Nutrient Detection Sensors in Seawater Based on ISI Web of Science Database
Abstract
Marine ecosystem is increasingly deteriorating. In order to assess anthropogenic influence and instigate appropriate remedial actions, it is still of great significance to develop the technology of sensors applied for nutrient detection (e.g., nitrate, phosphate, and silicate) in seawater. This brief review shows an important direction for the development of nutrient detection sensors in seawater and also the limitations and challenges based on data from the ISI Web of Science database. Being different from previous review papers, in this short critical review paper (1) we unified the unit of limit of detection (LOD) for making the comparison within different researches possible; (2) only the literatures focusing on the technological development of sensors in seawater were used; and (3) not only the detection methods but also the detected analytes and publication years were discussed to supply more valuable information for the development of nutrient sensors applied in seawater. In total, 109 literatures were collected with regard to technological development. The quantity of literatures has increased most during 2011-2020. For analytes, literatures related to nitrate, phosphate, ammonium, and phosphate will continue to increase with more accurate data. For detection methods, spectrophotometry, colorimetry, fluorimetry, and electrochemistry are the most widely used sensors. LODs show thousands of orders. In general, there are lower LOD to nitrite and ammonium and fluorimetry method. Now, for analytes, nitrate (1.0983) > silicate (0.5495) > phosphate (0.4823) > ammonium (0.1324) > nitrite (0.0568). For detection methods, microfluidics (1.7617) > electrochemistry (1.2607) > colorimetry (0.4462) > spectrophotometry (0.2941) > fluorimetry (0.0558). This result indicated that the development level of detection methods is closer for nitrate, nitrite, phosphate, and silicate. For ammonium, spectrophotometry has significantly lower LOD than electrochemistry (p < 0.05), and fluorimetry also has significantly lower LOD than electrochemistry (p < 0.05). Our results imply that sensors with accurate LOD should be developed in the future. In addition, more detection methods should be considered by future sensors.
1. Introduction
Despite the fact that oceans cover more than 70% of our planet and have a profound impact on global climate, weather patterns, human health, agriculture, and commerce [1, 2], human ability to make sustained measurements of ocean processes is limited and much of the oceans remain largely unexplored [3, 4]. Meanwhile, marine ecosystem is increasingly deteriorating due to continuous development and utilization of oceans by industrial pressures and growing population [5, 6]. These ecosystems of coastal zones, estuaries, and gulfs have been gradually destroyed with different extents, such as dumping of waste, construction of harbours, dredging, and extraction processes [7, 8]. Furthermore, frequently occurred marine natural disasters (e.g., tsunamis and red tides) bring substantial social impacts and economic losses [9, 10]. Therefore, the work of ocean environmental monitoring is urgent and monitoring the concentration changes and spatiotemporal distribution of nutrients in ocean environments is significant. In order to assess the impacts of these activities and instigate appropriate remedial actions, it is of great significance to develop the technology of sensors applied for ocean environmental monitoring, including the detection of chemical elements (e.g., nitrate, nitrite, phosphate, ammonium, and silicate), physical elements (e.g., pH, DO, and heavy metals), and biology (e.g., phytoplankton, benthonic animal, and fish).
Determining the distributions and variations of chemical elements in oceans is key to fully understanding global geochemical cycles, evaluating seawater pollution, and forecasting the occurrence of harmful algal blooms (HABs) [11, 12]. Although a wide variety of elements are essential to life in oceans, only a relatively small number of essential elements (e.g., nitrate, phosphate, and silicate) are termed as nutrients [13]. These nutrients are essential for the survival of marine organisms, such as promoting the growth of biology and microorganisms. Besides, accurate quantification of these nutrients is also necessary for forecasting the occurrence of harmful red tides and comprehending the dynamics of marine ecosystems [14]. On the contrary, inadequate nutrients will restrict the growth of phytoplankton and excessive nutrients are prone to cause eutrophication and even further lead to harmful algal blooms, extreme depletion of DO, and even death of aquatic organism [12, 15].
Traditional nutrient monitoring is difficult to determine the distributions and variations of nutrients to support ocean environmental monitoring [16]. Because they are measured by manual processing of sampling: water is collected at known locations and times, preserved and transported to laboratory for analysis by standard detection methods such as spectrophotometry, colorimetry, or fluorescence [17]. Nevertheless, these traditional methods (1) cannot satisfy the long-term in situ monitoring demands of nutrient detection in seawater and (2) are rather costly and time-consuming with expensive and bulky high-tech instruments and professional operators. Moreover, these obtained data may be not accurate enough because these seawater samples may undergo unexpected reactions during the long-time operation [14].
Currently, sensors and sensing systems are applied for nutrient monitoring to obtain primary data, to assure time-series observations on remote permanent platforms [1]. Consequently, in situ nutrient sensors, the device placed on a mobile platform such as a submersible vehicle, have a unique and important role in ocean environmental monitoring [17]. Over the years, the development of low-cost portable devices that can be employed for onsite and continuous analysis of nutrients has been attracting scientific attention [18]. Now, various nutrient sensors, including electronic sensors, chemical sensors, and biosensors, have been widely used for continuous observation in estuaries and seawater [19, 20]. And these nutrient sensors have been greatly improved during the past decades on accuracy, operability, sustainability, and other aspects [21–23]. Because of the unavoidable limitation of in situ seawater environment, the development of sensor technology has to be long duration of use, less wastewater output, low energy consumption, less reagent consumption, small volume, and strong ion selectivity. Some products with mature technology in market include Micro-Lab, EcoLAB2, and CYCL Phosphate sensors (Wetlabs, USA), SUNAV2 (Satlantic, Canada), and WIZ sensors (SYSTEA, Italy) [24, 25]. However, the main bottlenecks that restrict the development of nutrient sensors are short duration, low precision, narrow range of detection concentration, and poor reproducibility [26, 27]. To be more specific, since sample pretreatment, such as enrichment and dilution, cannot be applied in the field detection of nutrients in seawater, an ideal chemical sensor needs to have a high precision and a wide measuring range. Taking precision of sensors (LOD, limit of detection) as another example, studies have shown that the nutrient concentrations in seawater before and after algal bloom in some oligotrophic zones are order of nmol/L, and the nutrient concentrations in the same sea area vary greatly in different sea areas and at different times, and the detected concentration ranges from nmol/L to μmol/L (a difference of 5 orders of magnitude) [1, 28]. Hence, it is still necessary to utilize more appropriate methods to increase the precision of nutrient sensors in seawater, such as the limit of detection (LOD).
Previous valuable review papers have systemically reviewed either one analyte (or one group of analytes) or one category/type of sensors in seawater [1, 8, 9, 17, 29, 30]. With the increase of new literatures on nutrient sensors, how to utilize these resources to better service scientific development has been an important work. In this short critical review, after a brief introduction of necessity and need for higher precision of nutrient sensors in seawater, we collected related literatures focusing on the technological development of nutrient sensors in seawater and excluded those literatures focusing on application. We summarized the research status of nutrient sensors in seawater by quantity of literatures and then discussed the sensitivity of sensors (LOD) from two aspects of analytes and detection methods. Finally, a statistic analysis was performed to see if there existed any significant differences among five different detection methods (spectrophotometry, chromatography, colorimetry, electrochemistry, and fluorimetry) within one nutrient (nitrate, nitrite, phosphate, ammonia, and silicate). Being different from previous review papers, in this short critical review paper (1) we unified the unit of LOD to make the comparison within different researches possible; (2) only the literatures focusing on the technological development of sensors in seawater were used; and (3) not only the detection methods but also the detected analytes and publication years were discussed to supply more valuable information for the development of nutrient sensors applied in seawater. This brief review shows an important direction for the development of nutrient detection sensors in seawater and also the existing limitations and challenges.
2. Materials and Methods
2.1. Database Compilation
We build this database using the following topics searched in core database of Web of Science (1985-2020): “sensor” and “seawater or sea water or saline water or marine water or salt water or ocean” and “nutrient or nitrate or nitrite or phosphate or ammonia or silicate” in July 2020. Here, for supplying more valuable information for the development of nutrient sensors applied in seawater, we just collected literatures focusing on the technological development of sensors, excluded those focusing on application. In total, 42.21% of the screened literatures were published during the last five years (2016-2020). For each literature, we extracted the information: tested analytes, detection method of sensors, LOD, and publication year. All tested analytes were categorized into phosphate, nitrite, nitrate, ammonium, and silicate. As for the detection methods, we included spectrophotometry (ultraviolet spectrophotometry, visible light spectrophotometry), fluorimetry, colorimetry (visual colorimetry, photoelectric colorimetry), chromatography, microfiber, microfluidics, electrochemistry, optofluidics, and carbon nanotube. We unified all the units of LODs as μM (μmol/L).
2.2. Data Analysis
We performed one-way ANOVA to test the differences of LODs among different detection methods within one analyte. All data were checked for normality before conducting the ANOVA tests and were log-transformed to meet normality and homogeneity assumptions [19]. If significant effects are present in the ANOVA, then Tukey’s test was used for post hoc analysis of significant differences among detection methods. All statistical analyses were performed by SPSS statistics software (IBM, 20.0).
3. Results and Discussions
3.1. Research Status of Nutrient Sensors in Seawater
To a certain extent, quantity of literatures could reflect scientific research status in this area. After screening from 538 literatures searched in the core database of Web of Science, we finally collected 109 literatures focusing on the technological development of nutrient sensors applied in seawater. Table 1 shows the summary of sensors for the nutrient detection in seawater. Here, we classified analytes mainly by phosphate, nitrate, ammonium, and silicate related to different detection methods with LOD and publication date.
| Analytes | Detection methods | LOD (μM) | Ref. | Year |
|---|---|---|---|---|
| Phosphate | Fluorimetry | 0.4000 | [31] | 2020 |
| Phosphate | Colorimetry | 2.4211 | [32] | 2020 |
| Nitrate | Chromatography | 0.2200 | [33] | 2020 |
| Nitrate | Microfiber | 2.7419 | [34] | 2020 |
| Nitrite | Colorimetry | 0.1000 | [35] | 2019 |
| Nitrate | Spectrophotometry | 0.0200 | [36] | 2019 |
|
Colorimetry |
|
[37] | 2019 |
| Nitrate | Spectrophotometry | 0.2000 | [38] | 2019 |
| Ammonium | Spectrophotometry | 0.1500 | [39] | 2019 |
| Ammonium | Fluorimetry | 0.0021 | [40] | 2018 |
| Nitrate | Spectrophotometry | 0.0074 | [41] | 2018 |
| Nitrate | Spectrophotometry | 0.1000 | [42] | 2018 |
| Phosphate | Spectrophotometry | 0.1000 | [43] | 2018 |
| Silicate | Electrochemistry | 0.5000 | [23] | 2018 |
| Nitrate | Electrochemistry | 0.0009 | [44] | 2018 |
| Nitrate | Electrochemistry | 0.9000 | [14] | 2018 |
| Ammonium | Spectrophotometry | 0.2000 | [45] | 2018 |
| Ammonium | Spectrophotometry | 0.0800 | [46] | 2018 |
| Ammonium | Fluorimetry | 0.0065 | [47] | 2018 |
| Nitrite | Electrochemistry | 0.2000 | [48] | 2018 |
| Silicate | Spectrophotometry | 1.6000 | [49] | 2018 |
| Ammonium | Fluorimetry | 0.1600 | [50] | 2018 |
| Phosphate | Spectrophotometry | 0.1000 | [43] | 2018 |
| Ammonium | Fluorimetry | 0.0010 | [51] | 2017 |
|
Spectrophotometry |
|
[52] | 2017 |
| Phosphate | Colorimetry | 0.0300 | [53] | 2017 |
| Nitrate | Electrochemistry | 0.3900 | [54] | 2017 |
| Nitrate | Electrochemistry | 0.8000 | [55] | 2017 |
| Phosphate | Colorimetry | 0.0526 | [56] | 2017 |
| Phosphate | Colorimetry | 0.0300 | [53] | 2017 |
| Phosphate | Colorimetry | 0.1000 | [57] | 2017 |
| Silicate | Optofluidics | 0.0451 | [58] | 2017 |
| Ammonium | Electrochemistry | 0.6400 | [59] | 2017 |
| Ammonium | Colorimetry | 0.0150 | [60] | 2017 |
| Nitrate | Electrochemistry | 0.8000 | [61] | 2016 |
| Nitrite | Fluorimetry | 0.1000 | [62] | 2016 |
| Ammonium | Fluorimetry | 0.0074 | [63] | 2016 |
| Phosphate | Spectrophotometry | 0.0014 | [64] | 2016 |
| Phosphate | Electrochemistry | 0.1000 | [65] | 2016 |
| Phosphate | Electrochemistry | 4.0000 | [66] | 2016 |
| Silicate | Electrochemistry | 0.5000 | [67] | 2015 |
| Nitrate | Microfluidics | 5.0000 | [68] | 2015 |
| Nitrate | Electrochemistry | 3.8000 | [69] | 2015 |
| Ammonium | Spectrophotometry | 0.0055 | [70] | 2015 |
| Ammonium | Fluorimetry | 0.0058 | [71] | 2015 |
|
Microfluidics |
|
[72] | 2015 |
| Phosphate | Fluorimetry | 0.0145 | [73] | 2014 |
| Ammonium | Spectrophotometry | 0.0036 | [74] | 2014 |
| Nitrate | Spectrophotometry | 0.0300 | [75] | 2014 |
| Ammonium | Fluorimetry | 0.0100 | [76] | 2013 |
| Phosphate | Colorimetry | 0.0520 | [77] | 2013 |
| Ammonium | Fluorimetry | 0.0007 | [78] | 2013 |
|
|
|
[79] | 2013 |
| Phosphate | Electrochemistry | 0.1900 | [80] | 2013 |
| Nitrate | Spectrophotometry | 0.2000 | [81] | 2013 |
| Nitrate | Spectrophotometry | 1.9355 | [82] | 2012 |
| Silicate | Electrochemistry | 0.1000 | [83] | 2012 |
|
Microfluidics |
|
[84] | 2012 |
| Ammonium | Carbon nanotube | 0.0100 | [85] | 2012 |
| Nitrite | Colorimetry | 0.0150 | [86] | 2011 |
| Nitrate | Spectrophotometry | 1.7000 | [87] | 2011 |
| Ammonium | Fluorimetry | 0.0130 | [88] | 2011 |
| Ammonium | Fluorimetry | 0.0010 | [89] | 2011 |
| Ammonium | Spectrophotometry | 0.0035 | [90] | 2011 |
| Ammonium | Fluorimetry | 0.0050 | [91] | 2011 |
| Phosphate | Electrochemistry | 0.1200 | [25] | 2011 |
| Ammonium | Colorimetry | 0.0150 | [92] | 2011 |
| Nitrate | Spectrophotometry | 0.2000 | [93] | 2010 |
| Nitrate | Spectrophotometry | 0.3000 | [94] | 2010 |
| Nitrate | Electrochemistry | 10.0000 | [95] | 2010 |
| Nitrite | Spectrophotometry | 0.1000 | [96] | 2009 |
| Nitrate | Electrochemistry | 0.014 | [97] | 2009 |
| Nitrate | Electrochemistry | 0.0002 | [98] | 2008 |
| Nitrate | Microfluidics | 4.5000 | [99] | 2008 |
| Silicate | Electrochemistry | 0.3000 | [100] | 2008 |
| Ammonium | Fluorimetry | 0.0011 | [101] | 2008 |
| Silicate | Electrochemistry | 0.3000 | [100] | 2008 |
|
Colorimetry |
|
[102] | 2008 |
| Nitrite | Spectrophotometry | 0.0001 | [103] | |
| Nitrate | Electrochemistry | 4.5000 | [104] | 2007 |
| Silicate | Electrochemistry | 1.0000 | [105] | 2007 |
| Nitrate | Spectrophotometry | 2.0000 | [106] | 2006 |
| Ammonium | Spectrophotometry | 0.0050 | [107] | 2005 |
| Nitrate | Electrochemistry | 1.0000 | [108] | 2005 |
| Phosphate | Fluorimetry | 0.0200 | [109] | 2003 |
|
Spectrophotometry |
|
[110] | 2003 |
| Nitrate | Spectrophotometry | 0.2000 | [111] | 2002 |
| Nitrate | Spectrophotometry | 0.0452 | [112] | 2002 |
| Nitrate | Spectrophotometry | 0.0452 | [112] | 2002 |
|
Fluorimetry |
|
[113] | 2000 |
| Nitrate | Spectrophotometry | 0.0226 | [114] | 1999 |
| Nitrate | Spectrophotometry | 0.1000 | [115] | 1998 |
| Nitrate | Electrochemistry | 0.1000 | [116] | 1994 |
From Table 2, we can see that the quantity of literatures has increased with the development of decades. In particular, during 2011-2020 in both analytes and detection methods, there are more literatures focusing on the technological development of sensors. Furthermore, this trend also means that more literatures may appear during the next ten years to meet the needs from all walks of life and from many different areas. Actually, nitrate determination was firstly taken by electrochemical methods in 1834 [117]. It has been attracting more attention in the world today from different perspectives. A review published in 2021 summarizes the advances in knowledge in terms of the modes of action of devices and deployment strategies, identifying the current limitations and future challenges for the electrochemical detection of nutrients in marine environments [118]. The application of electrochemical sensors including potentiometric, voltammetric, and field-effect transistor sensors for nitrate, nitrite, ammonium, and phosphate determination in aqueous environments was reviewed [16]. The recent advances in ISE sensing platforms for environmental water analysis from on board to in situ approaches were also reviewed [119].
| Category | In total | Years | 2011-2020 | 2001-2010 | 1994-2000 |
|---|---|---|---|---|---|
| Analytes | |||||
| Nitrate | 39 | 1994-2020 | 21 | 14 | 4 |
| Nitrite | 12 | 2000-2019 | 8 | 3 | 1 |
| Phosphate | 21 | 2003-2020 | 18 | 3 | 0 |
| Ammonium | 26 | 2005-2019 | 24 | 2 | 0 |
| Silicate | 11 | 2003-2018 | 7 | 4 | 0 |
| Detection methods | |||||
| Spectrophotometry | 38 | 1998-2019 | 24 | 12 | 2 |
| Colorimetry | 18 | 2008-2020 | 15 | 3 | 0 |
| Fluorimetry | 19 | 2000-2020 | 15 | 2 | 2 |
| Electrochemistry | 24 | 1994-2018 | 15 | 8 | 1 |
| Chromatography | 1 | 2020 | 1 | 0 | 0 |
| Microfiber | 1 | 2020 | 1 | 0 | 0 |
| Microfluidics | 6 | 2008-2020 | 5 | 1 | 0 |
| Optofluidics | 1 | 2017 | 1 | ||
| Carbon nanotube | 1 | 2012 | 1 | ||
In terms of quantity, the above obvious result may mainly attribute to (1) emerging technologies. In 2003, Thouron developed a system with three analysers to measure phosphate, nitrate, and silicate together [110]. And Zhang et al. fabricated a self-supported electrode to detect ammonia based on electrodepositing platinum-polypyrrole on Ni foam [120]. New technologies also promote the improvement of old ones. For example, scientists improved the sensitivity measurement of nitrate concentration based on new dispersion turning point (DTP) theory [34]. Meanwhile, the emergence of new technologies also brings more detection methods. For example, phosphate was detected by interfacial barrier effects of p-n junction on electrochemistry [121]. (2) Some problems previously hard to study are solved. It was well known that not extensively researches had studied voltammetric sensors for nitrate detection in seawater, because the LOD data from sensors was usually above 1 μM, higher than the nitrate concentrations in seawater, particularly the concentration of nitrate at the ocean surface (at a nanomolar level) [122]. Nine years later, Legrand et al. developed an electrode by electrodepositing silver nanoparticles on a gold disc electrode to test nitrate in synthetic seawater [54]. The sensor showed a limit of quantification of 0.39 μM and a linear range of 0.39-50 μM. The peak current intensity remained at 95% of the initial value after regular detection of 25 μM nitrate for about 26 days. And (3) more and more emerging contaminants interference is still a technical challenge. For example, the challenges of nitrate biosensors based on reductases include oxygen interference [123], low electron transfer efficiency [124, 125], and high cost and low storage temperature, which promote relevant research and generate more literatures.
Although the quantity of relevant literatures has been increasing steadily, the research status within both analytes and detection methods is very varied. For analytes, we can see that more research focused on nitrate, phosphate, and ammonium. This result should attribute to seriously increasing marine environmental and ecological problems, such as frequent red tide, green tide, and other ecological disasters [9, 10, 126]. Hence, for a period of time, literatures related to nitrate, phosphate, ammonium, and phosphate will continue to increase with more accurate data and new detection methods.
For the detection methods, we found a similar trend as one previous review paper that spectrophotometry, colorimetry, fluorimetry, and electrochemistry are the most commonly used sensors for the detection of nutrient detection in seawater [127]. These traditional and classic detection methods have been attracting a lot of attention for years, followed by emerging chromatography, microfiber, microfluidics, optofluidics, and carbon nanotube (Table 2). Then, other electrochemical sensors have been used for water analysis. As we know, we widely used electrochemical sensors as one of the most promising analytical tools for the rapid detection of nitrate due to its high sensitivity, quick response, ease of operation and miniaturization, low sample and power consumption, low reagent consumption, and easy combination with automation devices [18, 128]. Nevertheless, some new methods also appeared with technical advantage. For example, a new method using a microfiber mode interferometric sensor to improve the sensitivity of nitrate concentration measurement in seawater based on dispersion turning point (DTP) theory is demonstrated [34]. Through interdisciplinary research within electronics, chemistry, materials science, etc., the application of microfluidic technology shows more advantages and gradually becomes a better way to further reduce energy consumption, reduce the amounts of reagents, and promote the miniaturization of sensors [129]. Therefore, more studies should focus on the development of new detection methods for clearer understanding of the nutrient in seawaters.
3.2. Sensitivity of Nutrient Sensors in Seawater
LODs in Table 1 show thousands of orders for different analytes and detection methods. Here, the average LOD of nutrient sensors included was μM. Among all the sensors checked, the most sensitive sensor was developed by Chen and Chumbimuni-Torres in which the indirect detection of nitrate through spectrophotometry and electrochemistry method was used [98, 103]. Above sensor could achieve an ultrasensitive LOD, as low as 0.1 nM [103]. By contrast, some other sensors are relatively “insensitive” with the LODs at the level of 2-10 μM, distributed from 2006 to 2016 [66, 68, 95, 104].
Figure 1(a) shows the average LOD of five nutrients at the level almost below1 μM with descending sequence: nitrate (1.0983) > silicate (0.5495) > phosphate (0.4823) > ammonium (0.1324) > nitrite (0.0568). Figure 1(b) only shows the average LOD of five detection methods with more than five literatures at the level almost below 1 μM (except for microfluidics at the level of 2 μM) with descending sequence: microfluidics (1.7617) > electrochemistry (1.2607) > colorimetry (0.4462) > spectrophotometry (0.2941) > fluorimetry (0.0558). For the rest four detection methods, chromatography, microfiber, optofluidics, and carbon nanotube have a LOD of 0.2200, 2.7419, 0.0451, and 0.01, respectively.

In general, both detection of nitrite and ammonium and fluorimetry method seem to have lower LODs (Figure 1). Nevertheless, we cannot just draw a simple conclusion that these related research develop best. This result could mainly attribute to the following: (1) some new literatures based on special detection technique usually increase the mean value with their own high LODs. For example, Yang et al. used a new technique to improve the sensitivity of sodium nitrate concentration measurement in seawater based on dispersion turning point [34]. Although the sensor shows advantages of easy to construct, low cost, high precision, and high sensitivity, which provides a new optical detection method for in situ detection of marine environment or low concentration substances sensing in other liquids, its LOD is 2.7419 μM. The same situation applies to Khongpet et al. who used a compact hydrodynamic sequential injection system for consecutive online determination of phosphate and ammonium, with a LOD 1.8947 μM [37]. On a long view, these technologies are constantly developing with lower LOD. And more literatures related to these technologies will finally lower the average LOD. Before we get too excited about that, we may have to face the situation that the emergence of other new technologies (in embryonic stage) may increase the mean value with their own high LODs, again. (2) Different detection methods have their own special value. For example, a variety of approaches have been used for the determination of phosphate in seawater, including colorimetric detection, fluorescent detection, and electrochemical detection. For all are reagent-based methods, phosphate cannot be detected directly. Therefore, autonomous systems tend to employ the colorimetric method rather than fluorescent or electrochemical methods [130]. Previous study has shown that when algal blooms erupt, the nutrient concentration in seawater is on the order of 10−9 mol/L [131]. So it is very necessary to utilize more appropriate methods to increase the accuracy of the sensor. In addition to accuracy, the nutrient concentration in the same sea area varies significantly at different times or in different sea areas and the ranges from 10−9 to 10−6 mol/L, with a difference of 5 orders of magnitude [131]. Hence, an ideal sensor should better have wide measurement range.
3.3. Comparison of Detection Methods
Figure 2 shows the result of analysis of one-way ANOVA for different detection methods (p < 0.05). In general, at the present stage, we could see that not all methods (NO DATA) can be used for detection within one nutrient in seawaters such as chromatography to nitrite, phosphate, ammonium, and silicate (Figure 2). Clearly, future studies should focus on the development of chromatography sensors applied in seawater.
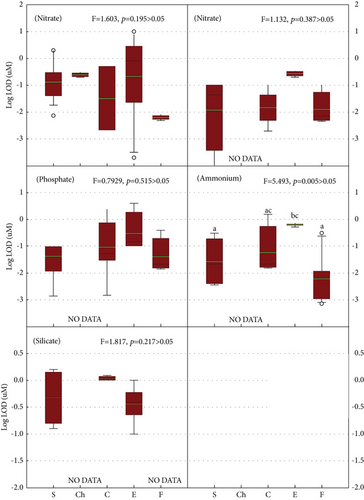
Furthermore, except ammonium (p < 0.05), there were no significant differences among different detection methods applied in any nutrient sensor. This result indicated that, for nitrate, nitrite, phosphate, and silicate, their development level of detection methods is closer to each other. For ammonium, spectrophotometry has a significantly lower LOD than electrochemistry (p < 0.05), and fluorimetry also has a significantly lower LOD than electrochemistry (p < 0.05). This result is in accordance with the consensus that electrochemical sensors have been widely used as one of the most promising analytical tools for the rapid detection of nitrate in environmental matrices due to low sample and power consumption, high sensitivity, quick response, and ease of operation and miniaturization [18, 128].
4. Conclusions
From 109 literatures, the general status of nutrient detection sensors in seawater including the research status, sensitivity, detection methods, and future challenges was reviewed, with most published during 2011-2020. For analytes, literatures related to nitrate, phosphate, ammonium, and phosphate will continue to increase with more accurate data. For detection methods, spectrophotometry, colorimetry, fluorimetry, and electrochemistry are the most widely used sensors. LODs show thousands of orders. In general, there are lower LOD to nitrite and ammonium and fluorimetry method. Now, for analytes, nitrate (1.0983) > silicate (0.5495) > phosphate (0.4823) > ammonium (0.1324) > nitrite (0.0568). For detection methods, microfluidics (1.7617) > electrochemistry (1.2607) > colorimetry (0.4462) > spectrophotometry (0.2941) > fluorimetry (0.0558). This result indicated that the development level of detection methods is closer for nitrate, nitrite, phosphate, and silicate. For ammonium, spectrophotometry has significantly lower LOD than electrochemistry (p < 0.05), and fluorimetry also has significantly lower LOD than electrochemistry (p < 0.05). Our results are expected to indicate that higher sensitivity sensors should be developed in the future. In addition, more detection methods should be considered by future sensors. We can see that although the stability, sensitivity, and detection limit of sensors have greatly improved, there are still some certain technical issues that restrict the large-scale use of this technology, including low reproducibility, low accuracy, narrow detection concentration ranges, and short continuous measurement time. Besides, good stability is important to an ideal sensor achieved by improving sensor antifouling ability, which is a hot topic partially in biocompatibility and blood compatibility [132]. So, it is a feasible solution to further explore new antifouling materials and approaches for maintaining long-term sensor durability and stability. In short, this brief review shows an important direction for the development of nutrient detection sensors in seawater and many limitations and challenges still existed. Clearly, much work still needs to be done in many areas of sensor development and for a variety of seawater environments.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors’ Contributions
Lina Cao and Hongyong Xiang have equal contributions.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant numbers 32071587, 32101310, and 41501566), Fellowship of China Postdoctoral Science Foundation (grant number 2021M692728), China Scholarship Council (grant number 202006625001), Science Foundation of Science and Technology of Education Department of Jilin (grant number JJKH20211293KJ), Natural Science Foundation of Heilongjiang (grant number YQ2021C031), University Nursing Program for Young Scholar with Creative Talents in Heilongjiang (grant number UNPYSCT-2020008), Postdoctoral Scientific Research Developmental Fund of Heilongjiang (grant number LBH-Q20166), and Program of Introducing Talents of Discipline to Universities (grant number B16011).


