[Retracted] Effects of Free Amino Acids, Metallothionein, and Chemical Forms of Heavy Metals on the Accumulation and Detoxification of Cadmium and Chromium in Chinese Goldthread (Coptis chinensis Franch.)
Abstract
Chinese goldthread (Coptis chinensis Franch.) is an important medicinal plant worldwide. However, its clinical safety is negatively impacted by high cadmium (Cd) and chromium (Cr) levels. Understanding the accumulation pattern and detoxification mechanism of heavy metals, such as Cd and Cr, is important for regulating their transportation and reducing their accumulation in plants. Free amino acids (FAAs), metallothioneins, and chemical forms of heavy metals are closely associated with heavy metal accumulation and detoxification mechanisms. In the present study, we evaluated the uptake of Cd and Cr in Chinese goldthread and characterized the changes in FAA levels, CcMT2, a metallothionein gene, expression under Cd and Cr stress, and Cr chemical forms in various tissues of Chinese goldthread. The majority of Cd and Cr was retained in the roots; this might be the tolerance strategy utilized by Chinese goldthread to protect the aboveground photosynthetic tissues. A chemical continuous extraction method was utilized to analyze the chemical forms of Cr, and the results suggested that Cr was primarily fixed by oxalate in all tissues, and after Cr treatment, Cr tended to combine with phosphate to form an insoluble complex that reduced the toxicity and bioavailability of Cr in Chinese goldthread. The accumulation of Cd and Cr was significantly positively correlated with valine, histidine, threonine, tyrosine, and serine levels and significantly negatively correlated with glutamic acid and alanine levels. The variation in proline levels under Cd stress was different than that under Cr stress. FAAs might affect the accumulation of and resistance to Cd and Cr through various detoxification strategies, such as chelation with heavy metals ions and conversion into the required products. Furthermore, CcMT2 was isolated for the first time and found to be dominantly expressed in leaves. CcMT2 was induced under Cd and Cr stress in all plant tissues, indicating its role in the accumulation of and tolerance to Cd and Cr.
1. Introduction
The accumulation of heavy metals in soil due to various industrial activities has a negative impact on the environment, agriculture, and human health [1, 2]. Among various heavy metals, cadmium (Cd) and chromium (Cr), especially in the form of Cr(VI), are frequently found in arable land where they are toxic to plant growth and pose a risk to human health through absorption and accumulation in the food chain [3, 4]. Although Cd and Cr are nonessential for plant growth and metabolism, they can accumulate in plant tissues via transporters shared with essential nutrients or nonselective channels [5–7]. Following their entry into plants, Cd and Cr cause retardation of plant growth stimulating the production of reactive oxygen species (ROS); damaging DNA, proteins, and plasma membrane integrity; triggering lipid peroxidation; displacing cofactors from transcription factors and enzymes; and inducing various physiological and ultrastructural changes [8–11]. Consumption of plants contaminated with Cd can lead to renal injuries, osteoporosis, diabetes, cardiovascular disorders, neuronal damage, and cancer [12]. Similarly, Cr is considered highly toxic to living organisms and is known to exert carcinogenic and mutagenic effects [4].
Plants have developed versatile mechanisms to tolerate certain levels of exposure to heavy metals. These mechanisms include cellular exclusion, compartmentalization, sequestration, antioxidant production, and chelation via specific ligands [6, 13]. Metallothioneins (MTs) are cysteine-rich proteins that are broadly distributed in microorganisms, plants, and animals [14]. MTs are ubiquitous heavy metal chelators and play a pivotal role in metal ion homeostasis and detoxification [15]. Plant MTs can be classified into four subfamilies based on the distribution of cysteine residues in their amino- and carboxyl-terminal regions [16]. It has been reported that different MT genes exhibit distinct tissue-specific expression in plants [17]: MT1 subfamily genes are primarily expressed in roots, MT2 subfamily genes in leaves, MT3 subfamily genes in mature leaves and ripening fruits, and MT4 subfamily genes in seeds [18]. MT proteins have a low molecular weight (Mw), typically 7–8 kDa, and high levels of cysteine (20%–30%), which contribute to effective chelation of heavy metals with –SH groups and sequestration of the resulting compounds [19]. In general, heavy metals induce MT synthesis in response to toxicity [8]. Thus, MT content and/or MT genes are potential biomarkers of heavy metal accumulation and tolerance in organisms [20]. Upon exposure to metals, plants often synthesize a set of diverse low-Mw substances, particularly specific free amino acids (FAAs) known as compatible solutes. FAAs serve as signaling molecules and play various important roles in plants by acting as osmolytes, radical scavengers, regulators of ion transport, and modulators of stomatal opening to detoxify heavy metals [13, 21]. Glutamic acid (Glu), proline (Pro), and histidine (His) also play functional roles in metal tolerance and detoxification in plants [13]. Amino acids bind to various metal ions through their chelating moieties, including amino (–NH2), carboxylic (–COOH), hydroxyl (–OH), and thiol (–SH) groups, all of which have charge donating and complex stabilizing abilities [22]. Notably, amino acids are essential for the synthesis of glutathione (GSH) and phytochelatins (PCs), which are effective heavy metal chelation ligands in plant cells [23]. Chemical forms of heavy metals play important roles in the internal mechanisms of plants that impart protection against heavy metal toxicity [24]. Metal ions absorbed by plants can be converted to different chemical forms, which determine their mobility and toxicity [25]. Conversion to nontoxic chemical forms is another important strategy employed by plants to alleviate heavy metal toxicity [26]. The chemical forms of Cr with low bioavailability have been shown to integrate with insoluble phosphate compounds or oxalate, thereby contributing to metal tolerance and detoxification [27].
Chinese goldthread (Coptis chinensis Franch.) is one of the most important traditionally used medicinal plants worldwide [28]. Its active ingredients include alkaloid compounds that possess antibacterial, antiviral, anti-inflammatory, and antitumor properties [29]. However, high levels of Cd and Cr in Chinese goldthread negatively impact its clinical safety [30–32]. Notably, Chinese goldthread has a strong enrichment effect on Cd [33]. Under normal conditions, plants retain <1 μg g−1 of Cr [9]; however, the level of Cr in Chinese goldthread is generally >1 μg g−1 [34]. To regulate and reduce the uptake of Cd and Cr by Chinese goldthread, it is important to understand the uptake, transport, accumulation, and detoxification of these heavy metals. Distribution patterns and chemical forms of heavy metals, FAAs, and MTs in plant tissues may reflect heavy metal accumulation and detoxification processes [20, 21, 35]. In a previous study, we examined the subcellular and tissue distribution of Cd and Cr in Chinese goldthread [30, 34]. However, the roles of FAAs, MTs, and the chemical forms in Chinese goldthread remain unclear. To address these research gaps, the present study aimed to (1) evaluate Cd and Cr uptake by Chinese goldthread, (2) analyze the chemical forms of Cr, (3) characterize the changes in FAAs levels in Chinese goldthread under Cd and Cr stress, and (4) isolate CcMT2 and assess its expression profile in different tissues and under Cd and Cr stress.
2. Materials and Methods
2.1. Plant Materials and Hydroponic Conditions
Plants and rhizosphere soil samples were collected from six townships (Huaping, Shuping, Xiaoshuhe, Zhongbao, Qingling, and Ganzhouhe) in Zhenping County, Shaanxi Province, which is one of the main production areas of Chinese goldthread in China. The plant samples were washed and dried to facilitate detection of Cd and Cr. After being allowed to dry naturally and removing impurities, the soil samples were passed through a 100-mesh sieve.
Hydroponic plants were used to determine different chemical forms of Cr and detect variations in FAA levels under Cd and Cr stress. Chinese goldthread plants were collected from Zhenping County, Shaanxi Province, China. All plants were cultivated hydroponically under similar conditions in dark and aerated containers in a solution containing the following constituents: 2 mM Ca2+, 3.5 mM K+, 1 mM Mg2+, 7 mM NO3−, 0.5 mM H2PO4−, 1 mM SO42−, 10 μM H3BO3, 0.1 μM CuSO4, 5 μM MnSO4, 0.5 μM ZnSO4, 0.1 μM CoCl2·6H2O, 0.1 μM Na2MoO4·2H2O, and 20 μM Fe-EDTA. After acclimatization for 4 weeks, the plants were exposed to 0 or 100 μM Cd, or 500 μM Cr(VI) in a hydroponic nutrient solution. Cd and Cr treatment levels were determined based on the results of previous pre-experimental studies (see Supplementary Information (available here)). Each treatment was performed in three replicates of 15 plants each. Plants were harvested following exposure to heavy metals at 0 h (control group, CK), after 24 h (Cd24h and Cr24h), and after 10 days (Cd10d and Cr10d). Plants were grown under natural light, with day/night temperatures of 25 °C/20 °C and day/night relative humidity of 70%/85%; the nutrient solution was renewed every 3 days, and the pH was maintained at 5.6 ± 0.3.
The leaves, rhizomes, and roots of Chinese goldthread were collected for the analysis of CcMT2. Hydroponic plant materials were used for the analysis of CcMT2 expression following Cd and Cr treatment. The hydroponic conditions and treatment levels of Cd and Cr were the same as described previously. The treated samples were harvested at 0, 2, 4, 8, 12, 24, and 72 h and 10 days after heavy metal stress. All collected samples were immediately frozen in liquid nitrogen and stored at − 80 °C until analysis.
2.2. Determination of Cd and Cr Concentrations
Roots and rhizomes of the collected plants were soaked in 20 mM EDTA-Na2 for 15 min to desorb surface-adsorbed metals and then washed thrice with deionized water. Cr and Cd levels were assessed according to a previously described method [30]. Briefly, all plant and soil samples were dried to a constant weight, digested in a microwave (Mars 5, CEM, USA) using HNO3/H2O2 (v/v = 3/1), and then allowed to stand overnight. The samples were digested until the liquid became clear and colorless. Cd and Cr levels in Chinese goldthread tissues and rhizosphere soil samples were detected using inductively coupled plasma-mass spectrometry (ICP-MS) (Varian ICP-820MS, Varian, Palo Alto, CA, USA).
For ICP-MS analysis, 0.5 g of sample was accurately weighed in a Teflon digestion vessel. Next, 3 mL of concentrated HNO3 and 1 mL of H2O2 (30%) were added, and the mixture was allowed to stand overnight. The vessels were closed and placed on the rotating turntable of a microwave oven (Mars 5, CEM, USA) and digested at 120 °C for 5 min, 160 °C for 5 min, and 180 °C for 25 min, with 15 min ramp to temperature at a maximum power of 1,000 W for 30 min and 0 W for 15 min for cooling. Each sample was prepared in triplicate. After cooling, the solutions were evaporated to a small volume and then transferred to 50-mL volumetric flasks. Next, 0.5 mL of HNO3 was added before adjusting the final volume to 50 mL with high purity deionized water. The ion lens settings, nebulizer gas flow rate, and torch position of the ICP-MS were optimized to maximize the ion signals of the elements studied while reducing the background signals. The argon gas utilized was of spectral purity (99.9998%). Certified standard reference material (SRM) NIST 1570a trace elements in spinach leaves and NIST 1547 peach leaves (National Institute of Standards and Technology, NIST, Gaithersburg, MD, USA) were used to assess the accuracy and precision of the method.
2.3. Extraction of Cr in Different Chemical Forms
Previous research has identified six chemical forms of Cd [30]. Here, we utilized the chemical continuous extraction method [36, 37] to analyze the chemical forms of Cr. The six forms of Cr were extracted using specific solutions in the following order: (1) 80% ethanol for extracting inorganic Cr (FE); (2) deionized water (d-H2O) for extracting water-soluble Cr (FW); (3) 1 M NaCl for extracting pectate- and protein-integrated Cr (FNaCl); (4) 2% acetic acid (HAc) for extracting insoluble Cr–phosphate complexes (FHAc); (5) 0.6 M HCl for extracting oxalic acid bound Cr (FHCl); and (6) residual Cr (FR). Frozen root, rhizome, and leaf tissues were homogenized using the abovementioned extraction solutions (w/v = 1/10) and then shaken for 22 h at 25 °C. The homogenate was centrifuged at 5,000 g for 10 min, and the first supernatant was obtained. The sediment was re-extracted twice using the same extraction solution and shaken for 2 h at 25 °C. The three supernatants were then pooled, and the sediment was extracted using the next solution following the sequence of solvents one at a time. All extractions were performed following the same steps and durations used in the first extraction. Each fraction of the pooled supernatant solution and residual fractions were then dried to a constant weight and digested in a microwave using HNO3/H2O2 (v/v = 3/1). Finally, the Cd and Cr levels were detected using ICP-MS.
2.4. Measurement of FAA Levels
Intracellular FAA levels were analyzed as previously described [3]. Briefly, 100 mg of freeze-dried leaf, rhizome, and root samples were thoroughly mixed with 2% (w/v) sulfosalicylic acid solution. The homogenate was centrifuged at 200 g at room temperature for 2 h and then centrifuged at 10,000 g for 15 min. After filtration using a 0.22-μm membrane, the supernatant was prepared for FAA analysis using an automatic L-8900 amino acid analyzer (Hitachi, Ltd., Tokyo, Japan). The analysis conditions were as follows: cation-exchange column, 4.6 mm × 60 nm; separation column temperature, 57 °C; reaction column temperature, 135 °C; flow rate of the buffer and ninhydrin, 0.35 mL min−1; dual-channel ultraviolet detection wavelengths, 440 nm and 570 nm; and injection volume, 20 μL.
2.5. Cloning of the Metallothionein Gene
2.5.1. RNA Isolation and First Strand cDNA Synthesis
Total RNA was isolated from frozen plant tissues using a Plat Total RNA isolation kit (DP441, TIANGEN BIOTECH Co., Ltd., Beijing, China). RNA purity was verified using a Nano drop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). After isolation, cDNA synthesis was performed using a PrimeScript™ RT Reagent kit (TaKaRa Bio, Inc., Shiga, Japan) following the manufacturer’s instructions. The resulting product was stored at −20 °C until further use.
2.5.2. Gene Isolation and Bioinformatics Analysis
The following specific primers were designed according to the published length of the metallothionein gene in Coptis japonica (AB257869.1): forward (Fw) primer: TCGTGTAATCCCTCTGCATTTCCA and reverse (Rv) primer: CTCTTAACACTCCCAGGGCTCAG. The amplification template used in this study was the complementary DNA (cDNA) that had been reverse transcribed via RNA extracted from Chinese goldthread. Polymerase chain reaction (PCR) was performed according to the manufacturer’s instructions. The PCR products were then gel purified using a DNA Gel Extraction Kit (Omega Bio-Tek, Norcross, GA, USA) following the manufacturer’s instructions. Next, the purified products were cloned into a pMD18-T vector (TaKaRa Bio, Inc., Shiga, Japan) for sequencing. Both ORF-finder and GENSCAN Web Server were used to confirm the open reading frame (ORF). The theoretical isoelectric point (pI) and Mw were predicted using the pI/Mw tool on the ExPASy server (http://web.expasy.org/computepi/). Phylogenetic trees were generated using MEGA X free software, and the neighbor-joining method was performed using 1000 bootstrap replicates. The amino acid sequences of other species were downloaded from the NCBI database.
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
qRT-PCR was performed using a CFX96 Real-Time PCR System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The 25-μL reaction mixture contained 12.5 μL of SYBR® Premix Ex Taq™ (TaKaRa Bio, Inc., Shiga, Japan), 2 μL each of forward/reverse primer, 2 μL of cDNA template, and 6.5 μL of RNase Free H2O. The PCR settings were as follows: 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, and 58.5 °C for 30 s. This program was followed by a melting curve analysis (65 °C–95 °C, with temperature increments of 0.5 °C every 5 s). The β-actin gene was used as the endogenous control. The Fw and Rv primers were CGCAGGAAAGTGTGGTTGTGGTGA and TACTTCTTGCACCCAGCGCAGCT, respectively.
2.7. Statistical Analysis
Relative gene expression levels were calculated using the delta-delta Ct (2−△△Ct) method. Data were compared using one-way analysis of variance followed by the least significant difference test. Pearson correlation analysis was used to examine relationships between variables. P values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA), and figures were created using GraphPad Prism 6 software (GraphPad Software, San Diego, USA).
3. Results and Discussion
3.1. Uptake of Cd and Cr by Chinese Goldthread and Rhizosphere Soil
The World Health Organization and green industry standards for the import and export of medicinal plants and preparations have established the maximum permissible limit of Cd in medicinal herbs as 0.3 mg kg−1 [38]. For Chinese goldthread, the rhizomes are the main medicinal part of the plant. As shown in Table 1, the Cd level in most rhizomes was >0.3 mg kg−1. Furthermore, the Cr levels found in all Chinese goldthread rhizomes were higher than those observed in general plants (<1 μg g−1) [9]. These results indicated that high levels of Cd and Cr accumulate in Chinese goldthread. Different plant tissues have different capacities for heavy metal accumulation. In general, the distribution of heavy metals in different parts of a plant is according to the following pattern: leaf < rhizome < root. As predicted, the roots accumulated higher levels of Cd and Cr than other plant tissues, with the highest Cd and Cr levels observed being 6.89 mg kg−1 and 4.47 mg kg−1, respectively (Table 1). Moreover, the TF values of Cd and Cr were <1. These results confirmed that Chinese goldthread accumulates high levels of Cd and Cr in its roots. These results also suggested Cd and Cr retention in the roots during long-distance translocation from the roots to the rhizomes and leaves, indicating that roots serve as an effective barrier to Cd and Cr translocation [39]. High Cd and Cr levels have been reported in the roots of other plants, such as Mentha aquatica, Solanum lycopersicum Mill., Brachiaria mutica, and Leptochloa fusca [9, 40, 41], with only a small proportion of heavy metals being translocated to the aerial parts. High retention of heavy metals, such as Cd and Cr, in the roots could be an important tolerance strategy utilized by Chinese goldthread to protect the aboveground photosynthetic tissues [40]. Notably, enhanced heavy metal sequestration in the roots requires greater negative charge of the cell wall to enable binding of heavy metal ions with positive charges [9]. Our previous studies revealed that the majority of Cd and Cr was sequestrated in the cell wall of the root cells [30, 34]. This finding suggests that more negative charges of the cell wall bind with the positive charges of Cd and Cr ions in Chinese goldthread to increase the Cd and Cr sequestration in the roots. In addition, the Cd BCF values were >1 in all plant samples, with a maximum of 17.52 and a minimum of 6.44 (Table 1). In contrast, the Cr BCF values were < 1 in all plant samples, except for one sample with a value of 1.64. This finding suggests that Chinese goldthread has different absorption capabilities for different heavy metals. Moreover, the Cd BCF values of >1 revealed that Chinese goldthread could enrich Cd from soil, which was consistent with the findings of a previous study [33].
| Heavy metal | Sampling location | Leaves (mg kg−1 DW) | Rhizomes (mg kg−1 DW) | Roots (mg kg−1 DW) | Total in plant (mg kg−1 DW) | Soil (mg kg−1 DW) | BCF | TF |
|---|---|---|---|---|---|---|---|---|
| Cd | Huaping | 0.20 ± 0.01 | 0.23 ± 0.01 | 3.40 ± 0.19 | 3.84 ± 0.18 | 0.39 ± 0.01 | 9.86 ± 0.45 | 0.06 ± 0.003 |
| Shuping | 0.23 ± 0.02 | 0.45 ± 0.01 | 4.08 ± 0.09 | 4.76 ± 0.11 | 0.30 ± 0.02 | 15.81 ± 0.69 | 0.05 ± 0.004 | |
| Xiaoshuhe | 0.86 ± 0.04 | 0.52 ± 0.02 | 4.83 ± 0.06 | 6.21 ± 0.12 | 0.35 ± 0.01 | 17.52 ± 0.98 | 0.16 ± 0.01 | |
| Zhongbao | 0.30 ± 0.02 | 0.22 ± 0.02 | 2.46 ± 0.04 | 2.99 ± 0.04 | 0.40 ± 0.01 | 7.41 ± 0.21 | 0.11 ± 0.01 | |
| Qingling | 0.44 ± 0.02 | 0.52 ± 0.02 | 6.89 ± 0.15 | 7.85 ± 0.18 | 1.22 ± 0.02 | 6.44 ± 0.04 | 0.06 ± 0.003 | |
| Ganzhouhe | 0.32 ± 0.02 | 0.41 ± 0.02 | 3.00 ± 0.24 | 3.73 ± 0.26 | 0.46 ± 0.01 | 8.11 ± 0.34 | 0.09 ± 0.01 | |
| Cr | Huaping | 0.39 ± 0.02 | 1.44 ± 0.10 | 2.13 ± 0.21 | 3.96 ± 0.13 | 9.35 ± 0.31 | 0.42 ± 0.02 | 0.11 ± 0.005 |
| Shuping | 0.48 ± 0.01 | 1.55 ± 0.05 | 1.63 ± 0.15 | 3.65 ± 0.11 | 5.56 ± 0.42 | 0.66 ± 0.05 | 0.15 ± 0.01 | |
| Xiaoshuhe | 0.44 ± 0.02 | 2.08 ± 0.20 | 2.42 ± 0.20 | 4.93 ± 0.35 | 6.79 ± 0.48 | 0.73 ± 0.005 | 0.10 ± 0.01 | |
| Zhongbao | 0.53 ± 0.03 | 1.50 ± 0.07 | 2.27 ± 0.17 | 4.30 ± 0.12 | 8.26 ± 0.44 | 0.52 ± 0.02 | 0.14 ± 0.01 | |
| Qingling | 0.35 ± 0.02 | 1.40 ± 0.05 | 1.74 ± 0.13 | 3.48 ± 0.18 | 7.87 ± 0.30 | 0.44 ± 0.03 | 0.11 ± 0.01 | |
| Ganzhouhe | 0.83 ± 0.03 | 3.58 ± 0.34 | 4.47 ± 0.14 | 8.88 ± 0.43 | 5.46 ± 0.27 | 1.64 ± 0.15 | 0.10 ± 0.01 | |
3.2. Chemical Forms
The migration ability, bioavailability, and toxicity of heavy metals depend on their chemical forms in plants [35]. Heavy metals can convert into various unavailable forms in plants, and high levels of these unavailable forms may induce low phytotoxicity [24]. Heavy metals in inorganic (extracted with 80% ethanol) and water-soluble (extracted with d-H2O) forms show strong migratory capacity and exhibit the greatest toxicity to plants, followed by pectate- and protein-integrated (extracted with 1 M NaCl) and insoluble phosphates (extracted with 2% HAc). In contrast, oxalates (extracted with 0.6 M HCl) and residues are least harmful to plants [36, 42]. The chemical forms of Cr are shown in Figure 1 and Supplementary Table S1. Among the control plant tissues, the form of Cr extracted with HCl (33.2%–47.8%) was predominant, followed by that extracted with 80% ethanol (33.2%–37.9%), residual (7.7%–16.9%), and d-H2O (4.3%–11.5%), whereas the forms extracted with NaCl and HAc were rather low. After Cr treatment, the Cr fraction extracted with HCl increased by varying degrees and was the predominant form in all plant tissues. Taken together, these data demonstrated that Cr was primarily fixed by oxalate and that the largest fraction of Cr existed in less mobile and less toxic forms in Chinese goldthread. Heavy metals can form complexes with organic acids, resulting in changes in their toxicity and migration ability [43]. Previous studies have shown that the formation of strong bonds between chelating metals and carboxylate groups and phenolic hydroxide groups of organic acids plays an important role in metal accumulation and detoxification in plants [44, 45]. Among organic acids, oxalate is a strong dicarboxylic acid anion and a good complexing agent for the binding of heavy metal cations [46, 47]. In Leersia hexandra Swartz, the high oxalic integrated Cr content in leaves might be related to Cr resistance [47]. Moreover, the acidic environment of the vacuoles is favorable for the formation of metalorganic acid complexes [44]. Our previous study demonstrated that when Chinese goldthread is subjected to Cr stress, Cr ions mainly enter the vacuoles after crossing the plant cell wall to protect cellular activities [34]. Therefore, it is possible that Cr binding with organic acids and its storage in the vacuoles reduce the toxicity and bioavailability of Cr in Chinese goldthread. A comparison between the Cr treatment and control groups revealed that the proportion of Cr extracted with HAc significantly increased by 271.4%–878.6% in leaves, 200.0%–286.0% in rhizomes, and 296.3%–348.2% in roots. These results indicate that Cr treatment causes Cr to combine with phosphate to yield an insoluble complex, which is consistent with the results of a study on rice plants [48, 49]. Therefore, detoxification of Cr in Chinese goldthread might be associated with an increase in the amount of Cr combined with phosphate along with low migration ability and bioavailability. Phosphate precipitated with heavy metals in the root cell vacuoles consequently traps heavy metals in the roots and affects their transportation to the aerial parts of plants [50]. The application of exogenous phosphorus (P) may enhance the amount of HAc-extractable metals and decrease the amount of 80% ethanol-extractable metals in rice roots as a result of detoxification of P while alleviating heavy metal stress [51].
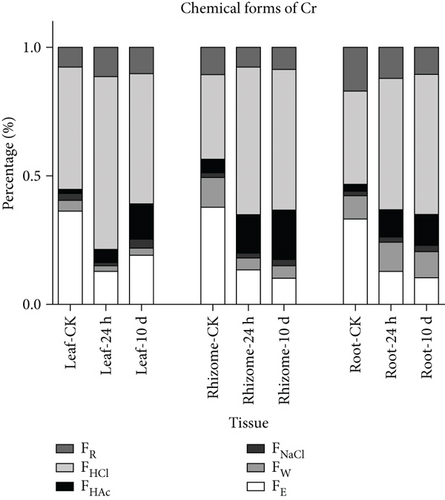
3.3. Effects of Cd and Cr on FAA Profiles
Since heavy metals can disrupt amino acid metabolism in plants, alterations in FAA levels play an important role in the mechanisms adopted by plants for coping with heavy metal stress [3, 52]. The effects of Cd and Cr stress on FAA levels in leaves, rhizomes, and roots are shown in Tables 2 and 3 and Supplementary Figures S1 and S2. The FAA levels varied among different plant tissues, with the highest FAA level in leaves and lowest in roots. Similarly, in the rice seedlings of Ageratum conyzoides L. and Crassocephalum crepidioides, the FAA levels in the aerial parts were higher than those in the roots [6, 13]. Notably, the exposure of Chinese goldthread to Cd and Cr had no obvious effect on the total FAA levels (Figure 2). Glu, serine (Ser), alanine (Ala), and aspartic acid (Asp) were the dominant FAAs in all control plant tissues and represented approximately 59% of the total FAA content in leaves, 53% in rhizomes, and 60% in roots. In control leaves, the most abundant FAA was Glu (22% of total FAAs), followed by Ser (13%), and Ala and Asp (12% each). In control group rhizomes, the predominant FAAs were Ser and Ala (15% each), followed by Asp (12%) and Glu (11%). In control group roots, the predominant FAAs were Glu and Asp (17% each), Ala (15%), and Ser (11%). In general, the abovementioned four amino acids were the predominant FAAs under Cd and Cr stress.
| FAAs | Leaf | Rhizome | Root | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CK | Cd24h | Cd10d | CK | Cd24h | Cd10d | CK | Cd24h | Cd10d | |
| Ile | 173.31 ± 6.90 | 95.69 ± 5.62∗∗ | 101.40 ± 6.68∗∗ | 154.00 ± 13.54 | 125.79 ± 8.06 | 148.66 ± 12.91 | 97.35 ± 7.64 | 79.10 ± 6.33 | 67.48 ± 4.08∗ |
| Leu | 224.90 ± 15.76 | 158.95 ± 13.39∗∗ | 87.67 ± 6.68∗∗ | 195.85 ± 17.83 | 148.24 ± 14.19∗ | 89.02 ± 5.63∗∗ | 86.07 ± 4.85 | 58.93 ± 5.93∗∗ | 33.07 ± 1.20∗∗ |
| Lys | 255.22 ± 11.60 | 207.95 ± 21.69 | 137.48 ± 11.84∗∗ | 208.63 ± 13.06 | 133.87 ± 11.63∗∗ | 99.05 ± 7.53∗∗ | 123.06 ± 9.40 | 95.08 ± 9.81 | 109.45 ± 9.55 |
| Phe | 171.47 ± 7.97 | 67.80 ± 4.90∗∗ | 133.18 ± 9.37∗ | 127.91 ± 12.71 | 68.41 ± 5.82∗∗ | 106.54 ± 9.71 | 44.07 ± 3.45 | 65.98 ± 5.57 | 94.44 ± 9.72∗∗ |
| Tyr | 65.84 ± 3.43 | 97.46 ± 9.15 | 146.08 ± 15.03∗∗ | 56.96 ± 5.65 | 89.85 ± 4.57∗ | 119.37 ± 12.4∗∗ | 31.62 ± 2.07 | 50.86 ± 2.77∗ | 95.22 ± 6.88∗∗ |
| Val | 269.91 ± 22.14 | 379.99 ± 15.84 | 421.08 ± 40.31∗ | 130.63 ± 13.39 | 178.16 ± 8.18∗ | 226.70 ± 12.29∗∗ | 93.39 ± 8.22 | 125.85 ± 5.13∗ | 168.89 ± 11.88∗∗ |
| Ala | 659.43 ± 27.07 | 300.82 ± 12.33∗∗ | 434.39 ± 24.18∗∗ | 545.29± 26.28 | 322.15 ± 16.15∗∗ | 341.23 ± 25.46∗∗ | 458.41 ± 19.37 | 359.19 ± 30.26∗ | 306.43 ± 15.49∗∗ |
| Arg | 289.09 ± 16.99 | 240.94 ± 24.35 | 145.24 ± 14.74∗∗ | 253.30 ± 23.81 | 323.19 ± 18.20 | 552.24 ± 29.61∗∗ | 191.16 ± 15.94 | 218.14 ± 15.01 | 283.58 ± 14.90∗∗ |
| Asp | 648.46 ± 36.18 | 428.25 ± 26.24∗∗ | 491.28 ± 36.01∗ | 457.63 ± 36.63 | 363.56 ± 17.14∗ | 325.30 ± 16.95∗ | 519.38 ± 21.15 | 438.64 ± 19.24∗ | 383.85 ± 26.16∗∗ |
| Glu | 1200.71 ±116.47 | 666.08 ± 20.38∗∗ | 865.91 ± 34.91∗ | 419.02 ± 21.76 | 299.06 ± 26.83∗∗ | 205.66 ± 13.77∗∗ | 513.70 ± 26.30 | 420.72 ± 26.00∗ | 379.26 ± 18.42∗∗ |
| His | 136.58 ± 7.07 | 185.21 ± 18.90∗ | 252.81 ± 12.43∗∗ | 98.93 ± 7.04 | 149.61 ± 10.63∗ | 198.61 ± 18.15∗∗ | 65.80 ± 5.12 | 97.67 ± 5.81∗ | 133.76 ± 11.10∗∗ |
| Pro | 353.86 ± 24.51 | 287.50 ± 15.69∗ | 222.02 ± 21.74∗∗ | 257.49 ± 17.56 | 175.45± 17.48∗∗ | 125.01 ± 7.03∗∗ | 267.00 ± 16.31 | 151.13 ± 13.90∗∗ | 119.75 ± 9.51∗∗ |
| Ser | 728.97 ± 51.58 | 903.13 ± 46.50 | 1205.92 ± 90.16∗∗ | 570.60 ± 48.13 | 721.70 ± 25.57∗ | 804.72 ± 45.11∗∗ | 346.89 ± 30.02 | 429.45 ± 31.12 | 585.69 ± 22.86∗∗ |
| Thr | 331.75 ± 14.94 | 412.09 ± 28.26 | 526.06 ± 42.36∗∗ | 258.15 ± 12.92 | 301.91 ± 27.93 | 484.39 ± 34.83∗∗ | 241.81 ± 23.89 | 345.57 ± 19.54∗∗ | 420.38 ± 12.71∗∗ |
- Asterisks denote significant differences ( ∗P < 0.05, ∗∗P < 0.01).
| FAAs | Leaf | Rhizome | Root | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CK | Cr24h | Cr10d | CK | Cr24h | Cr10d | CK | Cr24h | Cr10d | |
| Ile | 173.31 ± 6.90 | 157.81 ± 13.35 | 206.91 ± 18.29 | 154.00 ± 13.54 | 187.54 ± 13.18 | 125.71 ± 10.06 | 97.35 ± 7.64 | 100.86 ± 6.99 | 56.06 ± 3.17∗∗ |
| Leu | 224.90 ± 15.76 | 271.71 ± 17.84 | 282.46 ± 14.28∗ | 195.85 ± 17.83 | 195.24 ± 16.74 | 177.63 ± 13.40 | 86.07 ± 4.85 | 113.21 ± 9.01∗ | 48.76 ± 2.52∗∗ |
| Lys | 255.22 ± 11.60 | 300.16 ± 23.96 | 234.56 ± 20.75 | 208.63 ± 13.06 | 211.78 ± 18.78 | 213.69 ± 16.87 | 123.06 ± 9.40 | 146.66 ± 11.36 | 75.93 ± 3.77∗∗ |
| Phe | 171.47 ± 7.97 | 86.63 ± 3.83∗∗ | 133.48 ± 11.45∗ | 127.91 ± 12.71 | 233.67 ± 20.43∗∗ | 110.36 ± 7.91 | 44.07 ± 3.45 | 44.98 ± 2.70 | 67.80 ± 3.59∗∗ |
| Tyr | 65.84 ± 3.43 | 101.72 ± 9.08∗ | 115.35 ± 7.40∗∗ | 56.96 ± 5.65 | 80.59 ± 5.03∗ | 129.95 ± 8.29∗∗ | 31.62 ± 2.07 | 60.49 ± 3.77∗∗ | 92.74 ± 5.73∗∗ |
| Val | 269.91 ± 22.14 | 367.56 ± 18.56∗ | 437.81 ± 19.77∗∗ | 130.63 ± 13.39 | 329.92 ± 26.76 | 360.63 ± 30.42∗∗ | 93.39 ± 8.22 | 178.92 ± 13.83∗∗ | 230.66 ± 15.14∗∗ |
| Ala | 659.43 ± 27.07 | 562.03 ± 21.88∗ | 367.46 ± 27.59∗∗ | 545.29 ± 26.28 | 426.09 ± 31.57∗ | 359.48 ± 17.28∗∗ | 458.41 ± 19.37 | 372.70 ± 21.17∗ | 157.14 ± 12.54∗∗ |
| Arg | 289.09 ± 16.99 | 147.70 ± 12.99∗∗ | 155.68 ± 12.54∗∗ | 253.30 ± 23.81 | 298.74 ± 23.48 | 394.20 ± 29.34∗∗ | 191.16 ± 15.94 | 226.18 ± 18.57 | 183.70 ± 14.00 |
| Asp | 648.46 ± 36.18 | 527.03 ± 26.50∗ | 817.66 ± 31.55∗∗ | 457.63 ± 36.63 | 364.22 ± 15.36∗ | 611.19 ± 19.15∗∗ | 519.38 ± 21.15 | 225.09 ± 17.74∗∗ | 606.87 ± 19.80∗ |
| Glu | 1200.71 ± 116.47 | 878.11 ± 53.61∗ | 781.62 ± 25.45∗∗ | 419.02 ± 21.76 | 320.91 ± 8.81∗∗ | 297.01 ± 17.88∗∗ | 513.70 ± 26.30 | 401.88 ± 29.15∗ | 290.90 ± 21.67∗∗ |
| His | 136.58 ± 7.07 | 181.88 ± 10.53∗ | 212.53 ± 17.09∗∗ | 98.93 ± 7.04 | 152.06 ± 4.67∗ | 196.75 ± 15.60∗∗ | 65.80 ± 5.12 | 93.26 ± 4.66∗ | 153.78 ± 10.20∗∗ |
| Pro | 353.86 ± 24.51 | 686.96 ± 31.36∗∗ | 867.28 ± 54.48∗∗ | 257.49 ± 17.56 | 684.96 ± 32.30∗∗ | 757.49 ± 58.47∗∗ | 267.00 ± 16.31 | 175.80 ± 6.62∗∗ | 165.90 ± 9.95∗∗ |
| Ser | 728.97 ± 51.58 | 832.90 ± 27.52 | 989.75 ± 83.97∗ | 570.60 ± 48.13 | 721.22 ± 21.69∗ | 839.07 ± 42.98∗∗ | 346.89 ± 30.02 | 428.74 ± 26.04 | 452.42 ± 20.62∗ |
| Thr | 331.75 ± 14.94 | 389.95 ± 22.65 | 479.13 ± 27.87∗∗ | 258.15 ± 12.92 | 353.68 ± 22.91∗ | 491.52 ± 39.73∗∗ | 241.81 ± 23.89 | 327.74 ± 19.54∗ | 391.67 ± 18.58∗∗ |
- Asterisks denote significant differences ( ∗P < 0.05 and ∗∗P < 0.01).
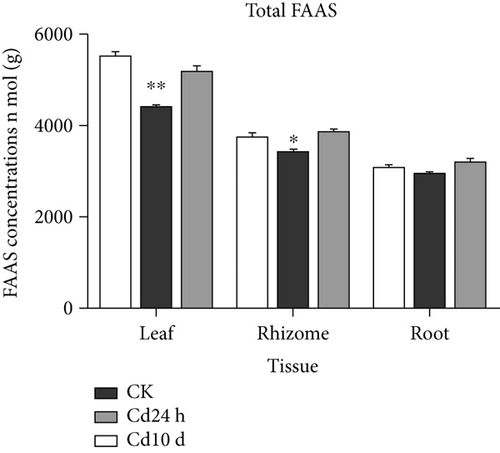
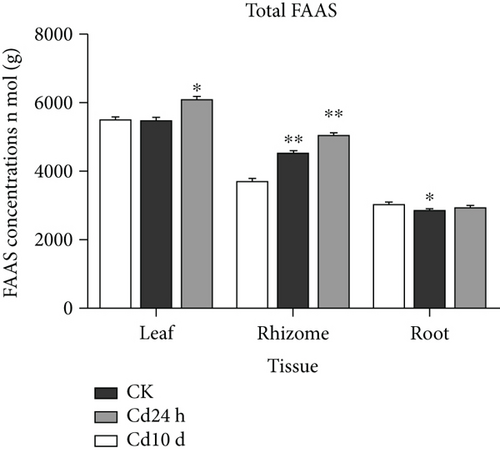
The addition of Cd and Cr altered FAA levels in Chinese goldthread (Tables 2 and 3). The results of the correlation analysis between heavy metal concentrations and FAA levels in leaves, rhizomes, and roots under Cd and Cr stress are shown in Table 4. The levels of valine (Val), His, threonine (Thr), tyrosine (Tyr), and Ser were significantly positively correlated with those of Cd and Cr in all plant tissues under heavy metal stress. Val is crucial for the balancing of the fluxes among different amino acids pathways. Increased accumulation of Val may promote stress-induced protein synthesis and maintain amino acid homeostasis [53]. Similarly, a high level of Val was reported to accumulate in tomato roots after Cd exposure [54]. His significantly contributes to the regulation of other amino acid biosynthesis and chelation and transportation of metal ions as well as plays a role in plant reproduction and growth [55]. His can act as a tridentate binding unit by means of a carboxyl group, amino group, and imidazole nitrogen, allowing it to form stable complexes with metal ions [56]. His has been reported to be significantly accumulated in all tissues of Maasai grass exposed to Cd and was implicated in the accumulation and detoxification of Cd [57]. Similarly, the accumulation of His in Chinese goldthread might be associated with detoxification of Cd and Cr. Thr is polar in structure and has sufficient electron donor functional groups to bind to heavy metals [22]. Yao et al. reported that ThrCd2+ has the lowest complex energy and the highest logβ value, indicating that it is more stable than other amino acid complexes with Cd [58]. An increase in the level of Thr under Cd stress was also observed in two Noccaea species plants [59].
| FAAs | Cd | Cr | ||||
|---|---|---|---|---|---|---|
| Leaf | Rhizome | Root | Leaf | Rhizome | Root | |
| Ile | −0.579 ∗ | 0.048 | −0.813 ∗∗ | 0.734 ∗∗ | −0.699 ∗ | −0.884 ∗∗ |
| Leu | −0.913 ∗∗ | −0.916 ∗∗ | −0.952 ∗∗ | 0.527 | −0.401 | −0.739 ∗∗ |
| Lys | −0.905 ∗∗ | −0.860 ∗∗ | −0.230 | −0.547 | 0.076 | −0.774 ∗∗ |
| Phe | −0.029 | −0.103 | 0.924 ∗∗ | 0.029 | 0.536 | 0.910 ∗∗ |
| Tyr | 0.910 ∗∗ | 0.895 ∗∗ | 0.978 ∗∗ | 0.682 ∗ | 0.934 ∗∗ | 0.957 ∗∗ |
| Val | 0.723 ∗∗ | 0.915 ∗∗ | 0.940 ∗∗ | 0.786 ∗∗ | 0.619 ∗ | 0.884 ∗∗ |
| Ala | −0.313 | −0.635 ∗ | −0.859 ∗∗ | −0.923 ∗∗ | −0.748 ∗∗ | −0.986 ∗∗ |
| Arg | −0.921 ∗∗ | 0.973 ∗∗ | 0.899 ∗∗ | −0.469 | 0.848 ∗∗ | −0.274 |
| Asp | −0.373 | −0.773 ∗∗ | −0.862 ∗∗ | 0.852 ∗∗ | 0.865 ∗∗ | 0.457 |
| Glu | −0.295 | −0.906 ∗∗ | −0.820 ∗∗ | −0.623 ∗ | −0.644 ∗ | −0.914 ∗∗ |
| His | 0.915 ∗∗ | 0.905 ∗∗ | 0.937 ∗∗ | 0.738 ∗∗ | 0.827 ∗∗ | 0.975 ∗∗ |
| Pro | −0.752 ∗∗ | −0.878 ∗∗ | −0.851 ∗∗ | 0.772 ∗∗ | 0.637 ∗ | −0.736 ∗∗ |
| Ser | 0.908 ∗∗ | 0.811 ∗∗ | 0.944 ∗∗ | 0.771 ∗∗ | 0.790 ∗∗ | 0.691 ∗ |
| Thr | 0.875 ∗∗ | 0.945 ∗∗ | 0.919 ∗∗ | 0.852 ∗∗ | 0.881 ∗∗ | 0.887 ∗∗ |
- ∗ and ∗∗ significantly different at p < 0.05 and 0.01, respectively.
In general, Glu and Ala levels were significantly negatively correlated with Cd and Cr accumulation in all plant tissues under Cd and Cr stress (Table 4). Glu is involved in nitrogen assimilation and transport within plants. Moreover, Glu mediates responses to environmental and abiotic stresses in higher plants [3, 12]. To alleviate Cd toxicity, plants synthesize a large number of defense elicitors by consuming Glu [60]. Glu is a primary amino donor for the synthesis of other amino acids. A sharp decline in Glu levels has been observed in rice plants under Cd stress [61, 62], and the level of Glu in rice grains was inversely correlated with Cd stress. This result might be attributable to the utilization of Glu to form Glu–Cd chelate, thereby reducing the levels of Glu and bioactivity and toxicity of Cd [12, 63]. Lader et al. described two mechanisms by which Glu chelates with heavy metal ions: One is a direct interaction between the metal and carboxyl group, and the other is electrostatic interactions caused by the carboxyl group [64]. In the present study, Glu may have been rapidly converted into the required products or Glu–metal chelate, leading to a decline in the level of Glu.
Pro is an indicator and protector during higher plant development under biotic and abiotic stresses, such as heavy metal exposure, drought, high salinity, and extreme temperatures [12]. The role of Pro in plants under heavy metal stress is multifaceted. It can form chelates with heavy metals, react with excessive oxygen free radicals to eliminate the harmful effects of ROS, and regulate osmosis and water balance in plant cells [65, 66]. Cd-induced accumulation of Pro was also observed in mung and wheat plants [67, 68]. However, no accumulation of Pro was noted in lettuce under Cd stress [69]. In the present study, variation in Pro levels under Cd stress differed from that under Cr stress. Under Cd stress, varying degrees of reduction were observed in Pro levels in all plant tissues. However, under Cr stress, Pro levels increased by 145.1% and 194.2% in leaves and rhizomes, respectively, compared with controls. These results imply that there are two distinct stress defense pathways in Chinese goldthread. The specific regulation mechanism of Pro under Cd and Cr stress in Chinese goldthread still needs to be studied further. Moreover, amino acid metabolism is known to be affected differently by different heavy metal treatments and vary with the plant species, genotype, and even different parts of the plant [13].
3.4. Isolation and Bioinformatic Analyses of CcMT2
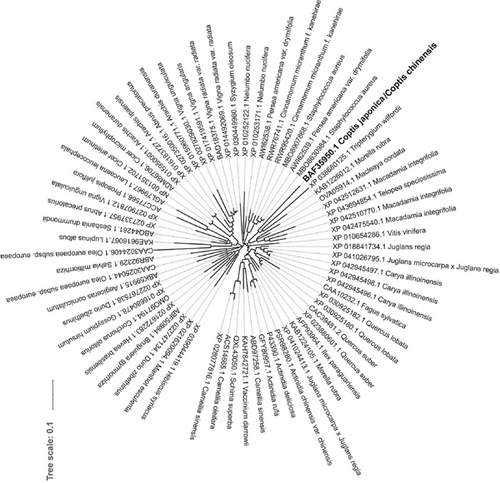
MTs play an important role in the regulation of plant growth and development and in plant responses to biotic and abiotic stresses, including heavy metal toxicity [1]. MTs are metal chelators for heavy metal homeostasis because they produce metal–thiolate complexes when combined with heavy metals in plant cells. This mechanism is closely associated with metal detoxification and stress resistance in plants [8]. To understand the function of MTs in Chinese goldthread, the metallothionein encoding gene CcMT2 was isolated. The full-length CcMT2 cDNA consisted of 402 nucleotides, including an ORF of 246 bp (stretched along 40–285 bp) that encoded a protein of 81 amino acids (Figure S3). The predicted CcMT2 weighed 8.17 kD with a theoretical pI of 4.65. Plant MTs can be classified into four subfamilies according to the protein alignment and phylogenetic tree analysis [1]. In the present study, the MT gene isolated from Chinese goldthread encoded a protein with two Cys-rich regions. The CcMT2 protein contains 14 Cys residues with one Cys-Cys, two Cys-X-Cys, and one Cys-X-X-Cys motifs at the N-terminal region and three Cys-X-Cys motifs at the C-terminal region. As this is typical of plant-type 2 MT genes, it was therefore named CcMT2. Analysis of the NCBI conserved domain database showed that CcMT2 contained the conserved domains of plant-type 2 MT genes and belonged to the MT2 superfamily (Figure S4). The phylogenetic trees showed that CcMT2 shared the same branch with the CjMT 2 (BAF35950.1) of C. japonica, TwMT2 (XP_038680125.1) of Tripterygium wilfordii, and MrMT2 (KAB1226012.1) of Morella rubra (Figure 3). Protein sequence alignment showed that CcMT2 has 100.0% identity with CjMT2, 64.4% with TwMT2, and 56.4% with MrMT2. Therefore, CcMT2 is speculated to have a similar function in Chinese goldthread. It has been suggested that the Cys residues of the alpha and beta domains and some residues of the spacer regions of CjMT 2 are important for Cd interaction [70] (Singh et al. 2019). Hence, the function of CcMT2 might be associated with accumulation of and tolerance to heavy metals, including Cd and Cr.
3.5. Expression of CcMT2 in Tissues and in Response to Heavy Metals
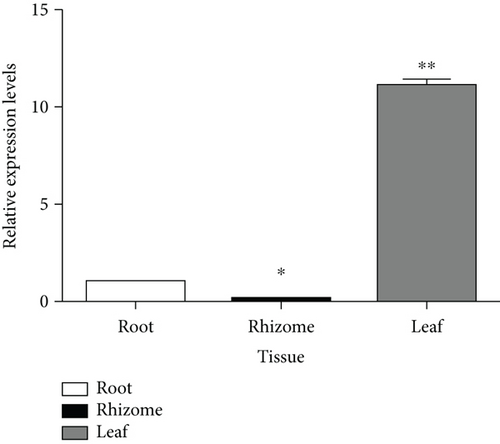
MT genes have distinct tissue-specific expression patterns in plants. In general, type-1 MT genes are expressed in roots, type-2 MT genes in leaves, type-3 MT genes in leaves and ripening fruit, and type-4 MT genes in developing seeds and reproductive organs [71]. To examine the tissue-specific expression pattern of CcMT2 in Chinese goldthread, qRT-PCR analysis was performed with total RNA extracted from leaves, rhizomes, and roots. The results revealed that CcMT2 was predominantly expressed in leaves and weakly expressed in rhizomes and roots, which were consistent with the general expression patterns of type-2 MT genes. The expression levels of CcMT2 in leaves showed an 11.2-fold increase compared with those in roots and a 77.2-fold increase compared with those in rhizomes (Figure 4). The tissue-specific expression pattern of CcMT2 was also consistent with that of type-2 MT genes in other plants, such as Nicotiana tabacum [1] and Jatropha curcas L. [72].
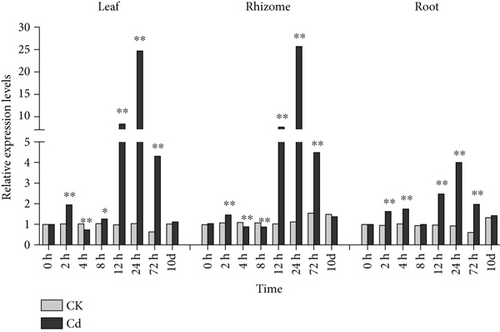
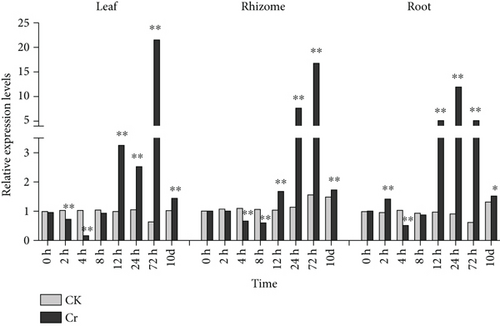
It has been reported that Cys residues in MTs can enhance plant tolerance to heavy metals [73] and play an important role in heavy metal detoxification [15]. When plants are exposed to heavy metals, MT gene expression is generally stimulated (Figures 5 and 6) [71]. The CcMT2 expression level in leaves significantly increased 8–72 h after Cd treatment and peaked at 24 h with a 2242.1% increase compared with controls. The expression level of CcMT2 under Cr stress decreased during the first 4 h, was substantially upregulated at 12–72 h and then peaked at 72 h with a 3211.4% increase compared with controls. The expression level of CcMT2 in rhizomes was suppressed within the first 8 h after heavy metal exposure but was considerably enhanced at 12–72 h. Expression levels peaked at 24 h under Cd stress and at 72 h under Cr stress and increased by 2173.2% and 978.9%, respectively, compared with controls. A similar pattern was observed in roots treated with Cd and Cr. The expression level of CcMT2 in roots was significantly induced by Cd and Cr stress at 12–72 h and peaked at 24 h, showing a 334.7% and 1189.0% increase, respectively, compared with controls. MT content and/or MT genes are potential biomarkers for heavy metal accumulation and defense in organisms [20]. As CcMT2 expression was upregulated in all plant tissues, these results suggest that CcMT2 is involved in the accumulation and detoxification of Cd and Cr in Chinese goldthread. Moreover, CcMT2 exhibited different response patterns to heavy metal stress in different plant tissues. This result is consistent with that observed in maize, indicating that plants have different regulatory mechanisms for different heavy metal stresses [23]. Sugarcane (Saccharum sinensis Roxb.) has been shown to upregulate the expression of MT genes that confer Cr tolerance [74], and this high expression of MT genes is consistent with the high heavy metal tolerance reported in Oryza sativa L. [8], Sedum alfredii Hance [75], and S. lycopersicum [71]. The particular mechanism through which CcMT2 influences Cd and Cr accumulation and detoxification warrants further research.
4. Conclusions
To address the issue of high Cd and Cr content in Chinese goldthread cultivation, the characteristics of Cd and Cr uptake, chemical forms of Cr, and variations in FAA levels and MT gene expression in Chinese goldthread following Cd and Cr treatment were analyzed. The results suggest that root retention of Cd and Cr might be an important tolerance strategy used by Chinese goldthread to protect photosynthetic tissues. Analysis of the chemical forms of Cr showed that Cr was primarily fixed by oxalate in all tissues; however, after Cr treatment, Cr tended to combine with phosphate to yield an insoluble complex. The low bioavailability and migration ability of the chemical forms of Cr help reduce Cr toxicity in Chinese goldthread. Variation in FAA levels under heavy metal stress revealed that some FAAs, such as Val, His, Glu, and Pro, might be involved in the accumulation of and resistance to Cd and Cr through various detoxification strategies. These strategies include balancing of the fluxes among different amino acids pathways, chelating with heavy metal ions through some specific groups, synthesizing a large number of defense elicitors, and reacting with excessive oxygen free radicals to eliminate the harm caused by ROS. The results also revealed that amino acid metabolism was affected differently by different heavy metal treatments and in different parts of the same plant. MT genes have been identified as biomarker candidates for heavy metal accumulation and tolerance in Chinese goldthread. In the present study, we isolated CcMT2 in Chinese goldthread for the first time and showed that it was predominantly expressed in leaves. CcMT2 was induced under Cd and Cr stress in all plant tissues, indicating involvement of the CcMT2 protein in the accumulation of and tolerance to Cd and Cr. The specific function of CcMT2 in Cd and Cr resistance requires further research. Taken together, our findings provide basic information regarding the accumulation patterns and detoxification mechanisms of Cd and Cr in Chinese goldthread, which could be used to reduce Cd and Cr content in this important medicinal plant in the future.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81903751), Natural Science Basic Research Project of Shaanxi Science and Technology Department (No. 2019JQ-877), Scientific research project of Shaanxi Administration of Traditional Chinese Medicine (No. 2019-ZZ-ZY018), Doctoral Research Fund project of Shaanxi University of Chinese Medicine, and the Innovative Team of Pharmacodynamic Substances and Applications of “Qinba Characteristic Qi-Medicines” in Taibai Mountains, Shaanxi University of Chinese Medicine. We express our gratitude to Shengli Wu (Xi’an Ande Pharmaceutical Co., Ltd) for assisting in sample collection.
Open Research
Data Availability
Additional supporting data to the article can be found in the supplementary material file.