Structural and Vibrational Investigation of Benzil-(1,2-Diphenylethane-1,2-Dione): Experimental and Theoretical Studies
Abstract
Single crystals of benzil commonly known as 1,2-diphenylethane-1,2-dione are grown by the slow evaporation method at room temperature. Gaussian 09 program is applied for theoretical calculations with B3LYP/6-311++G(d,p) basis set. The structure is optimized, and the energy, structural parameters, vibrational frequencies, IR, and Raman intensities are determined. Complete natural bonding orbital (NBO) analysis is carried out to analyze the intramolecular electronic interactions and their stabilization energies. From the second-order perturbation theory analysis of the benzil molecule, it is observed that there exists a hyperconjugative intramolecular stabilization energy between 17.45 and 22.76 KJmol-1. HOMO-LUMO analysis has been performed to identify the charges transferred within the molecule. The energy gap is calculated to be 2.919 eV and thus establishes the soft nature of the molecule. The molecular electrostatic potential (MEP) of the grown crystal was analyzed using the B3LYP method with 6-311++G(d,p) basis set. First-order hyperpolarizability calculations reveal the nonlinear optical microscopic behavior of the benzil molecule with nonzero values. The total value of the first-order hyperpolarizability (βtot) is of the order of 41.5246 × 10−31 esu, which is found to be 11.135 times that of urea. Hence, benzil can be referred to as a good material for nonlinear optical applications.
1. Introduction
Benzil-1,2-diphenylethane-1,2-dione(C6H5CO)2) is an organic compound consisting of two phenyl rings, and C-O groups are used as photoinitiators as well as free radicals in polymer chemistry. Benzil has potential applications in biological metabolism and clinical medicine. Benzil derivates exhibit radical scavenging and antibacterial and hypertensive [1], antiprotozoal [2], antiproliferative, and antimitotic [3] activities. Benzil derivatives have versatile applications in the pharmaceutical industry, and various heterocyclic compounds such as triazine, quinoxaline, and imidazole can be synthesized from 1,2-diketones [4–8]. It is also used as a nanocatalyst in the application of imidazole derivatives [9]. Recently, it is reported that benzil derivatives acted as photosensitive agents and photoinitiators due to its antitumor activity [10, 11]. The effective chemoselective synthesis of benzil derivatives was reported by Jadhav et al. [12]. The insect antifeedant activity and growth inhibitory studies on a few benzyl derivatives were reported by Sreelatha et al. [13]. There are few reports available for the benzil-type phosphorescent molecules earlier [14–16]. Wadkins et al. reported the new benzyl (diphenylethane-1,2-dione) analogs as inhibitors of mammalian carboxylesterases [17]. The conductivity property of benzyl derivatives for electronic applications was reported by Stanculescu et al. [18]. Synthesis and antitumor activity of benzil-related natural Z-stilbene was reported by Mousset et al. [19]. Topal et al. reported the computational investigation of benzoin derivatives from benzil compounds [20]. Benzil derivatives are used in a wide range of organic synthesis, especially in the construction of heterocyclic compounds. The structure of benzil was reported earlier [21–23]. Saranraj et al. have grown benzil crystal by unidirectional SR method and reported its structural, optical, electrical as well as dielectric properties [24]. Shankar and Varma have grown this crystal by the Bridgman–Stockbarger method and studied the dielectric dispersion as well as the piezoelectric response of their grown crystal [25]. The benzyl compound and its various derivatives are reported by Chigare et al. [26]. The quantum chemical computational as well as experimental studies of vibrational spectra and structure on benzil and its 18O- and d10-labeled derivatives were reported by Kolev and Stamboliyska [27]. Lopes et al. investigated benzyl for equilibrium conformers using low-temperature matrix-isolation FT-IR spectroscopy with the support of extensive DFT calculations [28]. True computational prediction of material properties before experimentation is needed for finding the new materials for its application. Theoretical computational studies enable us to go well beyond what is known experimentally and can guide future experimentation. In continuation of research on benzil derivatives by the researchers, in this report, we synthesized that benzil (1,2-diphenylethane-1,2-dione), the structure, and vibrational spectrum are reported theoretically by density functional theory. In addition to the above studies, other properties like NBO, NLO, and HOMO-LUMO have also been carried out and explained in this communication.
2. Materials and Methods
The benzil-(1,2-diphenylethane-1,2-dione) compound under investigation was purchased from a Sigma-Aldrich Chemical Company, USA, with a stated purity of 99% and used without further purification. Water is used as the solvent, and the dissolved solution is kept at room temperature for evaporation to get transparent crystals of benzil. The obtained benzil crystals are then subjected to single-crystal XRD. In the present investigation, DFT calculations were performed with a hybrid functional B3LYP at 6-311++G(d,p) basis set using Gaussian 09 program [29]. The structure is optimized with minimum energy. The harmonic frequencies, infrared, and Raman intensities were calculated by the B3LYP method with an identical basis set. All the computed frequencies are scaled by 0.9665. The absence of imaginary values of wavenumbers on the calculated vibrational spectra confirms that the deduced structure corresponds to the minimum energy. The NBO and HOMO-LUMO orbital energies were investigated, and the visual interpretation of the above-mentioned properties was done by using Gauss View 3.1 program [30, 31].
3. Results and Discussion
3.1. Structural Analysis
3.1.1. Data Collection
A good crystal of dimension 0.20 × 0.20 × 0.20 mm was selected for the data collection. Intensity data were measured on Bruker SMART APEX CCD detector diffractometer [32] with graphite monochromatic MoKα radiation at room temperature. The data collection was covered over a hemisphere of reciprocal space by a combination of three sets of exposures each having a different φ angle for the crystal and exposure of the 30 s covered at 0.3° in ω. The crystal to detector distance was 4 cm, and the detector swing angle was 24°. Coverage of the unique set was 100% complete. Crystal decay was monitored by repeating 30 initial forms at the end of the data collection. The duplicate reflection was found to be negligible. The intensity data were reduced, and Lorentz and polarization corrections were applied.
3.1.2. Structure Solution and Refinement
The structure was solved by direct methods procedures using SHELXS97 [33] program. A total of 4072 reflections (E > 1.2) was available for the phase set with the lowest combined figure of merit (CFOM = 0.0845) revealing that all the 8 nonhydrogen atoms have an initial R-factor of 0.126. The trial structure was first refined isotropically. All H atoms were calculated in the difference Fourier maps. The hydrogen atoms were geometrically fixed and ride on their parent atoms. The final R-value is 0.0443. The structure was refined by full-matrix least-squares on F2.
3.1.3. Crystal Structure Analysis
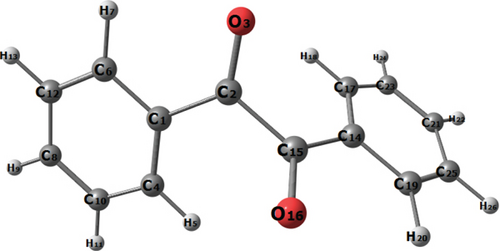
The crystal data and other relevant details are given in Table S1. The atomic coordinates of the nonhydrogen atoms with their equivalent thermal displacement parameters are presented in Table S2. The anisotropic displacement parameters are listed in Table S3. The atomic coordinates and their isotropic displacement parameters involving hydrogen atoms are given in Table S4. The hydrogen bond interactions are presented in Table S5. Figure S1 and Figure S2 show the ORTEP plot and packing diagram of the chosen molecule. Single crystal X-ray diffraction analysis reveals that benzil crystallizes in a trigonal system with noncentrosymmetric space group P3121 and the calculated lattice parameters are a = 8.4095(2)Å, b = 8.4095(2)Å, c = 13.6695(4)Å, α = 90°, β = 90°, γ = 120°, and V = 837.19 (Å3). X-ray analysis confirms the molecular structure and atom connectivity for the title compound (Figure 1). The obtained values are listed in Table 1 and are very well matched with the reported structures as well as the literature values [21, 22]. The one-half molecule in the asymmetric unit is located on a twofold rotation axis. The phenyl ring adopts a planer conformation. The crystal packing of the benzil compound is through weak C1-H1···O1 intermolecular interactions and also the crystal packing of the benzil compound C3-H3···O1 hydrogen bonds link molecules to form inversion dimers, with an ring motif. The optimized structure with a global minimum is given in Figure 1. All C-H bond lengths are found to be 0.93Å, and theoretically, this value is found to be 1.084Å. All C-C bond lengths within the phenyl ring are found to be within the range 1.364-1.380Å. However, carbon in the phenyl ring attached to the C-O group has 1.474Å, and the C-C bond that connects the two C-O groups attached to phenyl ring has the highest bond distance of 1.523Å. Theoretically, this value is computed to be 1.543Å. Two C-O bond distances have a bond length of 1.214Å. The small variation between experimental and theoretical values is due to the theoretical calculations are done in the gas phase.
| Atom | Experimental | B3LYP/6-311++G(d,p) | Atom | Expt | B3LYP/6-311++G(d,p) |
|---|---|---|---|---|---|
| Bond distance (Å) | Bond distance (Å) | ||||
| C(1)-C(2) | 1.379 | 1.388 | C(5)-C(4) | 1.368 (5) | 1.392 |
| C(1)-H(1) | 0.930 | 1.084 | C(5)-H(5) | 0.93 | 1.084 |
| C(2)-H(2) | 0.930 | 1.084 | C(6)-C(1) | 1.380 (4) | 1.403 |
| C(3)-C(2) | 1.370 | 1.397 | C(6)-C(5) | 1.388 (4) | 1.402 |
| C(3)-H(3) | 0.930 | 1.084 | C(6)-C(7) | 1.473 (4) | 1.487 |
| C(4)-H(4) | 0.930 | 1.084 | C(7)-O(1) | 1.213 (3) | 1.214 |
| C(3)-C(4) | 1.363 | 1.393 | C(7)-C(7) ∗ | 1.523 (6) | 1.543 |
| Bond angle (°) | |||||
| C(1)-C(6)-C(5) | 119.7 | 119.49 | C(2)-C(1)-C(6) | 119.7 (3) | 120.26 |
| C(1)-C(6)-C(7) | 120.9 | 118.12 | C(2)-C(1)-H(1) | 120.1 | 119.94 |
| C(5)-C(6)-C(7) | 119.4 | 122.36 | C(6)-C(1)-H(1) | 120.1 | 120.26 |
| O(1)-C(7)-C(6) | 123.4 | 123.46 | C(4)-C(5)-C(6) | 120.1 (3) | 120.08 |
| O(1)-C(7)-C(7) ∗ | 117.0 | 117.47 | C(4)-C(5)-H(5) | 119.9 | 119.81 |
| C(6)-C(7)-C(7) ∗ | 119.4 | 119.29 | |||
| Torsion angle (°) | |||||
| C(5)-C(6)-C(7)-O(1) | -3.6 (4) | -4.371 | C(7)-C(6)-C(5)-C(4) | 178.7 (3) | 177.53 |
| C(1)-C(6)-C(7)-C(7)∗ | -8.8 (3) | -4.371 | C(4)-C(3)-C(2)-C(1) | 0.4 (6) | 0.0647 |
| C(5)-C(6)-C(7)-C(7)∗ | 172.4 (2) | 177.537 | C(6)-C(1)-C(2)-C(3) | -0.6 (6) | -0.1437 |
| C(5)-C(6)-C(1)-C(2) | 0.5 (5) | 0.115 | C(2)-C(3)-C(4)-C(5) | 0.0 (6) | 0.0422 |
| C(7)-C(6)-C(1)-C(2) | -178.3 (3) | 178.262 | C(6)-C(5)-C(4)-C(3) | -0.1 (5) | -0.0697 |
| C(1)-C(6)-C(5)-C(4) | -0.2 (4) | -0.008 |
- Symmetry transformations used to generate equivalent atoms: ∗y, x, −z.
(1) Data Collection. SMART [34]; cell refinement: SAINT [34]; and data reduction: SAINT program(s) are used to solve structure: SHELXS97 [33] program(s) are used to refine structure: SHELXL97 [33]; molecular graphics: PLATON [35]; and software are used to prepare material for publication: SHELXL97 and PARST [36].
3.2. Vibrational Analysis
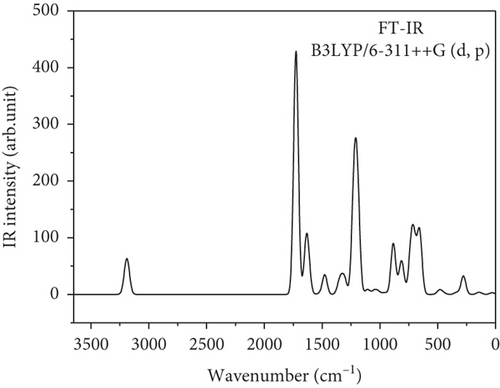
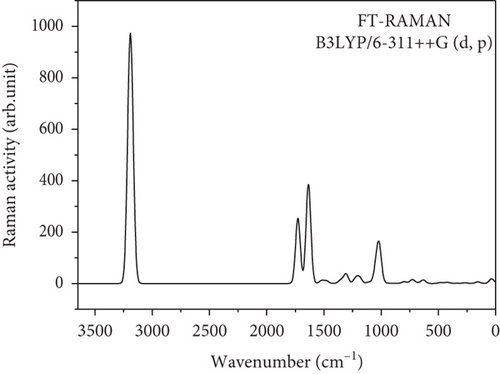
Computational chemistry is used to calculate the structure and properties of the molecule. It seemed to be intuitive to give the theoretical vibrational computation of the benzil molecule to understand the various functional groups present in the system. Normal coordinate vibrational analysis of benzil was reported by Ivanov et al. [37]. Kayadibi et al. reported the theoretical investigation of a few benzyl compounds [38]. There will be 72 modes of vibration (3N-6) which include C-C, C-O, and C-H vibrations in the benzil molecule. There are 25 stretching modes of vibration, 24 bending modes of vibration, and 23 torsion modes of vibration that occur in this benzil molecule. Theoretically obtained FT-IR and FT-Raman vibrational spectrum is given in Figures 2 and 3, respectively. Table 2 list the wavenumbers (cm-1) and relative intensities of calculated Fourier infrared and Raman spectra of the benzil molecule. C-H stretching modes of vibrational frequencies are usually found in the region 3300–2850 cm-1 [39–40]. There are 10 such asymmetric and symmetric frequencies computed between 3009 and 3047 cm-1. The highly intense characteristics vibrations of carboxyl groups occurred in the region 1870-1540 cm–1. There are two such peaks computed at 1641 and 1640 cm-1 by the DFT method. The most informative out plane bending vibration of C-H, i.e., H-C-C-C torsion is computed in the region 818–963 cm-1. Usually, C-C phenyl group ring vibrational modes occurred at 642, 996, 1009, and 1048 cm-1 is due to the resonance enhancement of the phenyl ring. Those peaks are computed at 662, 995, 1012, and 1052, respectively. C-C stretching and C-C-H vibrational frequencies are computed in the region 1500-800 cm-1. Usually, C-H, N-H, and O-H vibrations are observed at higher frequencies than C-C and C-O bond vibrations. Bending vibrations of C-C-C are computed in the range 1000–900 cm-1. At 726 and 624 cm-1, C-C-O bending mode of vibration is computed. Below 695 cm-1, torsion modes of C-C-C-C are computed. The possibility of DFT interpretation of the molecule may broaden the application span of vibrational spectroscopy [39–41].
| Unscaled frequency (cm-1) | Scaled frequency (cm-1) | IR intensity (arb.unit) | Raman intensity (arb.unit) | Assignment description |
|---|---|---|---|---|
| 3207 | 3047 | 2.006 | 206.277 | 4C-5H asymmetric stretching |
| 3207 | 3047 | 3.768 | 16.0875 | 17C-18H asymmetric stretching |
| 3199 | 3039 | 0.247 | 244.675 | 6C-7H asymmetric stretching |
| 3199 | 3039 | 18.27 | 33.9746 | 19C-20H asymmetric stretching |
| 3188 | 3029 | 4.248 | 235.243 | 8C-9H; 10C-11H asymmetric stretching |
| 3188 | 3029 | 22.02 | 61.5706 | 21C-22H; 23C-24H asymmetric stretching |
| 3179 | 3020 | 12.32 | 90.0344 | 23C-24H; 25C-26H symmetric stretching |
| 3179 | 3020 | 6.23 | 156.361 | 23C-24H; 25C-26H symmetric stretching |
| 3167 | 3009 | 0.253 | 78.6184 | 23C-24H; 25C-26H symmetric stretching |
| 3167 | 3009 | 0.343 | 28.3318 | 23C-24H; 25C-26H symmetric stretching |
| 1727 | 1641 | 132 | 208.069 | 3O-2C stretching |
| 1726 | 1640 | 297.3 | 45.2167 | 16O-15C stretching |
| 1637 | 1555 | 68.55 | 81.8839 | 6C-12C stretching |
| 1636 | 1554 | 21.46 | 291.67 | 19C-25C stretching |
| 1617 | 1536 | 5.562 | 12.5458 | 8C-10C stretching |
| 1617 | 1536 | 20.61 | 5.586 | 21C-23C stretching |
| 1521 | 1445 | 0.817 | 1.3596 | 22H-21C-25C bending |
| 1521 | 1444 | 0.726 | 9.7679 | 22H-21C-25C bending |
| 1479 | 1405 | 0.456 | 10.0279 | 9H-8C-10C bending |
| 1478 | 1404 | 34.11 | 0.3019 | 9H-8C-10C bending |
| 1357 | 1289 | 8.508 | 0.2942 | 7H-6C-12C bending |
| 1357 | 1289 | 0.01 | 10.1592 | 5H-4C-10C bending |
| 1342 | 1275 | 11.54 | 3.3852 | 1C-4C; 17C-14C stretching |
| 1337 | 1270 | 9.918 | 0.2745 | 1C-4C; 17C-14C stretching |
| 1307 | 1242 | 26.91 | 36.8482 | 2C-1C stretching |
| 1224 | 1163 | 175.6 | 16.7525 | 14C-15C stretching |
| 1206 | 1145 | 1.419 | 4.8215 | 2C-1C; 14C-15C stretching |
| 1197 | 1137 | 157.2 | 6.2535 | 11H-10C-8C bending |
| 1185 | 1126 | 0.472 | 9.4713 | 26H-25C-21C bending |
| 1184 | 1125 | 22.52 | 5.5839 | 13H-12C-8C bending |
| 1108 | 1052 | 0.216 | 5.1251 | 23C-17C stretching |
| 1107 | 1052 | 8.46 | 0.0626 | 23C-17C stretching |
| 1065 | 1012 | 0.744 | 5.4911 | 15C-2C stretching; 12C-8C-10C; 25C-21C-23C bending |
| 1048 | 995 | 5.853 | 2.0747 | 12C-8C stretching |
| 1040 | 988 | 0.882 | 62.6533 | 12C-8C stretching; 1C-4C-10C bending |
| 1016 | 966 | 4.14 | 6.6288 | 19C-25C-21C bending |
| 1016 | 965 | 0.154 | 119.219 | 6C-12C-8C bending |
| 1013 | 963 | 0.02 | 0.1948 | 13H-12C-8C-10C torsion |
| 1013 | 963 | 0.003 | 0.3665 | 13H-12C-8C-10C torsion |
| 998.6 | 949 | 0.263 | 0.0184 | 7H-6C-12C-8C torsion |
| 998.6 | 949 | 0.359 | 0.3017 | 9H-8C-12C-6C torsion |
| 960 | 912 | 1.636 | 0.0426 | 5H-4C-10C-8C torsion |
| 955.4 | 908 | 1.393 | 1.5291 | 11H-10C-8C-12C torsion |
| 884.6 | 840 | 89.38 | 0.3707 | 9H-8C-12C-6C torsion |
| 860.9 | 818 | 0.383 | 0.2135 | 5H-4C-10C-8C torsion; 7H-6C-12C-8C torsion; |
| 860.8 | 818 | 0.381 | 0.0109 | 13H-12C-8C-10C torsion; 24H-23C-25C-21C torsion; |
| 817.2 | 776 | 47.64 | 0.1776 | 3O-1C-15C-2C torsion |
| 799.2 | 759 | 15.58 | 6.9508 | 11H-10C-8C-12C torsion |
| 731.8 | 695 | 37.37 | 0.5538 | 6C-12C-8C-10C torsion |
| 729.9 | 693 | 40.62 | 13.1122 | 6C-12C-8C-10C torsion |
| 705.1 | 670 | 47.32 | 2.5976 | 12C-8C-10C; 25C-21C-23C bending |
| 697.6 | 663 | 20.14 | 0.4057 | 6C-12C-8C-10C torsion |
| 697.3 | 662 | 10.99 | 0.1175 | 6C-12C-8C-10C torsion |
| 657.1 | 624 | 108.4 | 1.966 | 3O-2C-15C bending |
| 630.3 | 599 | 0.703 | 6.3203 | 8C-10C-4C; 23C-17C-14C bending |
| 630.3 | 599 | 0.679 | 6.4854 | 8C-10C-4C; 23C-17C-14C bending |
| 486.8 | 463 | 6.798 | 2.8116 | 2C-4C-6C-1C torsion |
| 460 | 437 | 3.223 | 1.4307 | 25C-21C-23C-17C torsion |
| 433.7 | 412 | 0.506 | 0.5274 | 25C-21C-23C-17C torsion |
| 421.9 | 401 | 1E-04 | 2.8041 | 25C-21C-23C-17C torsion |
| 409.2 | 389 | 0.016 | 0.0106 | 21C-23C-17C-14C torsion |
| 407.3 | 387 | 0.001 | 1.1796 | 12C-8C-10C-4C torsion |
| 336 | 319 | 3.643 | 0.8309 | 25C-21C-23C-17C torsion |
| 278.1 | 264 | 32.4 | 0.1769 | 17C-14C-15C; 2C-1C-6C bending |
| 265.6 | 252 | 0.176 | 2.3752 | 17C-14C-15C; 2C-1C-6C bending |
| 158.9 | 151 | 0.237 | 3.1713 | 14C-15C-2C; 15C-2C-1C bending |
| 156 | 148 | 0.006 | 2.9025 | 2C-4C-6C-1C torsion |
| 147.7 | 140 | 2.412 | 0.2295 | 2C-4C-6C-1C torsion |
| 129.8 | 123 | 1.541 | 0.4346 | 14C-15C-2C; 15C-2C-1C bending |
| 40.32 | 38.3 | 0.685 | 6.2676 | 15C-2C-1C-4C torsion |
| 38 | 36.1 | 0.041 | 5.7474 | 15C-2C-1C-4C torsion |
| 25.68 | 24.4 | 2.34 | 6.8719 | 14C-15C-2C-1C torsion |
3.3. Natural Bond Orbital Analysis (NBO)
The energy differences between the donor and acceptor are given as Ei and Ej, respectively. Whereas Fij is the Fock matrix element between i and j orbitals of NBO. Figure 4 shows the natural bonding atomic charges of benzil computed at B3LYP/6-311++G(d,p). The carbon atoms in the phenyl ring have negative charges whereas the carbon atoms attached to oxygen atoms have a positive charge and are found to be a higher value than in the phenyl ring. All the hydrogen atoms have a positive charge of around 0.2e. Several significant interactions are provided in Table 3 which signifies the second-order perturbation theory analysis of the benzil molecule. In this molecule, mainly LP⟶π∗, π⟶π∗ occurs between bonding and antibonding orbitals. NBO analysis shows that hyperconjugative interaction energy has a higher value during π⟶π∗, LP⟶π∗ transitions than σ⟶σ∗ and σ⟶π∗. The π⟶π∗ interactions between bonding and antibonding orbitals are π (C1 − C4)⟶π∗(C6 − C12); π (C6 − C12)⟶π∗(C8 − C10) π(C8 − C10)⟶π∗(C1 − C4); π(C14 − C17)⟶π∗(C19 − C25) 20.05,21.73, 22.76, and 20.05 kJmol-1, respectively. The strongest interaction LP⟶π∗ involves lone pairs, and nearly vacant antibonding orbitals such as LP(2)O3⟶π∗(C1 − C2) and π∗(C2 − C15) and LP(2)O16⟶π∗(C2 − C15) and π∗(C14 − C15) have high E(2) values of 17.51 and 21.84 kJmol-1, respectively. These interactions contribute the greatest role to stabilizing the molecule. Thus, it is observed that there exists a hyperconjugative intramolecular stabilization energy between 17.45 and 22.76 KJmol-1. Similar values are seen due to the mirror reflection of the molecule.
| Donor (i) | Acceptor (j) | E(2) (Kj.Mol-1) | E(j)-E(i) (a.u) | F(i.j) (a.u) |
|---|---|---|---|---|
| BD(2)C1-C4 | BD∗(2)C2-O3 | 19.77 | 0.28 | 0.070 |
| BD(2)C1-C4 | BD∗(2)C6-C12 | 20.05 | 0.29 | 0.069 |
| BD(2)C1-C4 | BD∗(2)C8-C10 | 17.93 | 0.28 | 0.64 |
| BD(2)C6-C12 | BD∗(2)C1-C4 | 18.67 | 0.28 | 0.065 |
| BD(2)C6-C12 | BD∗(2)C8-C10 | 21.73 | 0.28 | 0.070 |
| BD(2)C8-C10 | BD∗(2)C1-C4 | 22.76 | 0.28 | 0.072 |
| BD(2)C8-C10 | BD∗(2)C6-C12 | 17.45 | 0.29 | 0.064 |
| BD(2)C14-C17 | BD∗(2)C15-O16 | 19.77 | 0.28 | 0.070 |
| BD(2)C14-C17 | BD∗(2)C19-C25 | 20.05 | 0.29 | 0.069 |
| BD(2)C14-C17 | BD∗(2)C21-C23 | 17.93 | 0.28 | 0.065 |
| BD(2)C19-C25 | BD∗(2)C14-C17 | 18.67 | 0.28 | 0.065 |
| BD(2)C19-C25 | BD∗(2)C21-C23 | 21.73 | 0.28 | 0.070 |
| BD(2)C21-C23 | BD∗(2)C14-C17 | 22.76 | 0.28 | 0.072 |
| BD(2)C21-C23 | BD∗(2)C19-C25 | 17.45 | 0.29 | 0.064 |
| LP(2)O3 | BD∗(2)C1-C2 | 17.51 | 0.71 | 0.101 |
| LP(2)O3 | BD∗(2)C2-C15 | 21.84 | 0.62 | 0.105 |
| LP(2)O16 | BD∗(2)C2-C15 | 21.84 | 0.62 | 0.105 |
| LP(2)O16 | BD∗(2)C14-C15 | 17.51 | 0.71 | 1.101 |
3.4. Frontier Molecular Orbital Analysis
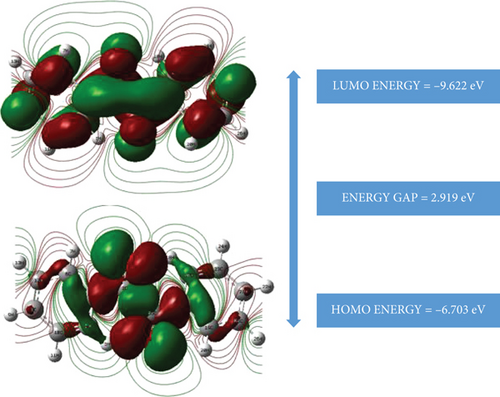
HOMO-LUMO is known as molecular orbitals which are collectively called frontier molecular orbitals. It helps to determine the energy band gap to know the electrical properties of the title compound. Some of the parameters like chemical potential, softness, electronegativity, and electrophilicity can be measured from these studies which in turn give an insight into the chemical reactivity of the compounds. At the time of molecular interactions in HOMO, the molecules donate electrons; thereby, their energies are corresponding to the ionization potential, on another hand, where the LOMO accepts electrons, and it corresponds to the electron affinity. The HOMO-LUMO gap helps to know the stability of the molecule. The value shows that the compound has high kinetic activity when compared to the one with a low value. Figure 5 shows the orbital composition of benzil. The energy value of HOMO is found to be -6.703 eV, and the energy value of LUMO is found to be -9.622 eV. The energy gap is calculated to be 2.919 eV. It shows that the title compound is a soft molecule. HOMO is localized mainly on the carboxyl groups attached to the phenyl ring, whereas LUMO is entirely occupied on the benzil molecule. The chemical descriptors such as hardness (η), chemical potential (μ), softness (S), electronegativity (χ), and electrophilicity index (ω) of benzil is calculated using the energy values of HOMO and LUMO. Based on Koopman’s theorem, the above parameters have been calculated and listed in Table 4 [44–46]. Hardness is the measure of the resistance of the system to change the electron cloud, while softness is the reciprocal of hardness, which is the measure of the ability to undergo electron density distribution. Electronegativity is the inverse of chemical potential. The minimum value of this electronegativity (0.122 eV) indicates the less tendency to gain electrons, and the high value of chemical potential implies the high reactive nature of benzil. Electrophilicity is a measure of the energy stabilization of a molecule when it acquires an additional amount of electron density from the environment. The high value of electrophilicity indicates the strong electrophilic nature of the molecule.
| EHOMO (eV) | -6.703 |
| ELUMO (eV) | -9.622 |
| Energy gap ΔE (eV) | 2.919 |
| Ionization potential (A) eV | +6.703 |
| Electron affinity (I) | +9.622 |
| Global softness (S) | 0.343 |
| Global hardness (η) | 1.459 |
| Chemical potential (μ) | 8.163 |
| Global electrophilicity (ω) | 22.835 |
3.5. First-Order Hyperpolarizability Calculations
Electronic nonlinearities in the most efficient organic materials are based on molecular units with highly delocalized electrons and extra electron donor and acceptor groups on opposite sides of the molecules. For many years, it has been known that certain organic materials have exceptionally large NLO and electrooptic effects. In contrast to inorganic materials, where lattice vibrations occurring in the frequency range of 1 MHz to 100 MHz play a prominent role, the NLO effect of molecular crystals is mostly determined by the polarizability of the electrons in the bonding orbitals. Organic materials are therefore well suited for high-speed applications, such as NLO with ultrashort pulses or high-data-rate electro-optic modulation. Optical frequency doubling, optical sum, difference-frequency generation, optical parametric amplification, linear electrooptic effects (Pockel’s effect), and photorefractive effects all require noncentral symmetry. The first derivative of polarizability (β) measures how the dipole is induced in a molecule in the presence of the electric field. The present crystal belongs to the noncentrosymmetric space group; hence, it is interesting to study the NLO properties. By utilizing the B3YLP/6-311++G(d,p) basis set in Gaussian 09 program, the total molecular dipole moment (m), linear polarizability (α), and first-order hyperpolarizability (b) are calculated in atomic units and are converted into electrostatic units. (α:1a.u = 0.1482 × 10−24 esu, β: 1a.u = 8.6393 × 10−30 esu). Table 5 lists the calculated parameters of an electric dipole moment μ (D), the average polarizability αtot (×10-24 esu), and first-order hyperpolarizability βtot (×10-31 esu). The dipole moment in the X and Y direction is computed to be zero, and in the Z direction, it is found to be -3.4881. The calculated total dipole moment is 3.4881 Debye. The biggest value of hyperpolarizability is observed in the βzzz direction, i.e., electron cloud is more in this particular direction. The total value of the first-order hyperpolarizability (βtot) is of the order of 41.5246 × 10−31 esu. Urea is a standard material to study of NLO properties of the compound holding the threshold value of α and β of 3.8312 × 10−24 esu and 0.37289 × 10−30 esu, respectively. The above computations reveal that the hyperpolarizability of benzil is 11.135 times that of urea and is expected to be a suitable candidate in the development of NLO materials [47–48]. Yadav et al. grew the benzil crystal by Czochralski method which has suitable application in piezoelectric, sensor, and telecommunication fields. Benzil single-crystal exhibits noncentro symmetric structure and has both nonlinear optical and piezoelectric properties. It has a ferroelectric transition at 83.5 K. These properties make its applicability in design of the interface between optical and electrical communication network [49].
| Parameters | B3LYP/6-311G(d,p) | Parameters | B3LYP/6-311G(d,p) |
|---|---|---|---|
| μx | 0 | βxxx | 0.0001 |
| μy | 0 | βxxy | -0.0003 |
| μz | -3.4881 | βxyy | -0.0001 |
| μ (D) | 3.4881 | βyyy | -0.0005 |
| αxx | -69.629 | βzxx | -31.1348 |
| αxy | -0.795 | βxyz | -17.2186 |
| αyy | -92.195 | βzyy | -15.0123 |
| αxz | 0.000 | βxzz | 0.0003 |
| αyz | -0.0001 | βyzz | 0.0002 |
| αzz | -98.7936 | βzzz | 6.8288 |
| αtot (e.s.u) | −86.873 × 10−24 | βtot (e.s.u) | 41.5246 × 10−31 |
3.6. Molecular Electrostatic Potential Studies
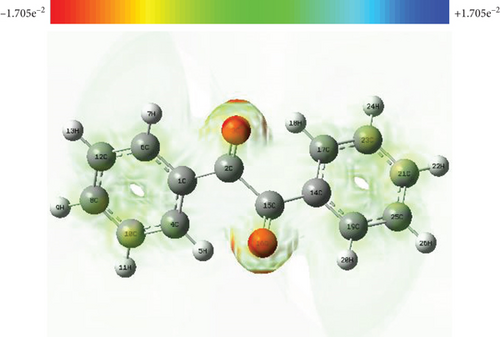
The molecular electrostatic potential (MESP) map is a three-dimensional representation of the charge distribution in molecules that allows the user to see the charge points in the molecule as well as the shape of the potential surface, which aids in the prediction of a variety of chemical properties [50]. With the use of a colour spectrum in the order red > orange > yellow > green > blue, GaussView converts the calculated electrostatic energy into an electron density model derived from the Schrodinger equation. e.s.u. ranges from −1.705 × 10−2 to +1.705 × 10−2 electrostatic potential. The colour red denotes an electrophilically active region, while the colour blue denotes a nucleophilically active region. The oxygen atoms at the carbonyls are deep red in colour in the present molecule (Figure 6), indicating that this is the most electrophilic area, where nucleophiles attack after the phenyl rings. The hydrogen atoms have deep blue patches around them, indicating that they are the most nucleophilic region in the molecule susceptible to electrophile attack [51, 52].
4. Conclusion
Single crystals of benzil-1,2-diphenylethane-1,2-dione were grown by slow evaporation method at room temperature and have been taken as the subject for experimental and theoretical investigation. Gaussian 09 program has been applied for theoretical calculations with B3LYP/6-311++G(d,p) basis set to study the vibrational and electronic properties of benzil molecule. The vibrational analysis explores the presence of various functional groups in the benzil molecule. NBO and HOMO-LUMO analysis has been performed and establishes the occurrence of charges transfer within the molecule. HOMO-LUMO energy gap is calculated to be 2.919 eV. First-order hyperpolarizability calculations reveal the nonlinear optical microscopic behavior of the benzil molecule with nonzero values, and it is found to be 11.435 times that of urea. Electrostatic potential establishes the strong electrophilic nature of the grown crystal. Above all the computations, we believe that the benzil compound is a suitable candidate for nonlinear applications as well as device fabrication and will be useful to the scientific community for further research.
Conflicts of Interest
The authors report there are no conflicts of interest.
Open Research
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and supplementary files.