[Retracted] Computation of Vertex Degree-Based Molecular Descriptors of Hydrocarbon Structure
Abstract
Topological indices are such numbers or set of numbers that describe topology of structures. Nearly 400 topological indices are calculated so far. The prognostication of physical, chemical, and biological attributes of organic compounds is an important and still unsolved problem of computational chemistry. Topological index is the tool to predict the physicochemical properties such as boiling point, melting point, density, viscosity, and polarity of organic compounds. In this study, some degree-based molecular descriptors of hydrocarbon structure are calculated.
1. Introduction
The invention of graph theory in 18th century was a biggest game changer in the field of mathematics by a Swiss Mathematician Leonard Euler (1702–1782). He used graphs to tackle the famous problem of Konigsberg bridge [1, 2]. In this study, is a simple undirected graph containing a set of vertices and an edges set [3]. The number of lines connected to a vertex is called a degree of a vertex and is denoted by Degu.
Topological indices investigate the features of graphs that persist constant after continual changing in graph. They describe symmetry of chemical structures with a number and then work for the improvement of QSAR and QSPR which both are employed to build a connection among the molecular structure and mathematical tools. These indices are useful to associate physiochemical properties of compounds (such as entropy, boiling and melting point, flammability, and many more).
Topological indices are invariants of structures, so they are independent of pictorial representation [4]. Among three categories of molecular descriptors, vertex degree-based indices are considerably significant. Medicine industries are producing new and advanced medicines which are effective for mankind and ecology. Graph theory and molecular descriptors are playing a significant role in analysing the physiochemical properties of organic compounds.
Hydrocarbon structure is an aromatic hydrocarbon and a unique structure composed of benzene through covalent bond. There are six sigma and six pi bonds in each benzene ring present in this compound. It is a nonpolar structure, and each benzene has a bond angle of 120°. It can be used in making of plastic, nylon, and dyes. It cannot be dissolved in water but in organic solvents. It has a sharp melting point because of the presence of benzene but does not have high boiling point. The structure is flammable and also show resonance.
1.1. Derivation of Degree-Based Topological Indices
1.1.1. First General Zagreb Index
Classes of Zagreb Indices. We have two Zagreb groups of indices, first Zagreb index and second Zagreb index denoted by M1 and M2 [6–8]. They are proposed in late seventies by Gutman and Tranjistic.
1.1.2. First Zagreb Index
1.1.3. Second Zagreb Index
Multiple and Polynomial Zagreb Indices. In 2012, advanced forms of Zagreb descriptors were suggested, with names 1st and 2nd multiple Zagreb descriptors given as PM1(G∗) and PM2(G∗) [8]. The polynomials are helpful to calculate Zageb index. 1st and 2nd Zagreb polynomial descriptors are denoted as M1(G∗, j) and M2(G∗, j).
1.1.4. First and Second Multiple Zagreb Indices
1.1.5. First and Second Polynomial Zagreb Indices
1.1.6. Modified Zagreb Index
1.1.7. Second Modified Zagreb Index
1.1.8. Reduced 2nd Zagreb Index
1.1.9. Atom Bond Connectivity Index
1.1.10. Atom Bond Connectivity Index of 4th Order
1.1.11. General Randić Connectivity Index
1.1.12. Randić Index
1.1.13. Reciprocal Randić Index (RRI)
1.1.14. RRR Index
1.1.15. GA Index
1.1.16. GA5 Index
1.1.17. Forgotten Index
1.1.18. General Sum Connectivity Index
1.1.19. SD Index
1.1.20. Harmonic Index
2. Topological Indices of Hydrocarbon Structure
In this study, numbers of molecular descriptors of the hydrocarbon structure are computed (Figure 1).
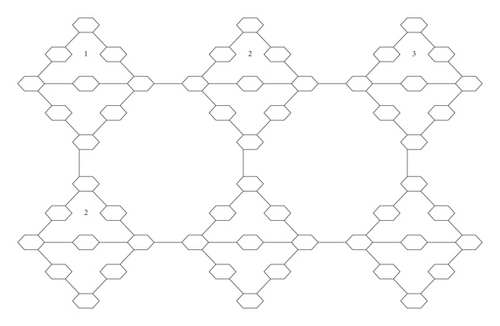
2.1. Description of Graph of Hydrocarbon Structure
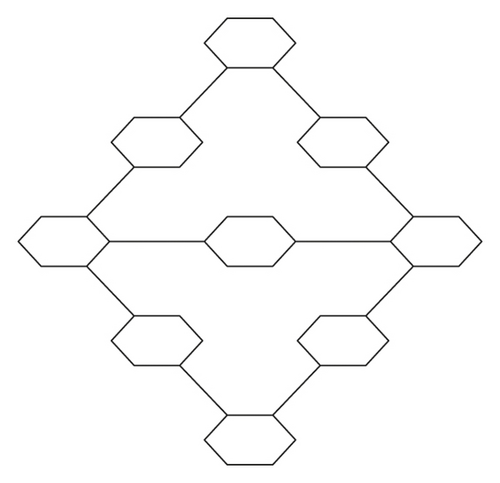
Chemical properties of graph shown in Figure 2 are given (Tables 1–4).
| Chemical formula | C54H34 |
|---|---|
| Exact mass | 682.27 |
| Molecular weight | 682.85 |
| Elemental analysis | C, 94.98; H, 5.02 |
| Degree of vertex | Number of vertices |
|---|---|
| 2 | 30pq + 2p + 2q |
| 3 | 24pq − 2p − 2q |
| Sum | 66pq − q − p |
| (Degp, Degq) where p, q εE (G) | Number of vertices |
|---|---|
| (2,2) | 112pq + 4p + 4q |
| (2,3) | 36pq − 4p − 4q |
| (3,3) | 18pq − p − q |
| Sum | 66pq − p − q |
| Degree of vertices | Number of vertices |
|---|---|
| (4, 4) | 2p |
| (4, 5) | 4p + 4q |
| (5, 5) | 12pq − 2p |
| (5, 7) | 22pq − 2p |
| (5, 8) | 2pq + 2p + 4q |
| (6, 7) | 6pq − 2p − 4q |
| (6, 8) | 6pq − 2p − 4q |
| (7, 7) | 2pq − p − q |
| (7, 8) | 8pq |
| (7, 9) | 2pq |
| (8, 8) | 2pq |
| (8, 9) | 4pq |
| Sum | 66pq − p − q |
Our concerned graph is shown in Figure 2, and it is denoted by G∗.
Theorem 1. G∗ is a graph of hydrocarbon structure, and its first general Zagreb index is given as follows:
Proof. Consider graph G∗, i.e., shown in Figure 2. G∗ has 54pq points in which 30pq + 2p + 2q of degree 2 vertices and 24pq − 2p − 2q of degree 3.
By applying the definition of Mα(G∗) (1),
Theorem 2. First Zagreb index of graph G∗ is given as follows:
Proof. of G∗ is divided into 3 groups.
-
holds 12pq + 4p + 4q arcs pq, here Degp = Degq = 2
-
has 36pq − 4p − 4q arcs pq, here Degp = 2, Degq = 3
-
contains 18pq − q − p arcs pq, here Degp = 3, Degq = 3
-
Consider
-
From equation (6), we get
Theorem 3. First and second polynomial and multiple Zagreb indices of (hydrocarbon structure) G∗ are given as follows:
- (1)
PM1(G∗) = 412 pq+4 p+4 q × 536 pq−4 p−4 q × 618 pq− q− p;
- (2)
PM2(G∗) = 412 pq+4 p+4 q × 636 pq−4 p−4 q × 918 pq− q− p;
- (3)
M1(G∗, j) = (12pq + 4p + 4q)j4 + (36pq − 4p − 4q)j5 + (18pq − p − q)j6;
- (4)
M2(G∗, j) = (12pq + 4p + 4q)j4 + (36pq − 4p − 4q)j6 + (18pq − p − q)j9;
Proof. is grouped in 3 edge partitions depending on end vertices degrees. contains 12pq + 4p + 4q edges, where Degp = Degq = 2. has 36pq − 4p − 4q edges pq, where Degp = 2 and Degq = 3. has 18pq − p − q lines pq, where Degp = 3 and Degq = 3. Consider , , and .
By utilizing the definition of PM1(G∗),
Now,
By utilizing M1(G∗, j) from (6),
From equation (7), we have
This completes the proof.
Theorem 4. Harmonic index, second Zagreb index, and reduced second Zagreb index of G∗ are as follows:
- (1)
Hyper-Zagreb index of graph G∗ is
(32) - (2)
Second Zagreb index is
(33) - (3)
Reduced 2nd Zagreb index is
(34)
Proof. is grouped in 3 partitions. holds 12pq + 4p + 4q edges, where Degp = Degq = 2. supports 36pq − 4p − 4q edges pq, where Degp = 2 and Degq = 3. keeps 18pq − q − p edges, where Degp = 3 and Degq = 3. Consider , , and .
From (8), we define HM(G∗) as
By using the definition of M2(G∗),
By substituting the values in equation (10),
With the help of (3) and (4), we have
Theorem 5. ABC index of graph hydrocarbon structure is given as follows:
Proof. G∗ encounters 66pq − p − q number of edges and 54pq vertices. Vertex count of degree 2 is 30pq + 2p + 2q and of degree 3 is 24pq − 2p − 2q. The cardinality arc group E of G∗ is 66pq − p − q. grouped into 3 disjoint arc groups, i.e., . has 12mn + 4n + 4m edges pq, where Degp = Degq = 2. supports 36pq − 4p − 4q edges pq, where Degp = 2 and Degq = 3. has 18pq − p − q arcs pq, where Degp = Degq = 3.
We use ABC(G∗) in (11) as
Theorem 6.
- (1)
ABC4(G∗) of G∗ is
(41) - (2)
GA5(G∗) is
(42)
Proof. The graph G∗ has 66pq − q − p number of edges. can be distributed into twelve disunite groups of edges.
, i = 4,5,6 … , 15. .
has 2n lines pq, where Sp = Sq = 4. supports 4p + 4q lines pq, where Sp = 4 and Sq = 5. contains 12pq − 2p edges, where Sp = Sq = 5. contains 22pq − 2p edges, where Sp = 5 and Sq = 7. keeps 2pq + 2p + 4q edges, where Sp = 5 and Sq = 8. E9(G∗) contains 6pq − 2p − 4q edges, where Sp = 6 and Sq = 7. contains 6pq − 2p − 4q edges, where Sp = 6 and Sq = 8. contains 2pq − p − q edges, where Sp = Sq = 7. holds 8pq edges, where Sp = 7 and Sq = 8. holds 2pq edges, where Sp = 7 and Sq = 9. The edge set keep 2mn edges, where Sp = Sq = 8. holds 4mn edges, here pq, where Sp = 8 and Sq = 9.
The index is defined in equation (12):
After substituting the values , we get
After simplification, we get
By utilizing the definition of GA5(G∗) from equation (18),
After substituting the values , we get
After simplification,
Theorem 7. Consider the following:
- (1)
The general Randić index of graph G∗ is given as follows:
(51) - (2)
Randić index of graph G∗ is
(52) - (3)
Reduced reciprocal Randić index of graph G∗ is
(53) - (4)
Reciprocal Randić index of graph G∗ is
(54)
Proof. G∗ encounters 66pq − p − q lines and 54pq vertices. Vertices of degree 2 are 30pq + 2p + 2q and of degree 3 are 24pq − 2p − 2q. The cardinality of of G∗ is 66pq − p − q. is divided into 3 dissociate edge groups that rely on the degrees of the end points, i.e., . has 12pq + 4p + 4q edges pq, where Degp = Degq = 2. has 36pq − 4p − 4q edges pq, where Degp = 2 and Degq = 3. has 18pq − p − q arcs pq, where Degp = Degq = 3.
We use general Randic index in (13) as
Now, we have
After simplification, we get
By the use of Randić index (14),
After simplification,
Definition of RRR(G∗) index from equation (16) is
Now, by utilizing the definition of reduced Randić index from equation (15),
Theorem 8. We have graph G∗ and its different indices are explained here.
- (1)
GA index is as follows:
(62) - (2)
Sum connectivity index is given as follows:
(63) - (3)
Forgotten index of G∗ is
(64)
Proof. G∗ encounters 66pq − p − q edges and 54pq vertices. Vertex count of degree 2 are 30pq + 2p + 2q and of degree 3 are 24pq − 2p − 2q. The cardinality line group of G∗ is 66pq − p − q. E(G∗) is classified into three disjoint edge groups, i.e., . has 12pq + 4p + 4q edges pq, where Degp = Degq = 2. has 36pq − 4p − 4q edges pq, where Degp = 2 and Degq = 3. has 18pq − p − q edges pq, where Degp = Degq = 3.
We use geometric arithmetic index in (17) as
After simplification, we obtain
From equation (20), we get
We use forgotten index in (19) as
Theorem 9. Let G∗ be graph:
- (1)
Symmetric division index is
(69) - (2)
Harmonic index is
(70)
Proof. G∗ encounters 66pq − p − q edge and 54pq points. Vertex counts of degree 2 are 30pq + 2p + 2q and of degree 3 are 24pq − 2p − 2q. The edge set E(G∗) splits into three distinct line groups.
has 12pq + 4p + 4q lines pq, where Degp = Degq = 2. has 36pq − 4p − 4q lines pq, where Degp = 2 and Degq = 3. has 18pq − p − q lines pq, where Degp = Degq = 3.
From equation (21), we obtain
From equation (22),
3. Conclusion
We find some topological descriptors of hydrocarbon structure. Randić index has well known applications in the study of physicochemical characteristics of alkane, for example, surface area, enthalpy of formation, boiling point, and melting point. The index has the most functional role in pharmacology. Similarly, forgotten index is helpful to figure out the strength of organic structures. Symmetric division index has productive applications to find heat formation of chemical structures. GA index can forecast biological activities of compounds. ABC index has an outstanding role in finding strain energy and stability of isoparaffins. Our results will be helpful to estimate the physicochemical properties of hydrocarbon structures.
4. Future Work
In future, we will find the distance-based and spectrum-based topological indices of hydrocarbon structures.
Conflicts of Interest
The authors declares that there are no conflicts of interest.
Acknowledgments
This project was supported by the Jahangirnagar University, Savar, Dhaka, Bangladesh.
Open Research
Data Availability
No data were used to support this study.




