Computational Study on the Microscopic Adsorption Characteristics of Linear Alkylbenzene Sulfonates with Different Chain Lengths on Anthracite Surface
Abstract
In order to explore the influence of different lengths of hydrophobic carbon chains on the diffusion characteristics of surfactants on the surface of anthracite, six linear alkyl benzene sulfonates with different hydrophobic carbon chain lengths were selected (mC, m = 8, 10, 12, 14, 16, 18; m represents the numbers of carbon atoms in the hydrophobic carbon chain), and molecular dynamics (MD) simulations were adopted. Models of surfactant-anthracite, surfactant-graphite layer, and water-surfactant-anthracite were constructed. After analyzing a series of properties such as adsorption energy, diffusion coefficient, radial distribution function (RDF), and hydrophobic tail order parameters, it was found that 12C had the highest adsorption strength on the surface of anthracite; the reason was that 12C had the highest degree of aggregation near the oxygen-containing functional groups on the surface of anthracite. Further studies had found that the hydrophobic tail chain of 12C had the strongest isotropy. The study fills the gap in the systematic study of the diffusion characteristics of linear alkylbenzene sulfonates (LAS) with different chain lengths on the surface of anthracite, enriches and develops the basic theory of coal wettability, and also provides technical ideas for the design of new surfactants and new dust suppression agents.
1. Introduction
Surfactants are amphiphilic compounds that contain both hydrophilic and hydrophobic groups. This special structure makes it one of the most versatile chemical products [1–8] (e.g., in the oil industry, cosmetics, coal industries, and polymer paint). In recent years, surfactants have also been widely used in high-tech fields. Such as electronic printing, microelectronics technology, nanomaterials, and biopharmaceutical research [9–17]. From the overall consumption of global surfactants, the consumption of anionic surfactants is the largest [18]. From the aspects of surfactant applicability, cost performance, and overall consumption level, anionic surfactant linear alkylbenzene sulfonates (LAS) are the most important surfactant series in the world [19–22].
LAS consists of a hydrophobic linear alkyl chain and a hydrophilic sulfonate group, which are, respectively, connected to the 1, 4 positions of the benzene ring [23]. Commercial LAS surfactants are usually complex mixtures of homologs [24] (i.e., different lengths of the alkyl chain). Heterogeneous components improve performance, making LAS widely used in different industries, such as cosmetics, detergents, and mineral extraction [25–28]. However, this heterogeneity also makes it more complicated to clarify the detailed functions of the pure LAS. So far, LAS mixtures have been extensively studied [29–33], but systematic studies on pure LAS are relatively rare.
Surfactants have been widely used in the coal industry, such as in the fields of coal flotation and coal dust suppression [34–41]. The research on the interface characteristics of coal relies on the basic theory of coal wettability [42–44]. A large number of experimental studies have shown that the surface active agent diffusion characteristics on the coal surface determine the wetting effect of coal [45–50]. However, the experimental methods have defects in explaining the structural changes, dynamics, and energy characteristics of the molecular adsorption process on the mineral surface.
With the development of science and technology, it is becoming a reality to use computer simulations to explain the macroscopic properties from a microscopic point of view [51–59]. In particular, MD simulation has proven to be a useful tool for analyzing coal interface properties at the molecular level [60–66]. Traditional experiments screen flotation agents and dust suppression agents by trial-and-error method. MD simulation can screen agents in batches more efficiently, save a lot of time and materials, and also help to understand the adsorption mechanism from a molecular perspective. So far, researchers have conducted a large number of MD simulation studies on the diffusion characteristics of surfactants on the coal surface [44, 67–71]. However, there are few researches on the diffusion characteristics of LAS on coal surface; moreover, there is a lack of research on the mechanism and structure of the chain length in the adsorption process. We studied the adsorption characteristics of the four isomers of the linear alkylbenzene sulfonate family (LAS) on the coal surface and systematically compared the adsorption characteristics and microscopic adsorption mechanism of the LAS family members on the coal surface. [71].
In the existing research on the influence of different hydrophobic tail chain length on the properties of surfactants, researchers mostly choose two or three surfactants to compare; most of the conclusions drawn are that various properties change linearly as the length of the hydrophobic alkyl chain changes [46]. We think this is unreasonable, because the selected types of surfactants are too few to get a comprehensive conclusion. Therefore, in this study, we selected six surfactants with hydrophobic chain length m = 8, 10, 12, 14, 16, 18 for research. Since the LAS with even number of carbon atoms in the hydrophobic chain are more widely used and the odd-even effect [72] causes a large difference between the odd-numbered carbon atoms and the even-numbered carbon atoms, we selected the LAS with even number of carbon atoms in the hydrophobic tail chain for research. As far as we know, this is the first attempt to systematically study and compare the diffusion characteristics of LAS family members with different carbon chain lengths on the surface of anthracite.
Surfactants with different hydrophobic chain lengths have different properties, which arouses the interest of a wide range of researchers. Acisli et al. [73] studied the adsorption capacity of five cationic surfactants with different hydrophobic chain lengths on montmorillonite and found that the hydrophilicity decreased with the increases of chain length. Ren et al. [74] study the adsorption behavior of different chain lengths Gemini surfactants on methyl orange and find that, with the increase of the chain lengths, the interlayer spacing, hydrophobicity, and dispersibility of organoclay increase significantly. Kun et al. [75] study the hydrophobic effect of three cationic surfactants with different hydrophobic chain lengths on bovine serum albumin and find that the initial step of the interaction is to attach the nonpolar carbon chain of the surfactant to the nonpolar region of the protein. Jiang et al. [76] study the effect of carbon chain lengths on the surface properties of salt-free cationic surfactants and conclude that the longer the hydrocarbon chain, the greater the hydrophobic effect. Díez-Pascual et al. [77] study the influence of surfactants with different chain lengths on the morphology of graphene and find that the thickness of the graphite flakes increases with the increase of the surfactant chain length, and the longer the molecular chain, the higher the surface coverage. Zhang et al. [78] use two Gemini surfactants (only the length of the hydrophobic tail is different) to study the adsorption of emerging pollutants after modified montmorillonite and find that an increase in the alkyl chain lengths can increase the mobility of molecules. Badache et al. [79] study the effects of different hydrophobic tail chain lengths on the interfacial properties of cationic surfactants and find that the surfactant with the longest chain has the lowest critical micelle concentration.
MD simulation was used for the calculation. Firstly, a binary system of surfactant-anthracite was constructed. The adsorption energy and diffusion coefficient of six LAS with different chain lengths were compared; a macroconclusion was drawn that the adsorption effect of 12C on the surface of anthracite was the best. Secondly a binary system of surfactant-graphite layer was constructed. Through coordination number and radial distribution function analysis, the microscopic reason for the highest adsorption strength of 12C was explained from the perspective of functional groups. In order to detect the tightness of the surfactant adsorption layer, a water layer was added to construct a water-surfactant-anthracite ternary system. Through the analysis of the RDF between oxygen atoms in the water and the order parameters of the hydrophobic tail chain, it was proved that the layer composed of 12C was the densest and had the strongest ability to “isolate” water molecules. This research fills the gap in the systematic study of the diffusion characteristics of LAS with different chain lengths on the surface of anthracite, expands the basic theory of wettability, and also provides ideas for the design and development of surfactants with new structures and new dust suppression agents.
2. Materials and Methods
2.1. Materials
This study selected the Jincheng anthracite that we had studied before (from the no. 3 coal seam of the Zhaozhuang coal mine in Jincheng). After hand-selecting and stripping the vitrinite, it was crushed and ground and passed through a 200-mesh sieve. The demineralized coal was obtained by pickling with HCl-HF-HCl. Carbonate, phosphate, clay, silica, and other minerals in coal were removed. The acid-treated coal sample was washed with excess distilled water until the pH of the filtrate became neutral. By measuring proximate analysis, ultimate analysis, carbon nuclear magnetic resonance (13C-NMR), high-resolution transmission electron microscopy (HRTEM), infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS), a molecular model of the average structural unit of Jincheng anthracite was initially constructed. Table 1 shows the results of proximate and ultimate analysis of Jincheng anthracite [80].
| Proximate analysis (%) | Ultimate analysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vda f | FCd | C | H | O | N | S |
| 0.66 | 23.05 | 12.86 | 67.05 | 91.51 | 3.89 | 2.10 | 1.71 | 0.79 |
- Mad: moisture content on an air-dried basis. Ad: ash content on dry basis. Vda f: volatile content (in ash-free form). FCd: fixed carbon content.
Analyzing the carbon skeleton parameters obtained from the 13C-NMR test results of demineralized coal, it was found that the bridge carbon and the peripheral carbon were relatively high. This indicated that the degree of condensation of the benzene ring of coal molecules was relatively high. Therefore, the aromatic structure of Jincheng anthracite was dominated by fused-ring aromatic hydrocarbons formed by seven benzene rings, and the degree of substitution of aromatic rings was relatively high. Table 2 shows the structural parameters of Jincheng anthracite samples. Combining the analysis of coal samples with FTIR and XPS [80], Jincheng anthracite contained less nitrogen and exists in the form of organic nitrogen and inorganic nitrogen; the sulfur in coal samples mainly existed in the form of organic sulfur, with less inorganic sulfur. The oxygen content in coal was less in the aromatic structure, and the oxygen-containing functional groups included hydroxyl and ketone groups. Finally, two structural units S1 and S2 containing hydroxyl group and ketone group were constructed (Figure 1(a)). The surfactant molecules used in this study were six kinds of LAS with different carbon chain lengths (denoted by mC, where m represents the number of carbon atoms in the tail chain, m = 8, 10, 12, 14, 16, 18); they were sodium octaalkylbenzene sulfonate, sodium decaylbenzene sulfonate, sodium dodecylbenzene sulfonate, sodium tetradecylbenzene sulfonate, sodium cetylbenzene sulfonate, and sodium octadecylbenzene sulfonate. The reason for selecting them was that the six surfactants had the same hydrophilic head group, but the hydrophobic tail chain length was different. We used the controlled variable method to study the effect of surfactant tail chain length on adsorption characteristics.
| xb | C | σ + 1 | P0 | B.L. | S.C. | MW | Mσ |
|---|---|---|---|---|---|---|---|
| 0.46 | 27 | 3.0 | 0.40 | 1.2 | 1.8 | 398 | 20 |
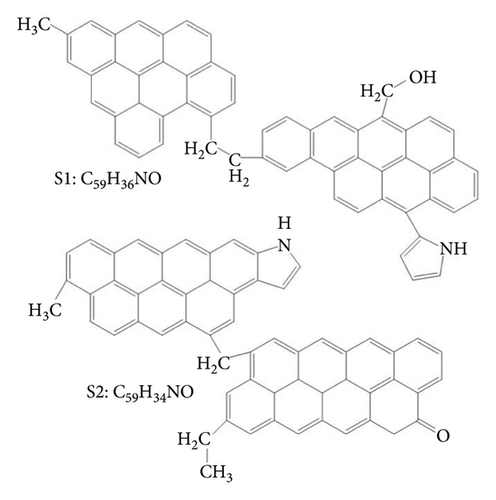
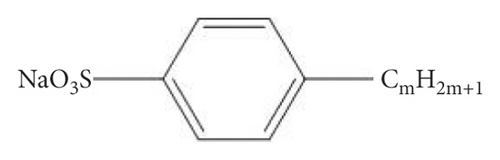
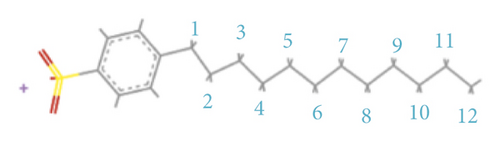
xb is the average value of the ratio of bridge carbon to peripheral carbon of aromatic compounds in coal (referred to as bridge carbon ratio); this value can reflect the degree of polycondensation of aromatic compounds, that is, the size of the aromatic nucleus. C represents the average number of carbon atoms in the aromatic layer. σ + 1 represents the average number of bridge bonds and side chains contained in each aromatic layer. P0 represents the percentage of bridge. B.L. represents the number of bridges and rings in each aromatic layer. S.C. represents the number of side chains in each aromatic layer. MW represents the average molecular mass of the aromatic layer. Mσ represents the average molecular mass of the side chain or 1/2 bridge chain.
2.2. Computer Simulation Details
In this study, we used the molecular structure of Jincheng anthracite proposed earlier by our team to build a coal model [80], as shown in Figure 1(a). Forty optimized coal molecules (ratio 1 : 1) were randomly stacked in a cubic unit cell (35 × 35 × 35 Å3) through the Amorphous Cell module of Materials Studio 6.0. Annealing algorithm was used to relax the coal structure. The initial temperature of anthracite cell was set to 298K and then rose once every 50K until the temperature reached 1098K. Five annealing cycles were performed, and the final temperature was 298K [67, 81]. On the top of the coal seam, a 12 nm thick vacuum layer was added to prevent the influence of periodic boundary conditions. Figure 2 shows the constructed anthracite surface model.
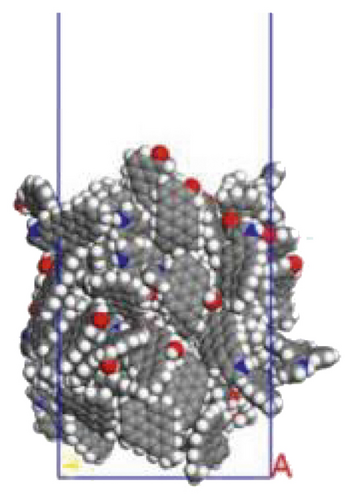
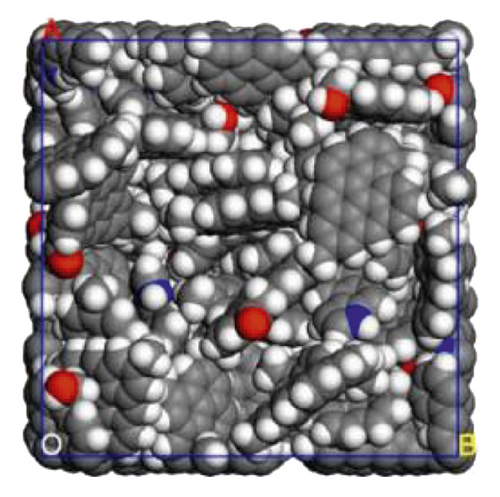
Surfactant-anthracite, surfactant-graphite layer, and water-surfactant-anthracite system models were constructed. 10 surfactant molecules made up the surfactant layer, while 1000 water molecules made up the water layer. The length and width of the surfactant layer and the water layer are consistent with the coal model, both being 35 Å. In this way, the models can be matched when constructing surfactant-coal and water-surfactant-coal systems. The intermolecular interaction was described by the polymer uniform force field. Before the MD simulation, geometric optimization was used to minimize the energy of all models. Subsequently, NVT ensemble (the constant-temperature, constant-volume ensemble) was used for MD simulation. Nose was used for thermostat [82, 83]. The time step was set to 1 fs. 1000 ps simulations were conducted to make the systems interact with each other fully. In all MD simulations, the Ewald algorithm was selected for long-range electrostatic interaction, and the accuracy was 0.001 kcal/mol, and the atom based algorithm was selected for the van der Waals interaction, and the cut-off distance was 1.25 nm. A 12 nm vacuum layer was added to the surface of each model to eliminate the mirror effect [84]. In the display of all models, the sodium atoms were hidden to show the adsorption form of surfactants more clearly.
3. Results and Discussion
3.1. Surfactant-Anthracite System
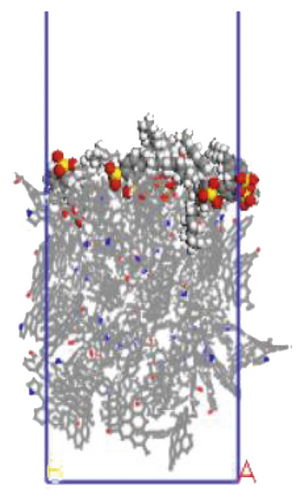
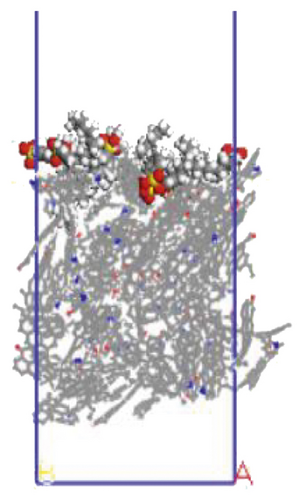
The surfactant layer was first placed on the surface of the anthracite with the build layer tool, and the MD simulation of 1000 ps was performed. Under the action of van der Waals force and electrostatic force, the surfactant layer kept approaching the anthracite coal and finally completed the adsorption. The final adsorption configuration is shown in Figure 3. In order to highlight the adsorption form of surfactants, the coal surface model was displayed in a line model, and the surfactant was displayed in a CPK mode. The initial configuration can be viewed in the support material, as shown in Figures S1–S6.
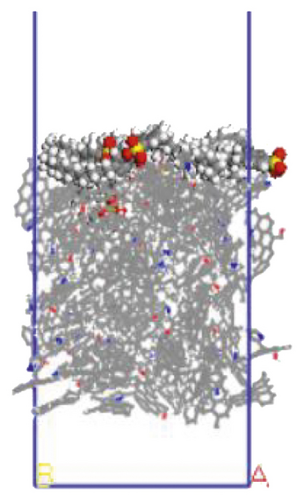
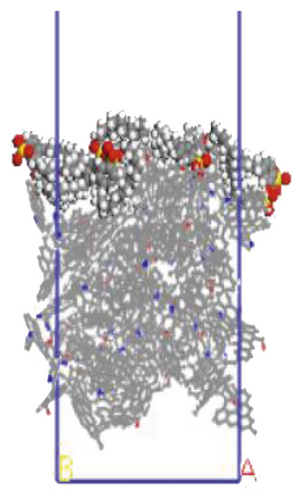
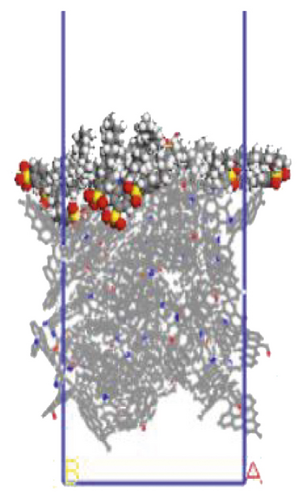
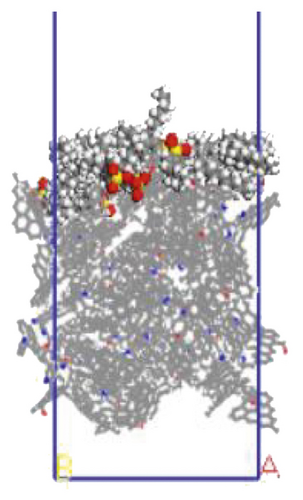
3.1.1. Adsorption Energy
We use a Perl script to extract the energy directly. The average of the last 10 conformational energies was calculated to arrive at the data in Table 3. Comparing the total adsorption energy data from Table 3, the 12C-anthracite system’s energy was the lowest, indicating that the 12C released the most energy during the adsorption process, the adsorption strength was the highest, and the degree of aggregation on the anthracite coal surface was the highest. This was due to the different molecular structure of surfactants. These six surfactants had the same polar head group and different hydrophobic tail chain length. When the hydrophobic chain length m increased from 8 to 12, the hydrophobic interaction between the hydrophobic tail chain and the anthracite was enhanced, and it was easy to self-aggregate. At this time, due to the short length of the hydrophobic tail chain, the steric hindrance effect was not obvious yet (the steric hindrance effect mainly refers to the steric hindrance caused by the proximity of certain groups in the molecule; it is a kind of intramolecular tension). At this time, the π-π stacking effect between the benzene ring and the aromatic structure of anthracite in the surfactant was relatively obvious, but as the carbon chain becomes longer, this stacking effect weakens. The total adsorption energy showed a linear decreasing trend, which was the same as other previous studies. When m = 12, the adsorption energy was the most negative, indicating that the chain length had the greatest influence on the aggregation behavior of surfactants; at this time, under the combined effect of steric hindrance, hydrophobic interaction, and π-π stacking between the benzene ring and the aromatic structure of anthracite, the surfactant had the densest aggregation and the adsorption strength was higher than other systems. As the length of the hydrophobic carbon chain continued to increase, the intermolecular steric hindrance was enhanced, and the π-π stacking effect was weakened, which was not conducive to the aggregation of surfactant molecules, resulting in a linear increase in the total adsorption energy. At the same time, the hydrophobic interaction between the tail chain and the anthracite continued to increase. When m = 18, the influence of the hydrophobic interaction was dominant, causing the adsorption energy to decrease again.
| Model | EV (kcal·mol−1) | EL (kcal·mol−1) | E (kcal·mol−1) |
|---|---|---|---|
| 8C-anthracite | −268.26 | −3.34 | −271.60 |
| 10C-anthracite | −290.06 | −2.47 | −290.53 |
| 12C-anthracite | −366.75 | −5.51 | −372.26 |
| 14C-anthracite | −269.37 | −3.18 | −272.55 |
| 16C-anthracite | −251.78 | −3.84 | −255.62 |
| 18C-anthracite | −266.59 | −3.79 | −270.38 |
The components of adsorption energy were further analyzed [54, 84, 87]. van der Waals interaction played a leading role in adsorption process, and electrostatic interaction was so small that it can be ignored. The range of electrostatic interaction was several nanometers [88]; although the simulation model satisfies the distance condition, due to the high degree of coalification of anthracite, low oxygen content, and low electronegativity, the charge difference between anthracite and anionic surfactant molecules was very small [89]. Therefore, the electrostatic interaction between the oppositely charged atoms was very weak.
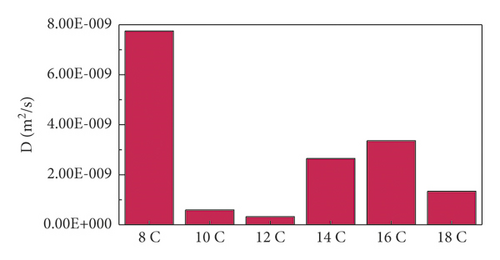
3.1.2. Diffusion Coefficient
As seen from Figure 4, the change trend of the diffusion coefficient was the same as that of the adsorption energy. The diffusion coefficient of 12C was the smallest. This was because the 12C had the strongest adsorption strength on the anthracite surface model, which limited its free diffusion ability. This result was consistent with the adsorption energy and contact surface area results. The diffusion coefficient of 8C was much greater than that of other surfactants. This was due to the shortest hydrophobic carbon chain of 8C, which led to the smallest hydrophobic interaction and the smallest intermolecular steric hindrance, resulting in the strongest free diffusion ability. This was consistent with the curvature analysis below.
3.2. Graphite Systems
Studies have found that oxygen-containing functional groups greatly affect the adsorption process [91]. In order to further explore the microscopic reasons for the highest adsorption strength of 12C on the surface of anthracite, the graphite layer was modified by hydroxyl and ketone groups, respectively.
Figure 5 shows models of the graphite layer modified by hydroxyl and ketone. The molecular structure of anthracite consists of an aromatic layer, which is very similar to the graphite flakes structure. Therefore, the surface of anthracite can be replaced by the surface of the graphite layer modified with oxygen-containing functional groups, which is more convenient and intuitive for the study of oxygen-containing functional groups [90, 92–96].
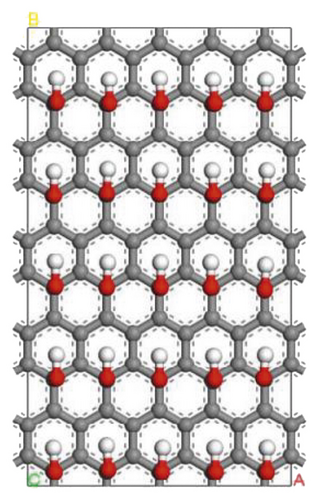
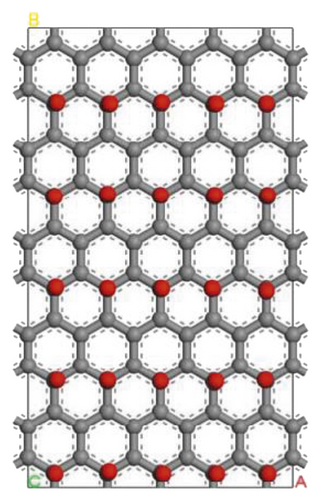
3.2.1. Adsorption Configuration
According to the construction method of the surfactant-anthracite system model in Section 2.1, 5 surfactant molecules were placed on the surface of the modified graphite layer, and the equilibrium was reached after 1000 ps molecular dynamics simulation. The final equilibrium configurations are shown in Figures 6 and 7.
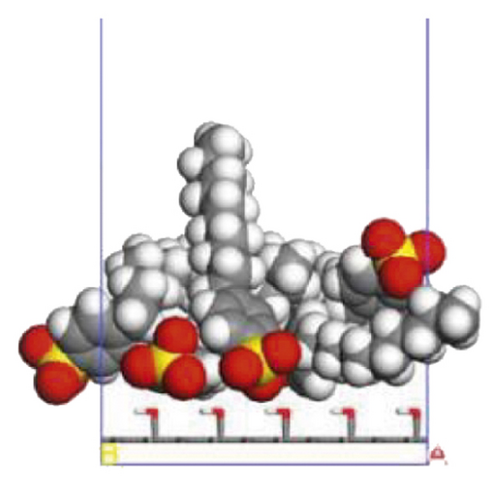
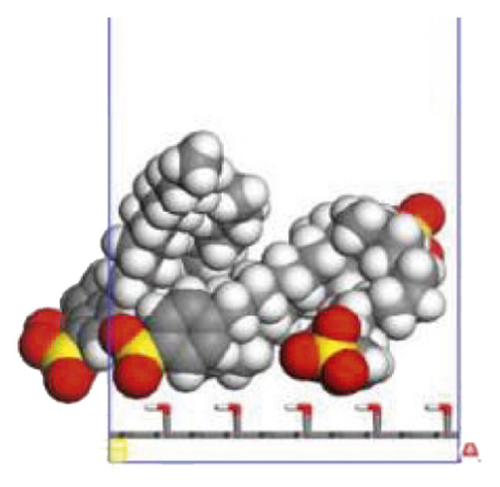

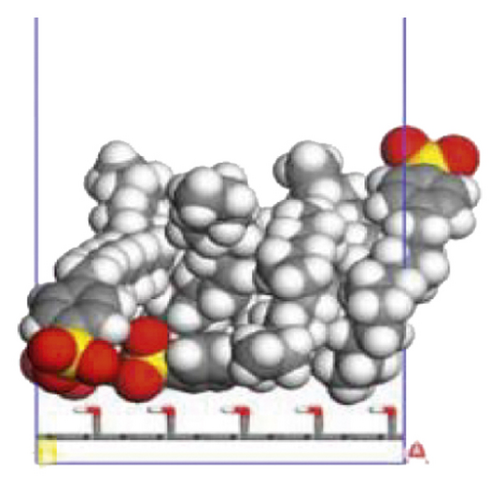

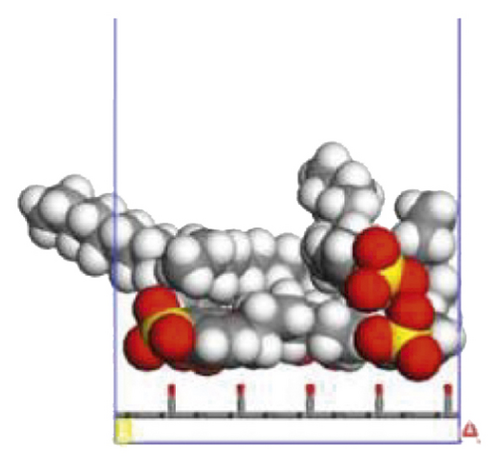
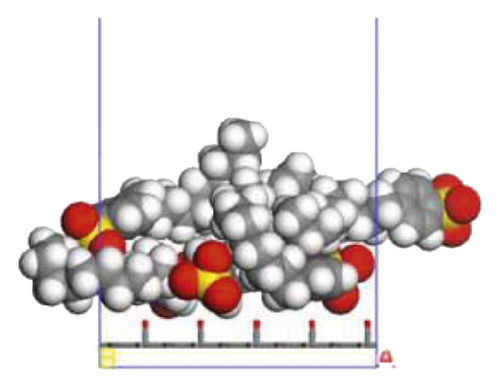
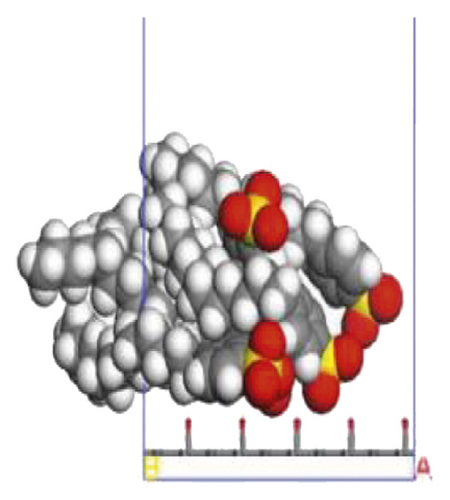
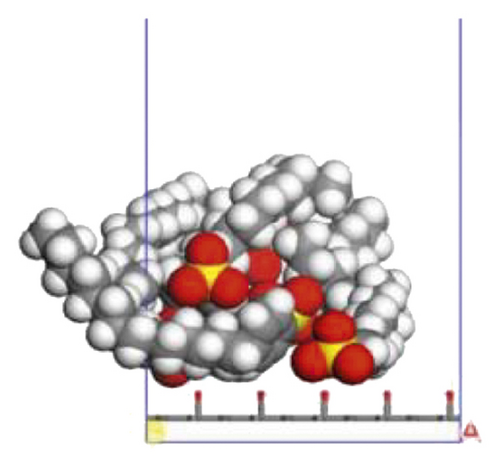
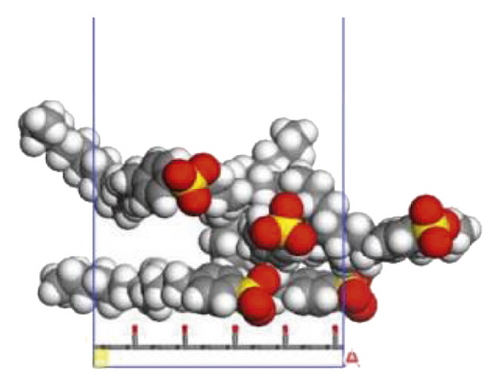
3.2.2. Radial Distribution Function
Figure 8 is the RDF between the oxygen atoms in the oxygen-containing group on the coal surface and the oxygen atoms in the surfactant.
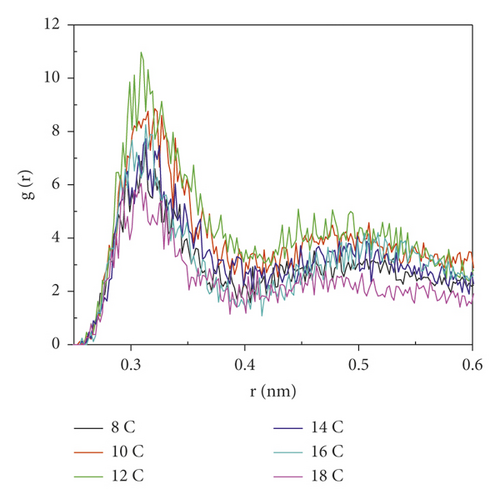
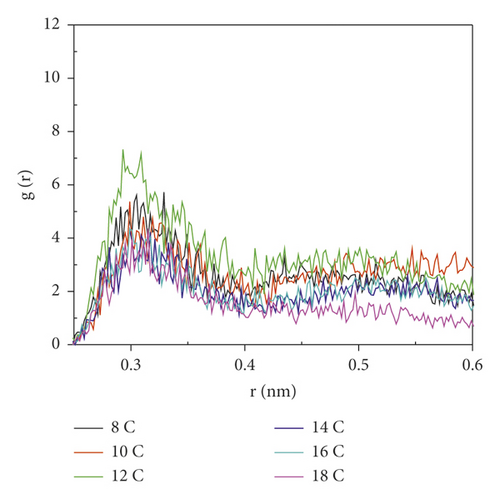
As seen from Figure 8, the RDF peak of 12C was the highest near the coal surface hydroxyl groups, indicating that the interaction between 12C and coal surface hydroxyl groups was the strongest, and 12C aggregates most densely near the hydroxyl groups. The RDF peak of 12C near the ketone group on the coal surface was much higher than that of other surfactants, indicating that 12C also had the highest degree of aggregation near the ketone group. The RDF peak of 18C was the smallest near the ketone group and the hydroxyl group. This was because the 18C carbon chain was the longest, and the hydrophobic interaction between the hydrophobic carbon chain and anthracite was the strongest. At this time, the π-π stacking effect of the benzene ring and the aromatic structure of anthracite in the surfactant was the weakest. Although the steric effect was also the strongest at this time, according to the analysis of the adsorption energy, the hydrophobic interaction in 18C was dominant; this led to better interweaving of the hydrophobic tail chain with the coal surface, which made it more difficult for the oxygen atoms in the head group to contact with the oxygen-containing functional groups.
In the formula, V is the periodic model volume and N is the total number of B atoms. Figure 9 shows the results.
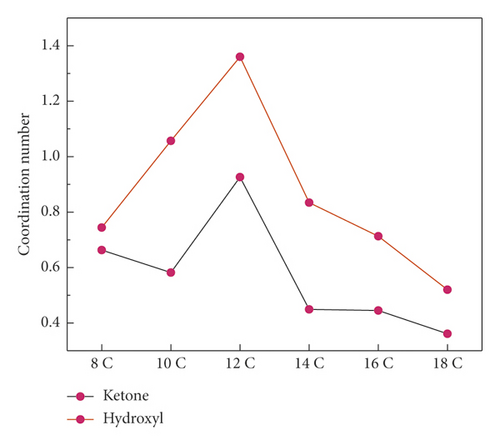
The coordination number result was consistent with the RDF analysis result, and the coordination number could reflect the result more intuitively. The coordination number of 12C near the hydroxyl group was 1.36, which was much higher than that of other surfactants, and the coordination number near the ketone group was 0.93, which was much higher than other surfactants. It showed that 12C had the highest degree of aggregation near the hydroxyl group and the ketone group. The coordination number of 18C near the hydroxyl group was 0.52, and the coordination number near the ketone group was 0.36, both of which were the lowest.
Through the abovementioned RDF and coordination number analysis, it was found that the reason for the highest degree of aggregation of 12C on the surface of anthracite was that it had the strongest ability to cover oxygen-containing functional groups on the surface of coal.
3.3. Water-Surfactant-Anthracite System
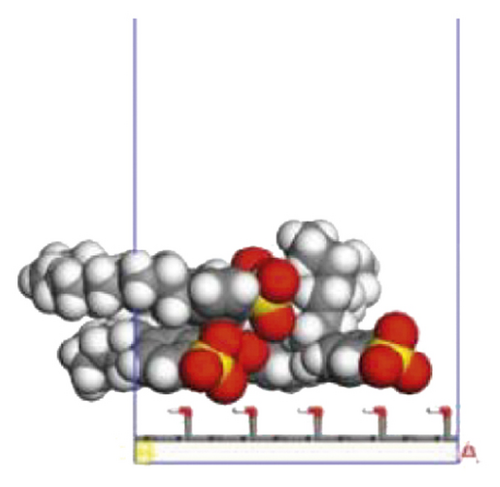
To further explore the the compactness of the layered structure and the relationship between the diffusion characteristics of surfactants in the aqueous solution, a water layer composed of 1,000 molecules was placed on the modified coal surface. After adding the water layer, the system became thermodynamically unstable. After the 1000 ps MD simulation, the system had regained equilibrium. Figure 10 shows the final conformation of the water-surfactant-anthracite system after MD simulation. After the surfactant layer covered the coal surface, it was equivalent to adding a water barrier layer [67]. The “isolation” effect made it difficult for water molecules to contact with anthracite. From Figure 10, the layered structure formed by 12C was the densest. The “isolation” ability of the layered structure to water molecules was used to evaluate the compactness of the layered structure. Furthermore, the microscopic reasons for the different degree of compactness of the layered structure were analyzed by the coordination number and RDF, the curvature of the hydrophobic tail chain, and the order parameters of the hydrophobic tail chain.
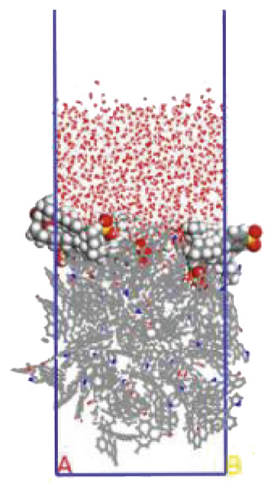
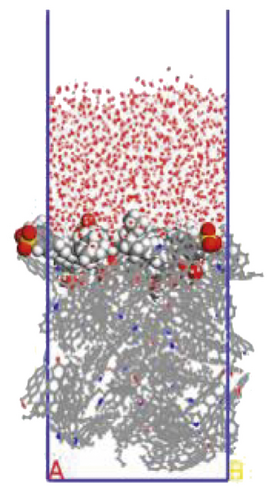
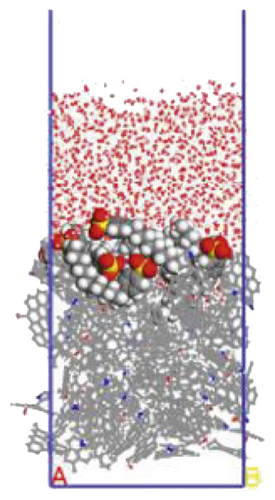
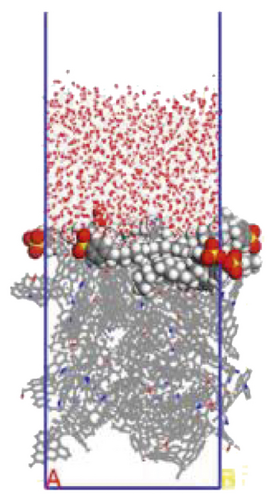
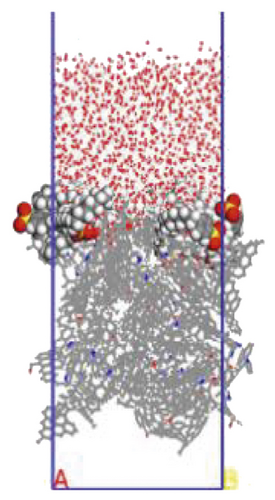
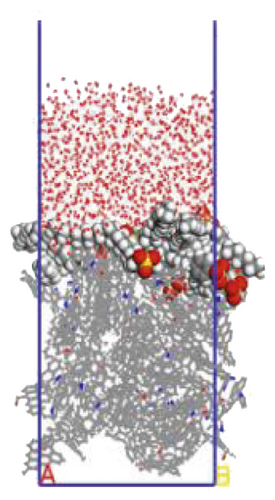
3.3.1. Radial Distribution Function
In order to determine the effect of the surfactant on the water molecules, the radial distribution function between the oxygen atoms (Ow − Ow) in the water molecules was calculated. As seen from Figure 11, the shape of the curve was not much different. Figure 11 showed that there was a peak between Ow atoms at about 0.29 nm, which was the average distance between oxygen atoms, but the peak heights were different. The RDF peak between Ow atoms in 12C was the lowest, indicating that the order of water molecules was the lowest in the system after 12C adsorption. It showed that the probability of finding Ow atoms around Ow atoms after 12C adsorption was the smallest at the same distance, indicating that the distance between Ow atoms was the largest. This was because the hydrophobic layer formed by 12C was the densest and had the strongest ability to “separate” the coal surface from the water layer, leading to more free diffusion of water molecules.
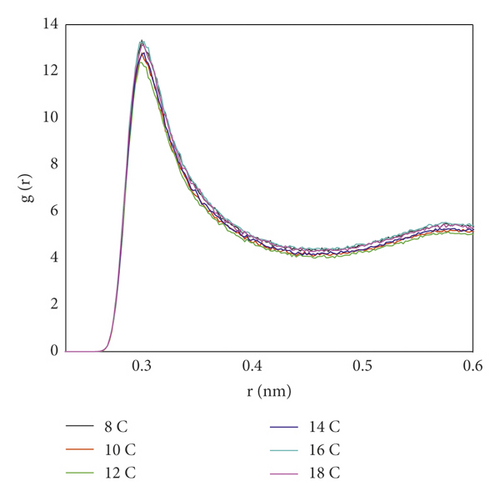
In order to compare the hydrophobic effect of the layered structure more intuitively, the coordination number between Ow was calculated, and the result is shown in Figure 12.
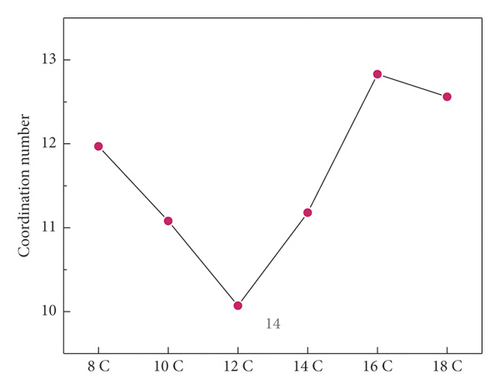
It can be seen that the coordination number of Ow after 12C adsorption was 10.07, which was the lowest among the six systems, which confirmed the above RDF conclusion. The change trend of coordination number was consistent with the above adsorption energy trend.
3.3.2. Hydrophobic Tail Order Parameters
θ was the angle between the hydrophobic tail chain and the Z axis. The order parameter could vary from −1/2 to 1. When the value of SCD was 1, the hydrophobic tail chain was arranged along the vertical direction of the interface. When the value of SCD was −1/2, the hydrophobic tail chains were arranged parallel to the interface. When SCD = 0, the tail chain distribution was isotropic [72, 106, 107]. The order parameters of different systems are shown in Figure 13.
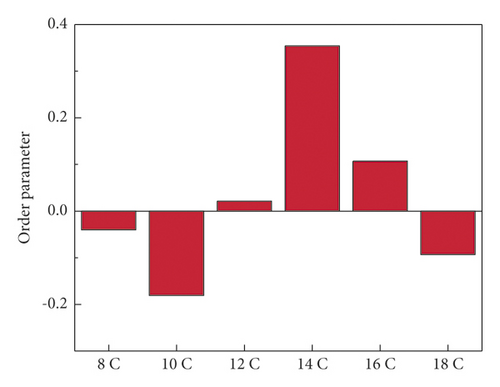
The SCD value of 12C was the closest to zero, indicating that its isotropy was the strongest. This also allowed its molecules to be combined from different directions, so that the molecules could be better interwoven together to form a denser layered structure. The SCD value of 10C was the smallest, indicating that its molecular chains tended to align along the interface; that is, the tendency to “lie flat” on the coal surface was the strongest. The SCD value of 14C was the largest, indicating that its molecular chains tended to be arranged perpendicular to the coal surface, that is, “standing” on the coal surface.
4. Conclusions
- (1)
By analyzing the adsorption energy and diffusion coefficient of the surfactant-anthracite system, it was found that the total adsorption energy of the 12C/anthracite system was the lowest; the diffusion coefficient of 12C was the smallest. It showed that 12C had the highest adsorption strength on the surface of anthracite.
- (2)
Through the analysis of coordination number and RDF in the surfactant-graphite layer system, it was found that the reason for the highest adsorption strength of 12C on the surface of anthracite was that it had the highest degree of aggregation near oxygen-containing functional groups on the surface of anthracite.
- (3)
Through the analysis of the coordination number and RDF of the water oxygen atoms of the water-surfactant-anthracite system, it was found that the layered structure formed by 12C in the solution was the densest, and the ability to “isolate” water was the strongest. After further analysis of order parameters, it was found that the 12C order parameter was the closest to zero, indicating that its isotropy was the strongest, which also allows its molecules to be combined from different directions, better intertwined, forming a denser layered structure, and better covering the coal surface.
- (4)
The systematic study of linear alkylbenzene sulfonates with different chain lengths on the surface of anthracite can better understand the influence of surfactant structure on the adsorption strength. This research fills the gap in the systematic study of the diffusion characteristics of LAS with different chain lengths on the surface of anthracite, expands the basic theory of wettability, and also provides ideas for the design and development of surfactants with new structures and new dust suppression agents.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (Grant No. 51974195).
Open Research
Data Availability
The data used to support the findings of this study are included within the article.