Mechanism of the Treatment of Irritable Bowel Syndrome with Sini Powder and Tong Xie Yao Fang Decoction Based on Network Pharmacology
Abstract
This study used a network pharmacology approach to investigate the potential active ingredients of Sini Powder and Tong xie yao fang decoction and the underlying mechanisms in irritable bowel syndrome (IBS) treatment. The potential active ingredients of Sini Powder and Tong xie yao fang decoction were obtained from TCMSP databases, and the potential targets of the active ingredients were predicted and analyzed by using the Swiss Target Prediction database. T Genecard, DisGeNET, and OMIM databases were processed to screen the potential therapeutic targets in IBS. The interaction of overlapped candidates between the potential biotarget of herb extracts and the potential therapeutic target of IBS were analyzed by STRING website and visualized by the Cytoscape V3.8.0 software. Gene ontology (GO) analysis and Kyoto Genomics and Genomics Encyclopedia (KEGG) pathway were processed to categorize and map the potential biofunctions and effects of these candidates by using David database. Result. There were 139 predicted active components and 248 related biotargets of Sini Powder and Tong xie yao fang decoction which were involved in IBS treatment, and 522 annotations and 101 related pathways are obtained by enrichment analysis (P < 0.01, FDR < 0.05). The underlying mechanisms of Sini Powder and Tong xie yao fang decoction may be related to neuroactive ligand-receptor interaction, calcium, cAMP, and HIF-1 signaling pathways. In conclusion, our results showed that the effect and mechanism of Sini Powder and Tong xie yao fang decoction in IBS treatment were in multi-ingredient, multitargets and multipathways, which would provide several potential and promising strategies for the further research and development of Sini Powder and Tong xie yao fang decoction on IBS treatment.
1. Introduction
Irritable bowel syndrome (IBS) is a dysfunctional gastrointestinal disease, which is characterized by recurrent abdominal pain, and associated with abnormal fecal form and/or abnormal frequency of defecation [1, 2]. The nationwide prevalence of Chinese IBS is 1.4%–11.5% in 2020 [3]. The high-risk factors of IBS at least including high fat, high bio-amine, and high carbohydrate diets, or intestinal infection [4, 5]. IBS patients usually suffered from anxiety, depression, and physical discomforts, which largely reduced their life quality [6]. Up to date, the pathophysiological mechanism of IBS remains as a complex context, accumulating evidence have been shown that visceral hypersensitivity, mental disorder, gut dysbiosis, gastrointestinal motility disorders, and intestinal low-grade inflammation contributed to dysfunction of gut-to-brain axis, which in turn played important roles in IBS initiation [7–11]. The therapeutic goals of IBS have so far been confined to ameliorate syndromes and improve patients’ life quality. Clinically, IBS treatments mainly includes diet, lifestyle, medical, mental, and behavioral interventions, besides, individualized management is more important and required [3, 12].
Traditional Chinese medicine has showed promising advantages in IBS treatment [13, 14]. Traditional Chinese medicine theory holds that liver stagnation and spleen deficiency play important roles in pathogeneses of IBS [15]. The combination of Sini Powder and Tong xie yao fang decoction has proven effective and been reported fewer adverse reactions in IBS treatment [16, 17]. Sini powder is derived from the treatise on febrile diseases and is composed of Chinese thorowax root, Radix Paeoniae Alba, trifoliate orange (Fructus Aurantii Immaturus), and Chinese licorice (Glycyrrhiza uralensis). Tong xie yao fang decoction is derived from Danxi’s Mastery of Medicine and is composed of Rhizoma Atractylodis, Radix Paeoniae Alba, Citri Reticulatae Pericarpium (Chenpi), and Saposhnikovia divaricate root. Both Sini powder and Tong xie yao fang decoction are based on the principle of reconciling the function of liver and spleen. The combination of two formulas can disperse the stagnated main and collateral channels in liver and spleen, which in turn to restore their normal function to ameliorate diarrhea, abdominal pain, and abdominal distension symptoms in IBS patients [16, 17]. However, the potential active ingredients of Sini powder and Tong xie yao fang decoction and the underlying mechanisms remain largely elusive.
In the present study, we explored the effective components, and then screened the hub targets and the relevant signaling pathways of Sini powder and Tong xie yao fang decoction based on network pharmacology analysis. Thus, our results would provide a theoretical reference for the study of its pharmacological mechanism and its clinical application.
2. Materials and Methods
2.1. Screening of Active Ingredients of Sini Powder and Tong xie yao fang Decoction and Target Prediction
The traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP) (https://tcmspw.com/) was used to retrieve Chinese medicines (Chinese thorowax root, Radix Paeoniae Alba, trifoliate orange Fructus aurantii Immaturus), and Chinese licorice (Glycyrrhiza uralensis), Rhizoma Atractylodis, Citri Reticulatae Pericarpium (Chenpi), and Saposhnikovia divaricata root, which are components of Sini powder and Tong xie yao fang decoction. The active components of these Chinese medicines were collected and screened based on oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18. The SDFs (structure data files) of the molecular structure of potential active components were obtained through the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), and their potential targets were predicted using the Swiss Target Prediction database (https://www.swisstargetprediction.ch/). Their potential targets were finally subjected to gene standardization through the UniProt database (https://www.UniProt.org/).
2.2. Prediction of IBS-Related Targets
Using “irritable bowel syndrome” as the keyword, searches, and screening were conducted using disease gene databases such as DrugBank (https://www.drugbank.ca/), DisGeNET (https://www.disgenet.org/), Genecards (https://www.genecards.org), OMIM (https://www.omim.org/), and TTD (https://db.idrblab.net/ttd/). The obtained target genes were introduced into the UniProt database for gene standardization.
2.3. Construction of the Active Ingredient-Target Network of the Drugs
The targets obtained in the steps described in “1.1” and “1.2” were imported into the Venny 2.1 website to create a Venn diagram, and the common targets of Sini powder and Tong xie yao fang decoction for IBS were obtained. Cytoscape 3.8.0 software was used to construct a drug active ingredient-potential therapeutic target network. The degree of node connectivity represents the number of edges connected to a certain point. The greater the value is, the greater the importance of the point in the topological structure. The degree of node connectivity was calculated using Cytoscape software, and the important effective ingredients and targets of Sini powder and Tong xie yao fang decoction in the treatment of IBS were clarified.
2.4. Construction of the Protein Interaction Network (PPI)
The common targets obtained with the procedure described in “1.3” were imported into the STRING database (https://string-db.org/), the species “homo sapiens” was selected, and the threshold was set to >0.7. Free targets were hidden to obtain PPI networks. The data were imported into Cytoscape 3.8.0 software for visualization, and the core targets in the PPI network were obtained. The Molecular Complex Detection (MCODE) plug-in in Cytoscape 3.8.0 software was used to perform a modular analysis of the core targets of the PPI network. Degree cutoff = 2, degree cutoff > 2, node score cutoff = 0.2, K-Core = 2, and Max.Depth = 100 were set, and the features of the targets were analyzed to select core target modules.
2.5. Functional Annotation of Common Target Genes Using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
The common targets obtained in step “1.3” were imported into the DAVID 6.8 database (https://david.ncifcrf.gov/) for GO and KEGG analysis. The screening conditions were set as P < 0.01 and FDR < 0.05. The top 20 enriched results were displayed, the results were visualized using the Image GP tool, and the analysis results were displayed in the form of bubble graphs.
3. Results
3.1. Screening of Active Ingredients of Sini Powder and Tong Xie and Target Prediction
According to the OB and DL parameters and after excluding the compounds that were not included in PubChem or for which the relevant target could not be predicted by the Swiss Target, the following active ingredients were screened from the TCMSP database: 14 Chinese thorowax root (including 3 duplicate compounds), 11 Radix Paeoniae Alba (including 4 duplicate compounds), 20 Fructus aurantii Immaturus (including 4 duplicate compounds), 87 Chinese licorice (including 6 duplicate compounds), 3 Rhizoma Atractylodis, 14 Divaricate Saposhnikovia root (including 3 duplicate compounds), and 4 Citri Reticulatae Pericarpium (including 3 duplicate compounds). A total of 139 active ingredients were finally obtained (Table 1). Chinese thorowax root, Radix Paeoniae Alba, Fructus aurantii Immaturus, Chinese licorice, Rhizoma Atractylodis, Divaricate Saposhnikovia root, and Citri Reticulatae Pericarpium are referred to as BR, PRA, AFI, GRER, AMR, SR, and CRP, respectively. The abovementioned 139 active ingredients were retrieved from the PubChem website and the Swiss Target Prediction database to obtain 9,846 targets. After screening and the removal of duplications, a total of 960 targets were obtained.
| Herb | MOL ID | Molecule name | ID | OB% | DL |
|---|---|---|---|---|---|
| Radix bupleuri | MOL001645 | Linoleyl acetate | BR1 | 42.1 | 0.2 |
| Radix bupleuri | MOL002776 | Baicalin | BR2 | 40.12 | 0.75 |
| Radix bupleuri | MOL000449 | Stigmasterol | BR3 | 43.83 | 0.76 |
| Radix bupleuri | MOL000354 | Isorhamnetin | A1 | 49.6 | 0.31 |
| Radix bupleuri | MOL000422 | Kaempferol | B1 | 41.88 | 0.24 |
| Radix bupleuri | MOL004598 | 3, 5, 6, 7-tetramethoxy-2-(3, 4, 5-trimethoxyphenyl) chromone | BR4 | 31.97 | 0.59 |
| Radix bupleuri | MOL004609 | Areapillin | BR5 | 48.96 | 0.41 |
| Radix bupleuri | MOL013187 | Cubebin | BR6 | 57.13 | 0.64 |
| Radix bupleuri | MOL004644 | Sainfuran | BR7 | 79.91 | 0.23 |
| Radix bupleuri | MOL004653 | (+)-anomalin | BR8 | 46.06 | 0.66 |
| Radix bupleuri | MOL004702 | Saikosaponin c_qt | BR9 | 30.5 | 0.63 |
| Radix bupleuri | MOL004718 | α-spinasterol | BR10 | 42.98 | 0.76 |
| Radix bupleuri | MOL000490 | Petunidin | BR11 | 30.05 | 0.31 |
| Radix bupleuri | MOL000098 | Quercetin | A2 | 46.43 | 0.28 |
| White peony root | MOL001918 | Paeoniflorgenone | PRA1 | 87.59 | 0.37 |
| White peony root | MOL001919 | (3S, 5R, 8R, 9R, 10S, 14S)-3, 17-dihydroxy-4, 4, 8, 10, 14-pentamethyl-2, 3, 5, 6, 7, 9-hexahydro-1H-cyclopenta[a]phenanthrene-15, 16-dione | PRA2 | 43.56 | 0.53 |
| White peony root | MOL001921 | Lactiflorin | PRA3 | 49.12 | 0.8 |
| White peony root | MOL001924 | Paeoniflorin | PRA4 | 53.87 | 0.79 |
| White peony root | MOL001925 | Paeoniflorin_qt | PRA5 | 68.18 | 0.4 |
| White peony root | MOL001928 | Albiflorin_qt | PRA6 | 66.64 | 0.33 |
| White peony root | MOL001930 | Benzoyl paeoniflorin | PRA7 | 31.27 | 0.75 |
| White peony root | MOL000211 | Mairin | A7 | 55.38 | 0.78 |
| White peony root | MOL000358 | Beta-sitosterol | A6 | 36.91 | 0.75 |
| White peony root | MOL000359 | Sitosterol | C | 36.91 | 0.75 |
| White peony root | MOL000422 | Kaempferol | B1 | 41.88 | 0.24 |
| Fructus aurantii immaturus | MOL013276 | Poncirin | AFI1 | 36.55 | 0.74 |
| Fructus aurantii immaturus | MOL013277 | Isosinensetin | AFI2 | 51.15 | 0.44 |
| Fructus aurantii immaturus | MOL013279 | 5, 7, 4′-trimethylapigenin | AFI3 | 39.83 | 0.3 |
| Fructus aurantii immaturus | MOL013428 | Isosakuranetin-7-rutinoside | AFI4 | 41.24 | 0.72 |
| Fructus aurantii immaturus | MOL013430 | Prangenin | AFI5 | 43.6 | 0.29 |
| Fructus aurantii immaturus | MOL013435 | Poncimarin | AFI6 | 63.62 | 0.35 |
| Fructus aurantii immaturus | MOL013436 | Isoponcimarin | AFI7 | 63.28 | 0.31 |
| Fructus aurantii immaturus | MOL013437 | 6-methoxy aurapten | AFI8 | 31.24 | 0.3 |
| Fructus aurantii immaturus | MOL001798 | Neohesperidin_qt | AFI9 | 71.17 | 0.27 |
| Fructus aurantii immaturus | MOL001803 | Sinensetin | AFI10 | 50.56 | 0.45 |
| Fructus aurantii immaturus | MOL001941 | Ammidin | A5 | 34.55 | 0.22 |
| Fructus aurantii immaturus | MOL013352 | Obacunone | AFI11 | 43.29 | 0.77 |
| Fructus aurantii immaturus | MOL002914 | Eriodyctiol (flavanone) | AFI12 | 41.35 | 0.24 |
| Fructus aurantii immaturus | MOL004328 | Naringenin | B2 | 59.29 | 0.21 |
| Fructus aurantii immaturus | MOL005100 | 5, 7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl) chroman-4-one | A3 | 47.74 | 0.27 |
| Fructus aurantii immaturus | MOL005828 | Nobiletin | A4 | 61.67 | 0.52 |
| Fructus aurantii immaturus | MOL005849 | Didymin | AFI13 | 38.55 | 0.24 |
| Fructus aurantii immaturus | MOL000006 | Luteolin | AFI14 | 36.16 | 0.25 |
| Fructus aurantii immaturus | MOL007879 | Tetramethoxyluteolin | AFI15 | 43.68 | 0.37 |
| Fructus aurantii immaturus | MOL009053 | 4-((2S, 3R)-5-((E)-3-hydroxyprop-1-enyl)-7-methoxy-3-methylol-2, 3-dihydrobenzofuran-2-yl)-2-methoxy-phenol | AFI16 | 50.76 | 0.39 |
| Licorice | MOL001484 | Inermine | GRER1 | 75.18 | 0.54 |
| Licorice | MOL001792 | DFV | GRER2 | 32.76 | 0.18 |
| Llicorice | MOL000211 | Mairin | A7 | 55.38 | 0.78 |
| Licorice | MOL002311 | Glycyrol | GRER3 | 90.78 | 0.67 |
| Licorice | MOL000239 | Jaranol | GRER4 | 50.83 | 0.29 |
| Licorice | MOL002565 | Medicarpin | GRER5 | 49.22 | 0.34 |
| Licorice | MOL000354 | Isorhamnetin | A1 | 49.6 | 0.31 |
| Licorice | MOL000359 | Sitosterol | C | 36.91 | 0.75 |
| Licorice | MOL003656 | Lupiwighteone | GRER6 | 51.64 | 0.37 |
| Licorice | MOL003896 | 7-methoxy-2-methyl isoflavone | GRER7 | 42.56 | 0.2 |
| Licorice | MOL000392 | Formononetin | GRER8 | 69.67 | 0.21 |
| Licorice | MOL000417 | Calycosin | GRER9 | 47.75 | 0.24 |
| Licorice | MOL000422 | Kaempferol | B1 | 41.88 | 0.24 |
| Licorice | MOL004328 | Naringenin | B2 | 59.29 | 0.21 |
| Licorice | MOL004805 | (2S)-2-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-8, 8-dimethyl-2, 3-dihydropyrano[2, 3-f]chromen-4-one | GRER10 | 31.79 | 0.72 |
| Licorice | MOL004808 | Glyasperin B | GRER11 | 65.22 | 0.44 |
| Licorice | MOL004810 | Glyasperin F | GRER12 | 75.84 | 0.54 |
| Licorice | MOL004811 | Glyasperin C | GRER13 | 45.56 | 0.4 |
| Licorice | MOL004814 | Isotrifoliol | GRER14 | 31.94 | 0.42 |
| Licorice | MOL004815 | (E)-1-(2, 4-dihydroxyphenyl)-3-(2, 2-dimethylchromen-6-yl)prop-2-en-1-one | GRER15 | 39.62 | 0.35 |
| Licorice | MOL004820 | Kanzonols W | GRER16 | 50.48 | 0.52 |
| Licorice | MOL004824 | (2S)-6-(2, 4-dihydroxyphenyl)-2-(2-hydroxypropan-2-yl)-4-methoxy-2, 3-dihydrofuro(3, 2-g)chromen-7-one | GRER17 | 60.25 | 0.63 |
| Licorice | MOL004827 | Semilicoisoflavone B | GRER18 | 48.78 | 0.55 |
| Licorice | MOL004828 | Glepidotin A | GRER19 | 44.72 | 0.35 |
| Licorice | MOL004833 | Phaseolinisoflavan | GRER20 | 32.01 | 0.45 |
| Licorice | MOL004835 | Glypallichalcone | GRER21 | 61.6 | 0.19 |
| Licorice | MOL004838 | 8-(6-hydroxy-2-benzofuranyl)-2, 2-dimethyl-5-chromenol | GRER22 | 58.44 | 0.38 |
| Licorice | MOL004841 | Licochalcone B | GRER23 | 76.76 | 0.19 |
| Licorice | MOL004848 | Licochalcone G | GRER24 | 49.25 | 0.32 |
| Licorice | MOL004849 | 3-(2, 4-dihydroxyphenyl)-8-(1, 1-dimethylprop-2-enyl)-7-hydroxy-5-methoxy-coumarin | GRER25 | 59.62 | 0.43 |
| Licorice | MOL004855 | Licoricone | GRER26 | 63.58 | 0.47 |
| Licorice | MOL004856 | Gancaonin A | GRER27 | 51.08 | 0.4 |
| Licorice | MOL004857 | Gancaonin B | GRER28 | 48.79 | 0.45 |
| Licorice | MOL004860 | Licorice glycoside E | GRER29 | 32.89 | 0.27 |
| Licorice | MOL004863 | 3-(3, 4-dihydroxyphenyl)-5, 7-dihydroxy-8-(3-methylbut-2-enyl)chromone | GRER30 | 66.37 | 0.41 |
| Licorice | MOL004864 | 5, 7-dihydroxy-3-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)chromone | GRER31 | 30.49 | 0.41 |
| Licorice | MOL004866 | 2-(3, 4-dihydroxyphenyl)-5, 7-dihydroxy-6-(3-methylbut-2-enyl)chromone | GRER32 | 44.15 | 0.41 |
| Licorice | MOL004879 | Glycyrin | GRER33 | 52.61 | 0.47 |
| Licorice | MOL004882 | Licocoumarone | GRER34 | 33.21 | 0.36 |
| Licorice | MOL004883 | Licoisoflavone | GRER35 | 41.61 | 0.42 |
| Licorice | MOL004884 | Licoisoflavone B | GRER36 | 38.93 | 0.55 |
| Licorice | MOL004885 | Licoisoflavanone | GRER37 | 52.47 | 0.54 |
| Licorice | MOL004891 | Shinpterocarpin | GRER38 | 80.3 | 0.73 |
| Licorice | MOL004898 | (E)-3-(3, 4-dihydroxy-5-(3-methylbut-2-enyl)phenyl)-1-(2, 4-dihydroxyphenyl)prop-2-en-1-one | GRER39 | 46.27 | 0.31 |
| Licorice | MOL004903 | Liquiritin | GRER40 | 65.69 | 0.74 |
| Licorice | MOL004904 | Licopyranocoumarin | GRER41 | 80.36 | 0.65 |
| Licorice | MOL004905 | 3, 22-dihydroxy-11-oxo-delta(12)-oleanene-27-alpha-methoxycarbonyl-29-oic acid | GRER42 | 34.32 | 0.55 |
| Licorice | MOL004907 | Glyzaglabrin | GRER43 | 61.07 | 0.35 |
| Licorice | MOL004908 | Glabridin | GRER44 | 53.25 | 0.47 |
| Licorice | MOL004910 | Glabranin | GRER45 | 52.9 | 0.31 |
| Licorice | MOL004911 | Glabrene | GRER46 | 46.27 | 0.44 |
| Licorice | MOL004912 | Glabrone | GRER47 | 52.51 | 0.5 |
| Licorice | MOL004913 | 1, 3-dihydroxy-9-methoxy-6-benzofurano(3, 2-c)chromenone | GRER48 | 48.14 | 0.43 |
| Licorice | MOL004914 | 1, 3-dihydroxy-8, 9-dimethoxy-6-benzofurano(3, 2-c)chromenone | GRER49 | 62.9 | 0.53 |
| Licorice | MOL004915 | Eurycarpin A | GRER50 | 43.28 | 0.37 |
| Licorice | MOL004917 | Glycyroside | GRER51 | 37.25 | 0.79 |
| Licorice | MOL004924 | (-)-medicocarpin | GRER52 | 40.99 | 0.95 |
| Licorice | MOL004935 | Sigmoidin-B | GRER53 | 34.88 | 0.41 |
| Licorice | MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one | GRER54 | 71.12 | 0.18 |
| Licorice | MOL004945 | (2S)-7-hydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-enyl)chroman-4-one | GRER55 | 36.57 | 0.32 |
| Licorice | MOL004948 | Isoglycyrol | GRER56 | 44.7 | 0.84 |
| Licorice | MOL004949 | Isolicoflavonol | GRER57 | 45.17 | 0.42 |
| Licorice | MOL004959 | 1-methoxyphaseollidin | GRER58 | 69.98 | 0.64 |
| Licorice | MOL004961 | Quercetin der. | GRER59 | 46.45 | 0.33 |
| Licorice | MOL000497 | Licochalcone a | GRER60 | 40.79 | 0.29 |
| Licorice | MOL004974 | 3′-methoxyglabridin | GRER61 | 46.16 | 0.57 |
| Licorice | MOL004978 | 2-((3R)-8, 8-dimethyl-3, 4-dihydro-2H-pyrano(6, 5-f)chromen-3-yl)-5-methoxyphenol | GRER62 | 36.21 | 0.52 |
| Licorice | MOL004980 | Inflacoumarin A | GRER63 | 39.71 | 0.33 |
| Licorice | MOL004985 | Icos-5-enoic acid | GRER64 | 30.7 | 0.2 |
| Licorice | MOL004988 | Kanzonol F | GRER65 | 32.47 | 0.89 |
| Licorice | MOL004989 | 6-prenylated eriodictyol | GRER66 | 39.22 | 0.41 |
| Licorice | MOL004990 | 7, 2′, 4′-trihydroxy–5-methoxy-3–arylcoumarin | GRER67 | 83.71 | 0.27 |
| Licorice | MOL004991 | 7-acetoxy-2-methylisoflavone | GRER68 | 38.92 | 0.26 |
| Licorice | MOL004993 | 8-prenylated eriodictyol | GRER69 | 53.79 | 0.4 |
| Licorice | MOL004996 | Gadelaidic acid | GRER70 | 30.7 | 0.2 |
| Licorice | MOL000500 | Vestitol | GRER71 | 74.66 | 0.21 |
| Licorice | MOL005000 | Gancaonin G | GRER72 | 60.44 | 0.39 |
| Licorice | MOL005001 | Gancaonin H | GRER73 | 50.1 | 0.78 |
| Licorice | MOL005003 | Licoagrocarpin | GRER74 | 58.81 | 0.58 |
| Licorice | MOL005007 | Glyasperins M | GRER75 | 72.67 | 0.59 |
| Licorice | MOL005008 | Glycyrrhiza flavonol A | GRER76 | 41.28 | 0.6 |
| Licorice | MOL005012 | Licoagroisoflavone | GRER77 | 57.28 | 0.49 |
| Licorice | MOL005013 | 18α-hydroxyglycyrrhetic acid | GRER78 | 41.16 | 0.71 |
| Licorice | MOL005016 | Odoratin | GRER79 | 49.95 | 0.3 |
| Licorice | MOL005017 | Phaseol | GRER80 | 78.77 | 0.58 |
| Licorice | MOL005018 | Xambioona | GRER81 | 54.85 | 0.87 |
| Licorice | MOL000098 | Quercetin | A2 | 46.43 | 0.28 |
| Rhizoma atractylodis macrocephalae | MOL000028 | α-amyrin | AMR1 | 39.51 | 0.76 |
| Rhizoma atractylodis macrocephalae | MOL000033 | (3S, 8S, 9S, 10R, 13R, 14S, 17R)-10, 13-dimethyl-17-((2R, 5S)-5-propan-2-yloctan-2-yl)-2, 3, 4, 7, 8, 9, 11, 12, 14, 15, 16, 17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | AMR2 | 36.23 | 0.78 |
| Rhizoma atractylodis macrocephalae | MOL000072 | 8β-ethoxy atractylenolide III | AMR3 | 35.95 | 0.21 |
| Radix saposhnikoviae | MOL011732 | Anomalin | SR1 | 59.65 | 0.66 |
| Radix saposhnikoviae | MOL011737 | Divaricatacid | SR2 | 87 | 0.32 |
| Radix saposhnikoviae | MOL011740 | Divaricatol | SR3 | 31.65 | 0.38 |
| Radix saposhnikoviae | MOL001941 | Ammidin | A5 | 34.55 | 0.22 |
| Radix saposhnikoviae | MOL011747 | Ledebouriellol | SR4 | 32.05 | 0.51 |
| Radix saposhnikoviae | MOL002644 | Phellopterin | SR5 | 40.19 | 0.28 |
| Radix saposhnikoviae | MOL000359 | Sitosterol | C | 36.91 | 0.75 |
| Radix saposhnikoviae | MOL000173 | Wogonin | SR6 | 30.68 | 0.23 |
| Radix saposhnikoviae | MOL000358 | Beta-sitosterol | A6 | 36.91 | 0.75 |
| Radix saposhnikoviae | MOL001494 | Mandenol | SR7 | 42 | 0.19 |
| Radix saposhnikoviae | MOL001942 | Isoimperatorin | SR8 | 45.46 | 0.23 |
| Radix saposhnikoviae | MOL003588 | Prangenidin | SR9 | 36.31 | 0.22 |
| Radix saposhnikoviae | MOL007514 | Methyl icosa-11, 14-dienoate | SR10 | 39.67 | 0.23 |
| Radix saposhnikoviae | MOL013077 | Decursin | SR11 | 39.27 | 0.38 |
| Orange peel | MOL000359 | Sitosterol | C | 36.91 | 0.75 |
| Orange peel | MOL004328 | Naringenin | B2 | 59.29 | 0.21 |
| Orange peel | MOL005100 | 5, 7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one | A3 | 47.74 | 0.27 |
| Orange peel | MOL005828 | Nobiletin | A4 | 61.67 | 0.52 |
3.2. Construction of the Active Ingredient-Target Network of the Drugs
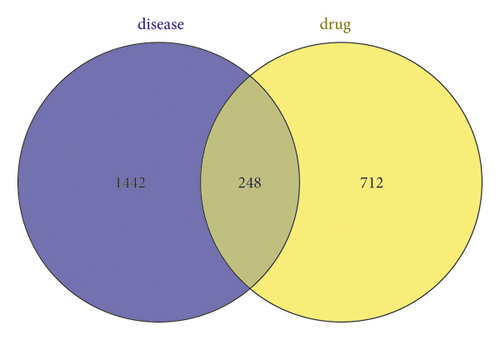
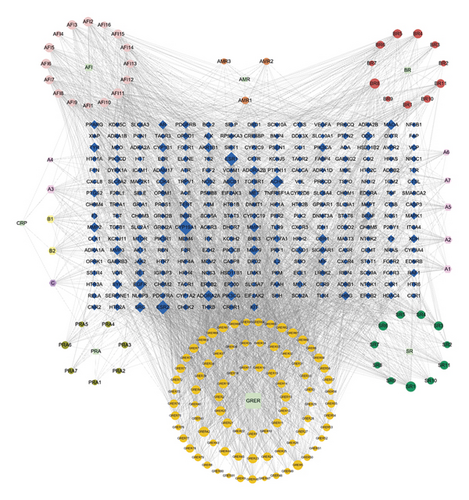
Five disease databases—DrugBank, DisGeNET, Genecards, OMIM, and TTD—were searched, and 57, 429, 768, 623, and 27 of target sites were obtained, respectively. After summarizing the abovementioned targets and removing duplicate targets, 1,690 disease targets were finally obtained. A total of 248 common targets were obtained by taking the intersection of the targets obtained in the steps described in sections “2.1” and “2.2” (Figure 1(a)). Cytoscape 3.8.0 software was used to construct a network diagram of traditional Chinese medicine-active ingredient-potential therapeutic targets (Figure 1(b)). The diagram contains a total of 394 nodes and 3,313 edges. The degree of potential therapeutic targets of IBS (i.e., the number of compounds involved in the regulation of this target) was calculated. The top 10 potential targets were cytochrome P450 family 19 subfamily A member 1 (CYP19A1), estrogen receptor beta (ESR2), ESR1, acetylcholinesterase (ACHE), epidermal growth factor receptor (EGFR), adenosine A2A receptor gene (ADORA2A), ATP binding cassette subfamily B member 1 (ABCB1), v-src avian sarcoma (Schmidt-Ruppin A-2), viral oncogene homolog (SRC), monoamine oxidase A (MAOA), and matrix metalloproteinase 9 (MMP9). Additionally, a potential active compound can correspond to multiple potential therapeutic targets, such as obacunone, praeruptorin B, 7-acetoxy-2-methyl isoflavone, alfalfa toxin, poncimarin, licochalcone B, wogonin, and quercetin. This reflects the characteristics of the network structure of a single drug with multiple targets and multiple drugs that have the same target in the active ingredient-target network diagram, indicating that Sini powder and Tong xie yao fang decoction use a multitarget, multipathway, and multistep approach to treat IBS.
3.3. Construction of the PPI Network between the Active Ingredients of Sini Powder and Tong Xie Yao Fang Decoction and IBS
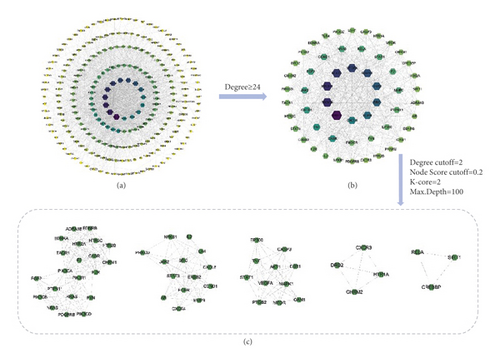
The 248 common targets obtained in the process described in “2.3” were imported into the STRING database to construct the PPI network. After the TSV file was obtained, it was imported into Cytoscape 3.8.0 software for visualization and network topology analysis. The PPI network contained a total of 232 nodes (16 target proteins are not involved in the interaction), and there were 1884 edges. The larger the degree value is, the larger the size of the hexagonal area and the darker the color (Figure 2(a)). The median degree value was 12, and a degree value ≥24 was used as the screening condition to obtain the core target proteins of Sini powder and Tong xie yao fang decoction in the treatment of IBS (Figure 2(b)). Of these, AKT Serine/Threonine Kinase 1 (AKT1, degree = 76); phosphatidylinositol-4, 5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA, degree = 67); phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1, degree = 66); signal transducer and activator of transcription 3 (STAT3, degree = 64); vascular endothelial growth factor (VEGF, degree = 62); mitogen-activated protein kinase 1 (MAPK1, degree = 60); H-Ras Proto-Oncogene; GTPase (HRAS, degree = 57); SRC (degree = 56); EGFR (degree = 54); and C-X-C motif chemokine ligand 8 (CXCL8, degree = 50) were obtained. The larger degree value of the target point reflects the high density of nodes and their surrounding nodes, which play important roles in the network. Therefore, these targets may be keys in the treatment of IBS. The MCODE plug-in of Cytoscape software was used for modular analysis of the screened core target proteins, and their characteristics were analyzed to screen the core target modules (Figure 2(c)). Selected modules have higher information transmission efficiency, and a single target has stronger interactions with other nodes.
3.4. GO Functional Enrichment and KEGG Pathway Enrichment Analysis
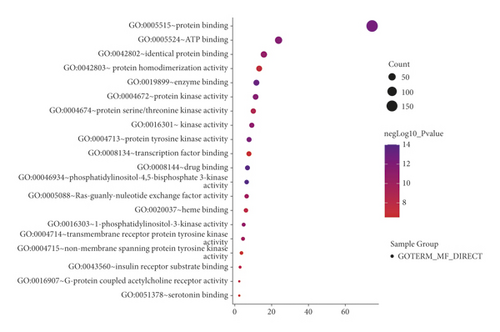
When P < 0.01 and FDR < 0.05, a total of 522 GO functions were enriched in common targets (including 79 molecular function items, 386 biological process items, and 57 cell component items). In terms of molecular functions, these functions were mainly enriched in protein binding, ATP binding, drug binding, enzyme binding, 5-hydroxytryptamine binding, protein kinase activity, protein serine/threonine kinase activity, protein tyrosine kinase activity, transcription factor binding, etc (Figure 3(a)). In terms of biological process, they were mainly enriched in the transcription of RNA polymerase II promoter, cell proliferation, gene expression, positive regulation of the ERK1 and ERK2 cascade, drug response, negative regulation of apoptosis process, protein phosphorylation, inflammatory response, and protein autophosphorylation, MAPK cascade, etc (Figure 3(b)). In terms of molecular components, they were mainly enriched in the plasma membrane, dendrites, synapses, and neuronal cell bodies (Figure 3(c)). The KEGG pathway enrichment screening resulted in 101 signaling pathways. The results suggest that cancer, neuroactive ligand-receptor interaction, proteoglycan in cancer, calcium ion signal, cAMP, hepatitis B, hypoxia-inducible factor 1-alpha (HIF-1), pancreatic cancer, and other signal pathways may be closely related to the pathogenesis of IBS (Figure 3(d)).
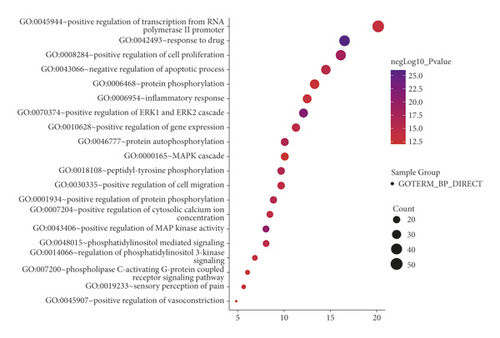

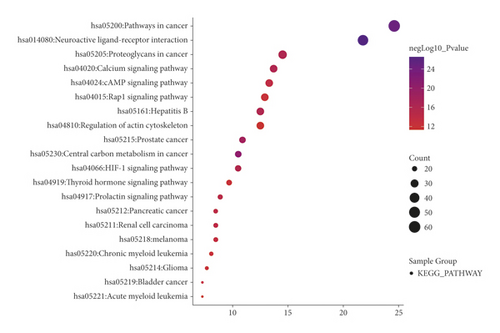
4. Discussion
Liver stagnation and spleen deficiency are important pathogenies of IBS in the theory of the traditional Chinese medicine. Sini powder and Tong xie yao fang decoction, which restrict the liver and support the spleen, have good indications and clinical efficacy for IBS [16, 17].
In the present study, the topological properties of the active ingredients-potential target network of Sini powder and Tong xie yao fang decoction were analyzed. We screened and picked up the ingredients with the highest degree value, such as obacunone, praeruptorin B, 7-acetoxy-2-methyl isoflavone, alfalfa toxin, poncimarin, licochalcone B, wogonin, and quercetin for the subsequent analysis. Obacunone is a natural compound that is widely present in plants in the Rutaceae family. It has anti-inflammatory, antitumor, antioxidative, and antipulmonary fibrosis effects. It has been confirmed in animal models that obacunone can inhibit the production of proinflammatory mediators [18]. It also alleviate colitis by improving the abnormal composition of intestinal microbiota and suppressing excessive activation of toll-like receptors (TLRs)/NF-κB signaling cascades to [19]. Licochalcone B can exert anti-inflammatory effects by inhibiting the phosphorylation of nuclear factor kappa B (NF-κB) P65 in the lipopolysaccharides (LPS) signaling pathway [20]. Wogonin is a pure natural flavonoid with a variety of biological activities, such as antiviral, anti-inflammatory, anticancer, neuroprotective, and antianxiety effects [21]. Previous studies have showed that wogonin can be used to treat colitis by inducing the expression of transcription factor HIF-1α through the protein kinase B/glycogen synthase kinase β (AKT/GSK3β) signaling pathway to increase interleukin 10 (IL-10) production [22]. Synergistic treatment using quercetin and 5-aminosalicylic acid improved symptoms and signs in a rat model of IBS after infection and improved the therapeutic effect [23]. As a coumarin-based component, praeruptorin B has been confirmed to have anti-inflammatory, antioxidant, antitumor, and analgesic activities [24, 25]. However, there are few reports on the treatment of IBS using praeruptorin B. In the present study, our results and the others suggest that the ingredients of the Sini powder and Tong xie yao fang decoction mainly served as anti-inflammatory mediators which indicate that the combined decoction might have a promising therapeutic value for the IBS treatment. Moreover, one potential valuable ingredient praeruptorin B was selected in the present study, which might be a promising target for IBS further research and drug discovery.
We next investigate the underlying mechanism of the valuable ingredients in the Sini powder and Tong xie yao fang decoction. Top ten core targets, such as AKT1, PIK3CA, PIK3R1, STAT3, VEGF, MAPK1, HRAS, SRC, EGFR, and CXCL8 were selected and further analysis. Previous studies have showed that overexpression of microRNA495 downregulated STAT3 to improve intestinal mucosal barrier function in ulcerative colitis [26]. Cell adhesion molecule 1 (CADM1) also would inhibit STAT3 signaling pathway to improve the intestinal barrier function of rats with IBS-D [27]. EGFR is a transmembrane receptor tyrosine kinase in the ErbB family that can promote intestinal development, regulate tight-binding protein expression, inhibit oxidative stress-induced apoptosis, and reduce intestinal epithelial colonization by inducing the autophosphorylation of RTK and the subsequent activation of the Ras/MAPK, PI3K/AKT, and PLC-γ/PKC signaling pathways, and it plays an active role in regulating intestinal permeability and promoting the integrity of the intestinal barrier [28]. VEGF is one of the most important factors in gastrointestinal mucosal remodeling, mucosal defense, and ulcer healing. The antiulcer drug sofalcone can prevent gastric mucosal injury through the upregulation of VEGF production mediated by the nuclear factor erythroid-2 related factor 2/hemeoxygenase-1 (Nrf2/HO-1) pathway [29]. Camellia oil can improve ketoprofen-induced gastrointestinal mucosal injury by upregulating HO-1 and VEGF [30]. The ERK/MAPK pathway can increase the expression level of intestinal tight junction proteins to enhance the intestinal barrier function in dogs with splenic asthenia syndrome [31]. Taken these together, the Sini powder and Tong xie yao fang decoction might improve intestinal barrier function, regulate intestinal permeability, exert a protective effect on the gastrointestinal mucosa, and inhibit the inflammatory response in IBS treatment.
Our results further showed that the top ten core genes mainly involved cell proliferation, inflammatory response, protein phosphorylation, ERK1/2 cascade, MAPK cascade, neurons, 5-hydroxytryptamine (5-HT) binding, and protein binding by GO functional enrichment analysis. 5-HT is a neurotransmitter in the intestinal tract that is considered an important signaling molecule. It mainly acts on various receptors on smooth muscle, enteric neurons, intestinal cells, and immune cells to regulate various intestinal functions. The symptoms of IBS (such as visceral sensitivity and gastrointestinal motility disturbance) are related to the level of 5-HT in the intestine. Previous studies have showed that an increase in 5-HT levels is correlated with diarrhea-dominant IBS. A decrease in the 5-HT level is correlated with constipation-dominant IBS [32]. The KEGG pathway enrichment analysis showed that the treatment of IBS with Sini Powder and Tong xie yao fang decoction involves multiple signaling pathways, such as cancer, neuroactive ligand-receptor interaction, calcium ion signaling, cAMP, HIF-1, and hepatitis B. The cAMP signaling pathway maintains the stability of the internal and external environment of intestinal cells, improves enteric nerve function, and regulates intestinal secretion and absorption [33]. PKA may mediate SP through cAMP signaling to regulate visceral sensitivity and enhance gastrointestinal motility [34]. In the rat model of diarrhea-dominant IBS, the expression levels of the L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in the colon was found to be elevated to different degrees, suggesting that the increase in the L-type voltage-gated calcium channel may be the molecular basis of colonic motility disorders [35]. The T-type voltage-gated calcium channel Cav3.2 is upregulated in IBS and participates in visceral hypersensitivity, which is closely related to the occurrence and development of IBS [36]. HIF-1α is a sensitive indicator of intestinal hypoxia and is related to the maintenance of the intestinal barrier. Studies have confirmed that intestinal epithelial HIF-1α is an important protective factor against colitis and can effectively alleviate inflammatory colonic injury [37]. Taken these together, the Sini powder and Tong xie yao fang decoction might regulate intestinal nerve function, improve visceral sensitivity and gastrointestinal motility, and maintain intestinal barrier function to treat IBS by integrating these multi-pathways.
In summary, this study investigated the potential material basis and mechanism of action of the Sini powder and Tong xie yao fang decoction in the treatment of IBS through a network pharmacology method. The main mechanism of Sini powder and Tong xie yao fang decoction in the treatment of IBS may be the regulation of intestinal 5-HT levels to improve intestinal nerve function, act on calcium channels to improve visceral sensitivity, maintain intestinal barrier function to provide mucosal protection, and inhibit inflammatory responses. In general, the drug has multi-ingredient, multitarget, and multipathway characteristics and plays a therapeutic role through the synergistic effect of multipathway system regulation. The present study had provided an alternative potential strategy for IBS treatment. However, more evidence from the IBS animal model and IBS patients should be evaluated in the future studies.
Disclosure
The fund providers had no role in the design of the study; collection, analysis, interpretation of data; writing the manuscript; and the decision to submit the manuscript for publication.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Hong Liu conceived and designed the study. Rong Tang, Xiaoqing Peng, Xiaohong Zhou, Zhimin Zheng, and Jiayu Yin acquired and analyzed the data. Rong Tang prepared the manuscript. Hong Liu submitted the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This work was supported by Basic and Applied Basic Research Fund Project of Guangdong Province (2020A1515110615), Medical Research Foundation of Guangdong Province (B2020043).
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




