesel was proved by the coefficient of determination R-squared between experimental and predicted values as illustrated in Figure 2; the variation is acceptable as the points are close to the regression line [33].
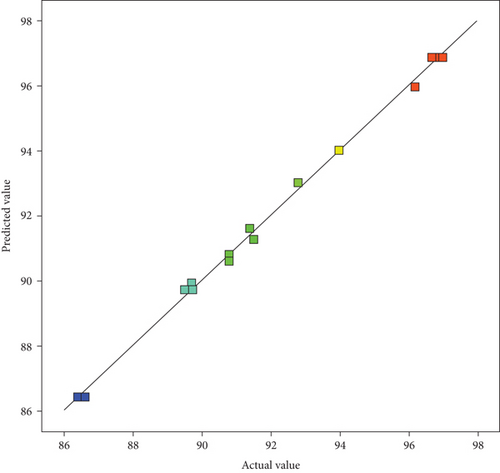
The regression analysis resulted in an R-squared value of 0.9977, which stands for a 99.77% (Table 5) influence of process variables on the yield of biodiesel.
| Performance index/coefficient of determination | ||||||
|---|---|---|---|---|---|---|
| R2 (R-squared) | R2-adjusted | R2-Pred. | Adeq Prec | Std. Dev. | Mean | C.V. % |
| 0.9977 | 0.9934 | 0.9661 | 44.8650 | 0.2861 | 92.00 | 0.3109 |
- X1: catalyst concentration; X2: methanol to oil molar ratio; X3: reaction time; Adeq Prec.: adequate precision; Std. Dev.: standard deviation; C.V.: coefficient of variation.
The quadratic model regression analysis was selected, and the analysis of the ANOVA result is indicated in Table 6. The result of probability (p value) from the analysis of the model is below 0.0001 presenting a high significance of the variables. Particularly in this study, the most substantial model term is the reaction time (X3) with an F-value of 462.46.
| Source | SS | df | MS | F-value | p value | |
|---|---|---|---|---|---|---|
| Model | 173.81 | 9 | 19.31 | 236.00 | < 0.0001 | Significant |
| X1 | 21.45 | 1 | 21.45 | 262.13 | < 0.0001 | |
| X2 | 7.41 | 1 | 7.41 | 90.57 | 0.0002 | |
| X3 | 37.84 | 1 | 37.84 | 462.46 | < 0.0001 | |
| X1X2 | 2.56 | 1 | 2.56 | 31.28 | 0.0025 | |
| X2X3 | 1.10 | 1 | 1.10 | 13.47 | 0.0144 | |
|
|
77.56 | 1 | 77.56 | 947.83 | < 0.0001 | |
|
|
22.77 | 1 | 22.77 | 278.25 | < 0.0001 | |
|
|
15.64 | 1 | 15.64 | 191.16 | < 0.0001 | |
| Residual | 0.409 | 5 | 0.082 | |||
| Lack of fit | 0.363 | 3 | 0.121 | 5.18 | 0.1661 | Not significant |
| Pure error | 0.047 | 2 | 0.023 | |||
| Cor total | 174.22 | 14 |
- SS: the sum of the square; df: degree of freedom; MS: mean square; X1: catalyst concentration; X2: methanol to oil molar ratio; X3:reaction time.
From Equation (3), it is seen that linear terms have a positive effect on the yield, whereas the quadratic terms with a negative sign have a negative impact on the yield that means with increasing the quadratic coefficient value, the yield of biodiesel will be decreased [1].
3.3. Individual Effect of Transesterification Reaction Parameters on Yield of Biodiesel
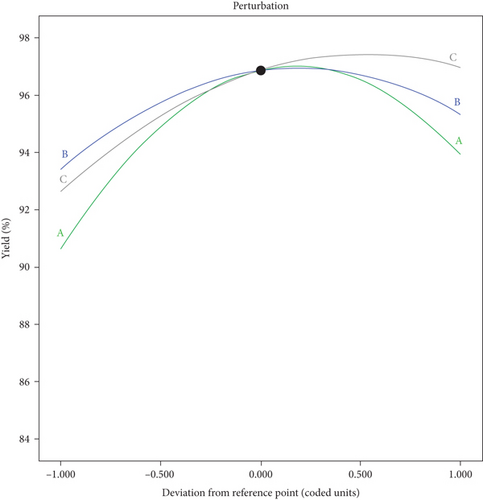
The effect of catalyst concentration, methanol to oil ratio, and reaction time is shown in Figure 3. A perturbation plot assists to identify the order of each process parameter and its impact while fixing the other process parameters at constant [1, 21]. The sensitivity of variable catalyst concentration (X1), methanol to oil ratio (X2), and reaction time (X3) on the yield of biodiesel is presented by the behavior of the curvature. The variable with a steeper slope has a greater impact on the yield of biodiesel as compared to the flatter slope [3, 34]. Thus, the plot describes that variable X3 has a high effect on the yield of biodiesel complied by X1 and X2. It shows that reaction time (X3) at the lower level until the middle value is more sensitive, whereas the sensitivity decreased between middle to a more prominent level. Also referring to the analysis of ANOVA, reaction time is the most significant variable, followed by catalyst concentration.
3.3.1. Interaction Effect of the Transesterification Reaction Parameters on Yield of Biodiesel
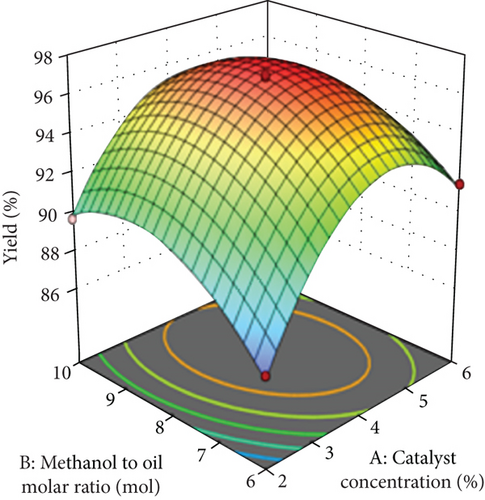
The 3D plots shown in Figure 4 were drawn to describe the effect of two independent variables on a yield of biodiesel. A corelation graph was plotted by keeping other parameters at zero level (middle point). The yield of biodiesel increased as the methanol to oil ratio and catalyst concentration increased up to a certain point and then diminished thereafter. Figure 4(a) indicated the combined effect of methanol to oil ratio and catalyst concentration on biodiesel yield, and it can be observed that increasing the methanol to oil ratio and catalyst concentration up to a certain value boosts it. The yield of biodiesel slowly diminishes when the catalyst concentration rises outside of 4%. The high amount of catalyst concentration will bring in emulsification and saponification of biodiesel during the refining process [35]. In addition, it is necessary to check suitable methanol to oil ratios to optimize the transesterification parameters [18]. If the quantity of methanol in the solution is too low, this will form more monoglycerides and diglycerides likened with a methyl ester. In contrast, if the quantity of methanol in the solution is too high, this will contribute to the solubility of the glycerol in the solution, which will complicate the isolation of methyl ester and glycerol [35, 36]. Hence, it is essential to use an appropriate quantity of methanol (8 mol/mol), since the microwave reactor is not a pen system, which does not allow for the large quantity of material [6], and thus, the quantity of methanol must be distinct to boost the yield of biodiesel [22].
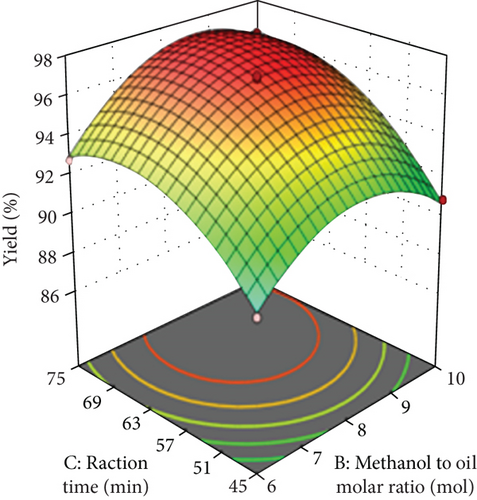
The influence of methanol to oil ratio and reaction time is shown in Figure 4(b). The reaction time and methanol to oil ratio were varied from 45 to 75 min and 6 to 10 w/w%, respectively. Overall, within the experimental model, the maximum yield of biodiesel was found at moderate transesterification parameters. Enhancing the reaction time even after the equilibrium state of the reaction mixture will not enhance the yield of biodiesel; thus, enhancing the reaction time may induce reverse transesterification to minimize the yield of biodiesel [37]. Hence, from this description, the optimized reaction time needed to produce the maximum yield of biodiesel was found to be 60 min.
3.4. Optimization of Process Parameters and Model Validation
The optimal values of the selected variables were obtained by numerical desirability optimization technique using Box-Behnken design approach by setting criteria for independent variables in the range and for the dependent variable (yield) maximize to obtain the best biodiesel yield. The transesterification parameters of the predicted solution for the yield biodiesel were catalyst concentration (3.14%), methanol to oil ratio (8.4 : 1), and reaction time (57.21 min) corresponding to 97.78% of biodiesel yield. Using this predicted value, experiments were triplicated, and the obtained results were 97.76%, 97.75%, and 97.78%, with the average of yield of 97.76% conversion. This result was greater than 97.43% of biodiesel produced from Ulva lactuca, marine macroalgae, at optimum conditions of 9 : 1 methanol to oil ratio, 8% catalyst concentration, 50 min reaction time, and 55°C temperature by Anantapinitwatna et al. [35] and 97.6% of biodiesel produced from Annona squamosa seed oil at the optimum condition of oil to methanol ratio (5.04), catalyst concentration (1.12%), sonication time (113 min), and temperature (57 °C) by Dehghan et al. [36]. The variation could be due to the production technique, plant compositions, and separation process [33]. Based on the results, it can be proved that the Box-Behnken model is good and can predict the optimal parameters of the microwave irradiation-assisted transesterification reaction for biodiesel production. This indicates that the Box-Behnken model allows an effective method to predict the optimum of experimental parameters.
3.5. Characterization of Cucurbita maxima Waste Oil Biodiesel
The physicochemical properties (moisture content, kinematic viscosity, density, acid value, iodine value, peroxide value, and oxidation stability) of the produced Cucurbita waste biodiesel are obtained according to standard methods [6, 11, 23, 32] as shown in Table 7. The properties of the produced Cucurbita waste biodiesel are in good correspondence with the defined biodiesel prosperity standards.
| Properties | Biodiesel | AOCS standard | Test methods |
|---|---|---|---|
| Moisture content (%) | 3.9 ± 1.0 | < 5 | AOCS cd 2C-30 (1996) |
| Kinematic viscosity (mm2/s) @ 40°C | 3.4 ± 12 | 1.9 – 6.0 | AOCS Dd 8-35 method |
| Density (g/cm3, 15 °C) | 0.88 ± 1.3 | 0.86−0.90 | ASTM D6751 method |
| Acid value (mg KOH/g) | 0.32 ± 0.1 | 0.5 | ASTM) D6751 method |
| Iodine value (g I2/100 g oil) | 76 ± 0.3 | <120 g I2/100 g | EN14214 method |
| Peroxide value (meq/kg) | 1.31 ± 0.2 | 1.25–3.96 | AOCS Dc 17b-35(1998) method |
The moisture content reflects the quality of the biodiesel produced. The higher level of moisture content in biodiesel as assigned by the AOCS standard is <5% in volume [34]. Higher moisture content in biodiesel may induce different problems such as microbial growth in fuel, transportation problem, and water accumulation [26]; this may be created by improper treatment during processing or by the assimilation of atmospheric moisture throughout storage [26]. Thus, the finding of this study is confirmed with the standard value of AOCS.
Viscosity is a key property of biodiesel since it shows the ability of a substance to flow [35] and has a strong link with the property of fuel injection [15]. The high viscosity of biodiesel will contribute to poor atomization and high droplet sizes of the fuel atomiser which contributes to operational problems [38]. Biodiesel can be utilized as diesel fuel only when its viscosity value rests down on the interval of 1.9 to 6.0 mm2/s AOCS [7]. The viscosity (3.4 ± 12 mm2/s; Table 7) in this study represents that the produced biodiesel had the acceptable viscosity value confirming to limitation of the AOCS standard indicating Cucurbita maxima waste as a good source of oil for biofuel production.
The amount of density must be kept within allowable limits to tolerate optimal air to fuel ratios for accomplished combustion [39] since a high level of density can contribute to particulate matter emissions and incomplete combustion [40]. In this study, the density of the biodiesel was 0.885 g/mL, which is confirmed to ASTM D6751 standards.
The acid value can play a significant role in the percentage of free fatty acid methyl ester of the final quality [41]. The high level of acid value for pure biodiesel, as defined by ASTM D6751 standard, is 0.5 mg KOH g–1 [26]. When oils, fats, and biodiesel go defective, there are some measurable problems, and thus, acid value rises as the fatty acid breaks away into shorter chain acids [42]; thus in this study, the acid value of the produced biodiesel was confirmed (Table 7).
The peroxide value tests the existence of oxidative moieties in the samples. The oxidative moieties commonly found in biodiesel are hydroperoxides generated when oxygen from the air reacts with fatty esters, and it is generally the first step in the oxidative abasement pathway of biodiesel [6]. In this study, 1.31 ± 0.2 meq O2 kg–1 of peroxide value was registered for biodiesel produced from Cucurbita maxima waste oil corresponding to the limit value.
The iodine value is an important parameter to determine the degree of unsaturation, oxidative rancidity, and chemical constancy properties of various oil and biodiesel [37]. According to EN14214 (European committee for standardization), the iodine value should be less than 120 g I2/100 g sample for the oil to be desirable as feedstock for biodiesel production [43]. Iodine value below 115 g/100 g is classified as nondrying oil, between 150 g/100 g and 130 g/100 g is semi drying oil, and above 130 g/100 g value is drying oil [43]. Hence, the value obtained in this study was 76 ± 0.3 g I2/100 g oil, which is confirmed to the EN14214 recommended value and classified as nondrying oil (Table 7).
3.5.1. Fourier Transform Infrared Spectrum Functional Group Analysis
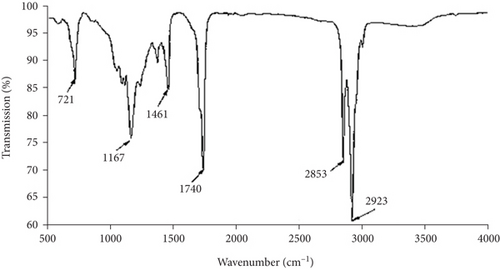
FTIR spectrum of methyl ester is shown in Figure 5. In the functional group −CO−O−CH3 of Cucurbita maxima waste oil methyl ester that was produced during the transesterification reaction, 2853 cm-1 and 2923 cm-1 were assigned to the asymmetric stretching and stretching vibrations of −CH3. On the other way, 1461 cm-1 matched the asymmetric deflecting vibration of −CH3 [4], while 1167 cm-1 was because of the stretching vibration from O−CH3; and 1740 cm-1 was attributed to the vibration of −C = O. The stretching C = O peak was 1742 cm-1 and was happened normally in free fat acid methyl ester, which is settled between 1700 and 1800 cm-1 of wavelength and is an ester [21]. The fingerprint region (1500–900 cm-1) that is the main spectrum from biodiesel has a peak at 1461 cm-1 linked to bending vibrations of −CH3 [1]. These outcomes reverberate the conversion of triacylglycerol which could be changed into methyl esters [36].
3.5.2. Gas Chromatography Fatty Acid Composition Analysis
Gas chromatography was utilized to test the fatty acid composition of Cucurbita maxima waste oil of methyl ester as indicated in Table 8.
| Fatty acid component | Carbon structure | Composition (%) |
|---|---|---|
| Saturated acid | ||
| Palmitic acid | C16:0 | 7.09 |
| Stearic acid | C18:0 | 3.14 |
| Behenic acid | C22:0 | 1.27 |
| Unsaturated acid | ||
| Oleic acid | C18:1 | 46.78 |
| Linoleic acid | C18:2 | 37.32 |
| Linolenic acid | C18:3 | 1.89 |
| Eicosenoic acid | C20:1 | 0.86 |
| Erucic acid | C22:1 | 1.65 |
| Total | 100 |
The composition analysis shown in Table 8 represents that the Cucurbita maxima waste oil methyl ester comprises a high level (88.5%) of unsaturated fatty acids like polysaturated and monosaturated fatty acid methyl esters. Oleic acid (C18:1) and linoleic acid (C18:2) were determined to be the dominant unsaturated fatty acid, respectively, and saturated fatty acids like palmitic acid (C16:0) was detected for 7.09% of the composition (Table 8). These values were higher than those explained for Cucurbita maxima seed oil (87.7%) by Montesano et al. [12]. Differences in Cucurbita maxima waste and seed compositions are the result of plants sources, characteristics, harvest conditions, species, cultivation zone, and degree of fruit maturation [44]. From this study, this major amount of unsaturated fatty acid ensures good flow attributes of fuel compared to high levels of saturated fatty acids, which restrict the application of biodiesel in cold countries because of poor cold flow [45]. Furthermore, oxidation debasement has a negative strike on kinematic viscosity and acid value when a high level of saturated fatty acid is present [26, 27].
4. Conclusion
The main issue in the present study is to produce and optimize methyl ester from Cucurbita maxima waste oil using microwave irradiation-assisted transesterification via the Box-Behnken design approach. The significant effect of transesterification parameters such as methanol to oil ratio, the concentration of catalyst (KOH), and reaction time on the percentage yield of methyl esters conversion was also determined. The experimental result indicated that the oil content of Cucurbita maxima waste was found to be 44.3%. The high coefficient of determination of the model displayed that it well corresponded to the experimental design. Thus, the results of the significance level indicated that reaction time and catalyst concentration were the most significant factors affecting the yield of biodiesel, respectively. It was predicted that the optimum transesterification conditions within the experimental range would be the catalyst concentration of 3.14%, the methanol to oil ratio of 8.40, and the reaction time of 57.12 min corresponding to 97.76% of biodiesel yield. This involves that empirical models deduced from Box-Behnken can be utilized to adequately report the link between the input and output factors. The physicochemical properties of the biodiesel reveal that the Cucurbita maxima waste could be applied as a potential source of material for methyl ester production. The high composition of fatty acid of Cucurbita maxima waste oil methyl ester is unsaturated fatty acid primarily oleic acid. The findings propose that the produced biodiesel from Cucurbita maxima wastes should be researched as a novel possible natural biofuel. This study concluded that Cucurbita maxima waste contains a reliable amount of oil that is extremely feasible for biodiesel production. Further investigation of biodiesel initiated from Cucurbita maxima waste oil can be extended by the testing of different catalyst (such as heterogeneous catalyst and metal oxide nanocatalyst) types and the influence of Cucurbita maxima waste oil methyl ester on the release of emissions in respect to other biodiesels.
Additional Points
Article Highlights. (i) The novel investigation of Cucurbita maxima waste oil as a potential source for the production of biodiesel (methyl ester). (ii) Optimization of important transesterification process parameters. (iii) Investigation high yield of biodiesel (97.76%) from Cucurbita maxima wastes. (iv) Determination of the properties of produced biodiesel from Cucurbita maxima waste oil.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of Interest
The author wishes to confirm that there is no conflict of interest associated with this paper for any material as well as financial.
Acknowledgments
The author would like to thank the School of Chemical Engineering, Jimma Institute of Technology, and Adama Science and Technology University for supporting the laboratory experimental materials and Holeta Agricultural Centre for supplying the raw materials for this work.
Open Research
Data Availability
The [experimental design, design expert software, data analysis, Fourier transforms infrared spectrum, and gas chromatography] data used to support the findings of this study are included within the article.