Bisphenol and Phthalate Migration Test from Mexican Meat Packaging Using HPLC-DAD Technique
Abstract
The objective of this work was to analyze the bisphenols’ and phthalates’ (PAEs) migration from meat packages (of sausages, Winnies, and ham found in Mexican markets) to a water simulant. The determination of these compounds was realized by high performance liquid chromatography (HPLC) and diode array detection (DAD) at a wavelength of 254 nm. The mobile phase utilized was a mixture of acetonitrile:H2O (70 : 30). Elution was performed isocratically at a temperature of 25°C and at a flow rate of 1 mL min−1. The LOQs obtained for BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA in µg mL−1 were 0.53, 2.09, 0.85, 1.45, 5.81, 1.03, 3.12, and 29.6, respectively. Calibration curves exhibited an adequate determination coefficient for all compounds (R2 >0.999). Excellent accuracy and precision in measurements (% RSD) were achieved. The recovery study showed good applicability of the method (percentage recovery 80% to 106%). The BPA, BADGE, DBP, and DOP concentrations found in samples exceeded the simulant migration limits (SMLs) established by the European Union. The contribution of the current investigation was to provide information related to the presence of bisphenols and PAEs in the package of meat products, highlighting the health risks associated with their exposure.
1. Introduction
Flexible plastic packaging is applied to a huge variety of products to protect food during all stages of the supply chain [1]. The vacuum packages are usually employed in the packaging of meat products and can be composed of thermoplastic material, which is popular because of its user-friendly, safe processing, and they can be melted, shaped, and cooled into a dimensionally stable part in a matter of seconds. However, the migration of monomers, oligomers, and food additives may occur because of direct contact with the food [2]. Some examples of these compounds are the bisphenols used as stabilizers in the manufacturing of polyvinyl chloride (PVC), and phthalates (PAEs), which are the diesters of phthalic acids and the most common plasticizers in plastic packaging [3, 4]. PAEs and bisphenols are not bound to the polymer matrix chemically and are known to migrate from food contact materials (paper, plastics, glass, metal, and printing inks) that protect food from physical damage and microbial spoilage [5].
PAEs can be readily dispersed into the foodstuff from food plastic containers during the processes of production, use, or disposal [1]. The exposure pathway for phthalates could be the ingestion of contaminated food and water, soil, and dust [6]. After entering the body, the PAEs are hydrolyzed to monoesters (more bioactive form) by esterases or lipases present in the salivary gland, intestine, and liver [7, 8]. The most frequent phthalates in foods are dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), di n-octyl phthalate (DOP), butyl benzyl phthalate (BBzP), diethyl hexyl phthalate (DEHP), phthalic acid (PA), among others [9].
Bisphenols are contained in a great number of products with varied applications (i.e., production of polycarbonates in the manufacture of food storage containers, food packaging, among others). At higher temperatures (sterilization, microwave heating), the resin can decompose, and as a result, the migration of bisphenols from packaging to food can be more intensive and rapid [5]. Bisphenol-A (BPA) belongs to category 1 of endocrine disruptive chemicals (EDCs) that are acutely toxic to living organisms, which is clear evidence concerning its endocrine-disrupting activity [10–12]. In the same way, Bisphenol-A-Dimethacrylate (Bis-DMA) shows estrogenic activity, and Bisphenol-A-Diglicyl Ether (BADGE) has been classified as a carcinogen and a mutagenic compound [13]. The potential target tissues are the central neural system, kidney, and liver [14].
Specifically, studies in different countries mention the relationship between ultraprocessed food intake and phthalate exposure [6], and bisphenol exposure was attributed mainly to food production, processing, packaging practices, food storage conditions, and animal feeding practices [15]. The migration phenomenon may lead to the contamination of the conditioned product through direct and indirect interactions that may occur with the food product. Guerreiro et al. corroborate the fact that these components migrate from the packaging material, and they are able to migrate into meat [2].
Among the ultraprocessed foods, it is found that meat products (sausages, ham, chorizo, and Winnies) are utilized widely in the preparation of fast foods. The long shelf life and ready-to-eat characteristics of these meat products make a potential delivery vehicle for PAEs and bisphenols in humans. In addition, this kind of food is often heated or served warm in the paper, cardboard, or plastic containers [16].
The European Union has established restrictions of use and migration limits of 0.6 mg BPA per Kg of food [5]. This directive is nowadays adjusted by new issues (1999/91/EC, 2001/61/EC, 2001/62/EC, 2002/16/EC, 2002/17/EC, and 2002/72/EC), in which established are the specific migration limits of 1 mg kg−1 in food for BADGE and their derivatives [17]. Bisphenol A-Dimetracrylate (BisDMA) is a derivate compound of BPA, and its maximum allowable limit has not been recognized. In 2020, the European Committee for Food Contact Materials and Articles submitted a technical guide based on the regulations for paper and cardboard packaging from Germany, France, Italy, and the Netherlands. It sets a specific migration limit (SML) of 0.3 mg kg−1 for DBP [18]. The European Commission in regulations No. 10/201110/2010 and 213/2018 define SMLs of each PAE from plastic in the food as follows: 60 mg kg−1 for DEP and DOP and 0.3 mg kg−1 for DBP [8]. While for phthalic acid and DHP, any regulation or SMLs in the consulted bibliography were not found.
Apprehension related to the health risks and the ubiquitous incidence of phthalates and bisphenols in foods inspires the development of reliable analytical approaches that allow their detection and quantification at trace levels [19]. Analytical schemes have been reported in the literature. The detection of very low concentrations can be carried out by high-performance liquid chromatography (HPLC) with diode array detection (DAD) [11, 20, 21] high performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) [10], cold fiber solid-phase microextraction (CF-SPME-GC-MS) [22], high performance liquid chromatography, ultraviolet detection (HPLC-UV) [23, 24], and liquid-liquid microextraction chromatography with mass spectrometry (LLME-GC-MS) [25–27].
Migration tests of food packages are usually realized through food simulants. Simulants are chosen as the models of the basic food categories, for example, aqueous, acidic, alcoholic, and fatty [5].
Since foods are the major sources of exposure to phthalates and bisphenols, information on the levels of these compounds in foods is important for human exposure assessment. The objective of this work was to analyze (1) the PAEs (DEP, DBP, DHP, DOP, and PA) and bisphenols (BPA, BADGE, and BisDMA) migration from meat packages (of sausages and ham collected from Mexican markets) to a food simulant (water) and (2) the validation and application of a selective, robust, and reliable methodology for their identification and quantification using an HPLC-DAD technique.
2. Methodology
2.1. Reagents and Solvents
The standards (purity 96%) of bisphenol A (BPA; CAS: 80-05-7), di-n-etyl-phthalate (DEP; CAS: 84-66-2), bisphenol A di-glicyl ether (BADGE; CAS: 1675-54-3), di-butyl-phthalate (DBP; CAS:84-74-2), bisphenol A dimetacrylate (BisDMA; CAS: 3253-39-2), di-hexyl-phthalate (DHP; CAS: 88-99-3), di-octyl-phthalate (DOP; CAS: 117-81-7), and phthalic acid (PA, CAS: 88-99-3) were provided by Sigma Aldrich (México).
Chromatographic grade acetonitrile and trifluoroacetic acid (TFA; CAS 76-05-1) were manufactured by Sigma Aldrich (México). Purified water was obtained from a Millipore purification system operating at a resistivity of 18.2 MV/cm.
2.2. Instrument
High Efficiency Liquid Chromatography was performed on an HPLC instrument (DIONEX UltiMate 3000, Thermo Scientific), coupled to a diode array detector. The wavelength (λ) was set at 254 nm. Data acquisition was carried out using Chromeleon version 7.2 software. A C18 column supplied by Acclaim-Tm-120 (4.6 mm × 15 cm packed with a carbon impregnated silica material) was used. The mobile phase utilized was a mixture of acetonitrile: H2O (70 : 30). Elution was carried out isocratically at a temperature of 25°C and at a flow rate of 1 mL min−1.
2.3. Figures of Merit
Figures of merit considered in this work were sensitivity, linearity, range, the limit of detection (LOD), the limit of quantification (LOQ), accuracy, and precision.
Individual bisphenols and phthalic acid ester stock solutions (800 µg mL−1) were prepared in acetonitrile and stored at –4°C in brown glass bottles. The same method was applied for PA in a separate flask.
To evaluate the linearity and range of the method, eight calibration curves were built, which were constructed with BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA peak area ratio obtained from the HPLC (y-axis) in relation to the corresponding concentrations (x-axis). Three injections from each concentration were analyzed under optimal conditions. Linear regression analysis was used to evaluate the linearity of the calibration curve using the least square linear regression method. The LOD was estimated from three times the standard deviation (s) of 10 replicates of the blank divided by the slope of the calibration curve, whereas the LOQ was calculated from 10 times (s) of 10 replicates of the blank divided by the slope of the calibration curve [27].
The interday repeatability was calculated based on five consecutive measurements of a standard solution (400, 260, 200, 240, 55, 260, 220, and 340 µg mL−1 for BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA, respectively) with the same measurement procedure and the same operating conditions. The intraday reproducibility was obtained with stated precision by three consecutive measurements of the same bisphenols and PAEs concentration mentioned above in a different analysis day.
2.4. Recovery Study
The accuracy of the assay method was determined by recovery studies. A volume of 300 µL of a known concentration standard of bisphenols and PAEs was added to the samples obtained after the migration test. The vials are placed into an autosampler, and automatically, the needle of the HPLC equipment goes through the septum of the vial, takes the volume of solution selected in the program, in this case, 5 μL, and then injects it into the chromatographic column. Percentage recoveries were calculated.
2.5. Sample Solution Preparation
Thirteen different kinds of meat packaging materials of commercial sausages, Winnies, and ham (A1, A2, A3, B1, B2, B3, C1, C2, C3, D1, D2, D3, and E1) were randomly collected from local Mexican suppliers. The selection criterion was based on high consumption and popularity among Mexican consumers. For confidentiality reasons, the studied products shall not be identified.
For the extraction of phthalates and bisphenols from the meat packaging, a microwave-assisted extraction methodology was implemented using microwave (Winia 0.7). Approximately, 300 to 500 g of sample were placed in 100 mL beakers, and 60 mL of 1% of H2O:TFA solutions were added to each one as dispersing solvent and extraction solvent, respectively. The contact between the samples and the extracting agent was improved by cutting the plastic wrap uniformly into small squares (0.5 cm2) and then being introduced in the microwave for one minute. After the extraction, the samples were allowed to cool to room temperature in beakers to avoid losses because of solvent evaporation. Once again, the samples were subjected to heat for 30 seconds. After being cooled for 10 minutes, they were stored in amber glass bottles, separating the package from the solvent. Each one of the samples was subjected to the extraction process in triplicate and two blanks were placed for each microwave run.
Subsequently, 15 mL of each one of the samples were transferred to a glass tube for centrifugation at 4.4 rpm, 25°C, and for 15 min, to obtain a phase separation. A nylon filter for chromatography (Titan3-Thermo 17 mm and 0.2 µm) was placed on the top of a pharmaceutical syringe, and 1.5 mL of each sample was taken and stored in a vial for HPLC. The vials were amber in color and had a 1.5 mL screw cap capacity. Subsequently, 300 µL of each sample was taken and placed in new vials.
3. Results and Discussion
3.1. Method Validation
The concentrations of the five phthalic acid esters and three bisphenols in 13 packaging samples were quantified using the linear regression of response against concentration. The areas of the chromatographic peaks were measured. Figure 1 shows a chromatogram of the eight standards. On each standard signal in the chromatogram, it was possible to get UV spectrums of pure compounds, which were used to compare with the identified compound in the samples (all identified compounds matched with the standard UV spectrum). A standard UV spectrum is compared with the identified compound in the sample (Figures S1 and S2 in Supplementary Materials).
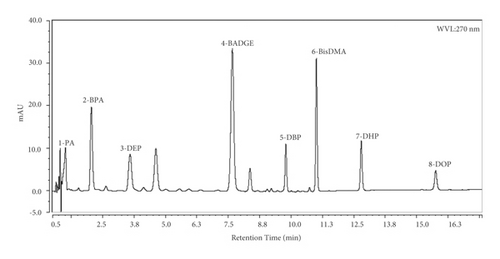
Table 1 shows the linear calibration ranges, the respective determination coefficient (R2), LODs, and LOQs for all analyses using the proposed method. The calibration plots are depicted in Figure 2.
| Phthalates | Calibration equation | R2 | Linearity range (µg mL−1) | LOD (µg mL−1) | LOQ (µg mL−1) |
|---|---|---|---|---|---|
| BFA | y = 0.1346x − 0.0068 | 1 | 6.25–800 | 0.16 | 0.53 |
| DEP | y = 0.1596x − 0.0832 | 1 | 4.1–520 | 0.63 | 2.09 |
| BADGE | y = 0.1666x − 0.0588 | 1 | 3.13–400 | 0.25 | 0.85 |
| DBP | y = 0.1086x − 0.0571 | 1 | 3.8–480 | 0.44 | 1.45 |
| BisDMA | y = 0.2611x − 0.1486 | 1 | 3.44–440 | 1.74 | 5.81 |
| DHP | y = 0.0721x − 0.0401 | 1 | 4.06–520 | 0.31 | 1.03 |
| DOP | y = 0.064x − 0.004 | 1 | 3.44–440 | 0.93 | 3.12 |
| Phthalic acid | y = 0.1605x − 0.3253 | 0.9999 | 10.63–680 | 8.91 | 29.61 |

LODs and LOQs were directly determined in the matrices investigated according to the International Conference on Harmonization: Validation of Analytical Procedure (i.e., an analyte that produces a chromatographic peak equal to three times (LOD) or ten times (LOQ) the standard deviation of the baseline noise) [28].
Table 2 shows a comparison between studies where the specific and simultaneous determination of bisphenols and phthalates was carried out. A migration test of these compounds was addressed using distiller water as a food simulant.
| aChromatographic analysis | Analyte | Solvents | Lineal range (µg mL−1) | LOD (µg mL−1) | RSD (%) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| HPLC-MS/MS | BPA | Methanol and water | 0.0005–0.1 | 0.05 | — | — | [10] |
| CF-SPME-GC-MS | DBP | — | 0.0002–0.006 | 0.0008 | 11.7 | — | [22] |
| HPLC-UV | DBP | Hexane and chlormentane | 1.01–106 | 0.5 | — | — | [23] |
| LLME-GC-MS |
|
Carbon tetrachloride |
|
|
2.2–7.8 | 80–100 | [25] |
| HPLC-DAD |
|
Water and acetonitrile |
|
|
|
|
Present work |
- aHPLC-MS/MS, high performance liquid chromatography coupled to tandem mass spectrometry; CF-SPME-GC-MS, cold fiber solid phase microextraction; HPLC-UV, high performance liquid chromatography and ultraviolet detection; LLME-GC-MS, liquid-liquid microextraction chromatography with mass spectrometry; HPLC-DAD, high performance liquid chromatography (HPLC) and diode array detection.
The sensitivity of the proposed method was lower than that obtained in different studies [10, 22, 23, 25] since they use detectors with greater sensitivity and preconcentration stages of the sample before the detection of analytes. For example, Moreira et al. (2014) used solid-phase microextraction (SPME), which is well-known for allowing analyte preconcentration and sample purification. It should be noted that the LOD of DBP was very close to that obtained by Jarosova and Bogdanoicova in 2016, where no pretreatments of the sample were used previously to the determination of this compound by HPLC-UV. However, the LODs and LOQs achieved were sufficient for determining such compounds in packaging materials, according to the regulations 10/2010 and 213/2018, which define the specific migration limit (SML) of each PAE/BPA from the plastic into the food (i.e., 60 mg kg−1 for DEP and DOP, 0.3 mg kg−1 for DBP, and 0.05 for BPA) [29, 30].
Adequate sensitivity was demonstrated in the selected concentration ranges for most of the analytes in comparison with other analytical techniques [23]. The linearity range for each compound was wide, which ensured the acquisition of reliable data for samples with low and high contents of bisphenols and PAEs. The determination coefficient (R2) was greater than 0.9999 in all cases.
The precision under interday repeatability and intraday reproducibility was assessed as the relative standard deviation (% RSD). The interday repeatability calculated for BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA was 0.11%, 0.19%, 0.06%, 0.05%, 2.26%, 0.02%, 0.07%, and 0.57%, respectively. However, the intraday reproducibility results were as follows: 1.95%, 2.27%, 2.27%, 2.27%, 2.10%, 1.41%, 1.31%, and 1.87% for BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA, respectively. For all bisphenols and PAEs studied in the analysis, % RSD values are lower than 20% as recommended by the validation of analytical procedures by high performance liquid chromatography for pharmaceutical analysis (figure S3 illustrates standard chromatograms used to calculate the method reproducibility) [28]. The preceding results suggested that the method demonstrated good precision and repeatability. These data showed values lower than those acquired in the chromatographic methodologies presented in Table 2.
3.2. Application of the Method (Recovery Study)
To demonstrate the applicability of the proposed method, a water sample was utilized, where the simulant migration of these eight compounds (PAEs and bisphenols) was performed. Thirteen different kinds of sausages, Winnies, and ham packaging materials were employed in the migration test (A1, A2, A3, B1, B2, B3, C1, C2, C3, D1, D2, D3, and E1). Recovery studies were conducted by analyzing 13 spiked samples at one fortified concentration after a simulant migration test. Furthermore, blank samples were analyzed that did not contain PAEs and bisphenols. Using the 2D ISO Plot and the 3D Plot sample chromatograms, it was possible to verify the purity of peaks or signals on the identified compounds on samples chromatograms (figures S4 and S5 in Supplementary Materials).
As shown in Tables 3 and 4, the recovery values of BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA in the 13 simulants migration samples were in the ranges of 87.6–92.4%, 88–106.2%, 80–105.3%, 87.9–92.9%, 92.1–98.5%, 87.7–92%, 87.7–91.5%, and 79.9–83.7%, respectively. In general, excellent recovery percentages were found for the analyzed components, which decreased the chances of losses during extraction and analysis (figures S6 and S7 presents a nonspiked E-1 sample chromatogram and spiked E-1 Sample chromatogram, respectively, in supplementary materials) and guaranteed the reliability of the optimized method [31]. About the DEP and DBP percentage recoveries, their results were in accord with the LLME-GC-MS method (80–100%) proposed by Mohebbi et al. [25].
| aPAEs and bisphenols | Spiking leves (mg L−1) | A1 | A2 | A3 | B1 | B2 | B3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | ||
| BFA | 0 | 52.7 ± 1.2 | — | 50.7 ± 0.2 | — | 52.5 ± 1.5 | — | 50 ± 0.1 | — | 50.4 ± 0.5 | — | 50.1 ± 0.1 | — |
| 400 | 422.3 ± 0.3 | 92.4 | 405.8 ± 1.45 | 88.8 | 420.6 ± 0.06 | 92.0 | 400.4 ± 0.5 | 87.6 | 403.9 ± 0.10 | 88.4 | 400.8 ± 0.15 | 87.7 | |
| DEP | 0 | 34 ± 1.2 | — | 32.2 ± 0.2 | — | 33.1 ± 0.7 | — | 32.2 ± 0.1 | — | 32.1 ± 1.3 | — | 32 ± 0.1 | — |
| 260 | 275.5 ± 0.1 | 92.9 | 261.1 ± 1.3 | 88.1 | 268.3 ± 1.3 | 90.5 | 261.1 ± 0.1 | 88.0 | 260.2 ± 0.2 | 87.9 | 259.3 ± 1.2 | 87.4 | |
| BADGE | 0 | 26 ± 1.8 | — | 24.8 ± 0.9 | — | 24.8 ± 1.8 | — | 24.8 ± 0.9 | — | 24.8 ± 0.9 | — | 24.8 ± 1.8 | — |
| 200 | 210.5 ± 0.1 | 92.3 | 200.8 ± 0.2 | 88 | 200.6 ± 0.9 | 87.9 | 200.6 ± 0.1 | 87.9 | 200.6 ± 0.9 | 87.9 | 200.6 ± 0.9 | 87.9 | |
| DBP | 0 | 31.3 ± 0.02 | — | 29.6 ± 0.01 | — | 30.6 ± 0.01 | — | 29.6 ± 0.01 | — | 29.6 ± 0.5 | — | 29.6 ± 0.02 | — |
| 240 | 254.2 ± 0.1 | 92.9 | 240.71 ± 0.01 | 87.9 | 248.1 ± 1.2 | 90.6 | 240.4 ± 0.1 | 87.8 | 240.52 ± 0.02 | 87.9 | 240.66 ± 0.01 | 87.9 | |
| BisDMA | 0 | 7.2 ± 0.3 | — | 6.7 ± 0.3 | — | 6.93 ± 0.04 | — | 6.67 ± 0.02 | — | 6.7 ± 0.2 | — | 6.7 ± 0.3 | — |
| 55 | 61.3 ± 0.2 | 98.5 | 57.82 ± 0.04 | 92.9 | 59.5 ± 0.2 | 95.5 | 57.3 ± 0.2 | 92.1 | 57.3 ± 0.3 | 92.1 | 57.3 ± 0.3 | 92.1 | |
| DHP | 0 | 34.7 ± 0.3 | — | 33.1 ± 0.4 | — | 33.7 ± 0.4 | — | 33.11 ± 0.04 | — | 33.12 ± 0.04 | — | 33.13 ± 0.03 | — |
| 260 | 273.1 ± 0.1 | 92.0 | 261.1 ± 0.5 | 87.7 | 266.3 ± 0.2 | 89.4 | 261.1 ± 0.2 | 87.7 | 261.1 ± 0.5 | 87.7 | 261.2 ± 0.5 | 87.7 | |
| DOP | 0 | 28.75 ± 0.01 | — | 27.616 ± 0.001 | — | 28.28 ± 0.04 | — | 27.59 ± 0.02 | — | 27.586 ± 0.002 | — | 27.54 ± 0.01 | — |
| 220 | 230.1 ± 0.3 | 91.5 | 220.9 ± 0.5 | 87.9 | 226.3 ± 0.3 | 90.0 | 220.8 ± 0.1 | 87.8 | 220.7 ± 0.1 | 87.8 | 220.4 ± 0.2 | 87.7 | |
| Phthalic acid | 0 | 85.7 ± 1.7 | — | 81.62 ± 1.9 | — | 85.8 ± 0.6 | — | 83.2 ± 0.2 | — | 82.7 ± 0.4 | — | 82.2 ± 1.4 | — |
| 300 | 336.7 ± 0.3 | 83.7 | 320.4 ± 0.1 | 79.6 | 337.1 ± 0.3 | 83.8 | 326.6 ± 0.1 | 81.1 | 324.6 ± 0.1 | 80.6 | 322.6 ± 0.3 | 80.2 | |
- aThe results are reported as BFA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and phthalic acid concentrations in the analyzed solutions. bResults are expressed as the mean value ± s (n = 3). Standard deviation, s.
| PAEs and bisphenols | Spiking leves (µg mL−1) | C1 | C2 | C3 | D1 | D2 | D3 | E1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | Mean | Rec. (%) | ||
| BFA | 0 | 50 ± 0.9 | — | 50.8 ± 0.2 | — | 50 ± 0.1 | — | 50 ± 0.9 | — | 50.8 ± 1.5 | — | 51.5 ± 1.5 | — | 52±±1.5 | — |
| 400 | 400.4 ± 1.2 | 87.6 | 406.5 ± 1.2 | 88.9 | 400.4 ± 1.5 | 87.6 | 400.3 ± 0.5 | 87.6 | 406.6 ± 0.3 | 89.0 | 412.7 ± 0.9 | 90.3 | 416.3 ± 1.5 | 91.1 | |
| DEP | 0 | 32 ± 0.8 | — | ≤LOD | — | ≤LOD | — | ≤LOD | — | ≤LOD | — | ≤LOD | — | ≤LOD | — |
| 260 | 260.1 ± 0.2 | 87.7 | 260.1 ± 0.7 | 100.2 | 268.3 ± 0.1 | 103.4 | 260.1 ± 0.7 | 100.2 | 260.1 ± 0.1 | 100.2 | 260.1 ± 0.8 | 100.2 | 275.5 ± 0.8 | 106.2 | |
| BADGE | 0 | 22.4 ± 0.2 | — | ≤LOD | — | ≤LOD | — | ≤LOD | — | ≤LOD | — | ≤LOD | — | ≤LOD | — |
| 200 | 181.6 ± 0.9 | 80 | 200.8 ± 1.8 | 100.6 | 200.6 ± 0.9 | 100.5 | 200.5 ± 0.9 | 100.3 | 181.6 ± 1.8 | 90.8 | 210.5 ± 1.8 | 105.2 | 210.5 ± 0.9 | 105.3 | |
| DBP | 0 | 29.6 ± 0.5 | — | 29.6 ± 0.5 | — | 29.6 ± 0.02 | — | 29.6 ± 0.1 | — | 29.6 ± 0.5 | — | 29.6 ± 0.02 | — | 29.6 ± 0.1 | — |
| 240 | 240.69 ± 0.01 | 87.9 | 240.6 ± 0.47 | 87.9 | 240.7 ± 0.02 | 87.9 | 240.76 ± 0.5 | 88.0 | 240.8 ± 0.1 | 88.07 | 240.45 ± 0.01 | 87.9 | 240.69 ± 0.01 | 87.9 | |
| BisDMA | 0 | 6.7 ± 0.3 | — | 6.7 ± 0.3 | — | 6.66 ± 0.01 | — | 6.7 ± 0.2 | — | 6.7 ± 0.1 | — | 6.7 ± 0.3 | — | 6.63 ± 0.01 | — |
| 55 | 57.32 ± 0.04 | 92.1 | 57.3 ± 0.2 | 92.1 | 57.33 ± 0.02 | 92.1 | 57.28 ± 0.02 | 92.03 | 57.3 ± 0.2 | 92.1 | 57.32 ± 0.02 | 92.1 | 57.1 ± 0.2 | 91.7 | |
| DHP | 0 | 33.11 ± 0.03 | — | 33.17 ± 0.04 | — | 33.13 ± 0.04 | — | 33.1 ± 0.3 | — | 33.1 ± 0.4 | — | 33.12 ± 0.04 | — | 33.1 ± 0.3 | — |
| 260 | 261.2 ± 0.4 | 87.7 | 261.2 ± 0.4 | 87.7 | 261.2 ± 0.4 | 87.7 | 261.1 ± 0.5 | 87.7 | 261.2 ± 0.3 | 87.7 | 261.2 ± 0.5 | 87.7 | 261.1 ± 0.4 | 87.7 | |
| DOP | 0 | 27.6 ± 0.6 | — | 27.6 ± 0.2 | — | 27.6 ± 0.4 | — | 27.56 ± 0.02 | — | 27.58 ± 0.01 | — | 27.60 ± 0.01 | — | 27.6 ± 0.02 | — |
| 220 | 220.8 ± 0.9 | 87.9 | 220.711 ± 0.4 | 87.8 | 220.87 ± 0.01 | 87.9 | 220.5 ± 0.5 | 87.7 | 220.8 ± 0.3 | 87.8 | 220.86 ± 0.01 | 87.8 | 220.87 ± 0.01 | 87.9 | |
| Phthalic acid | 0 | 81.9 ± 1.7 | — | 81.8 ± 0.6 | — | 82.3 ± 2.1 | — | 82.9 ± 1. | — | 83.8 ± 0.9 | — | 83.03 ± 0.81 | — | 85.8 ± 1.5 | — |
| 300 | 321.7 ± 0.1 | 79.9 | 321.2 ± 0.2 | 79.8 | 323.0 ± 0.1 | 80.2 | 325.9 ± 0.3 | 81.0 | 329.0 ± 0.3 | 81.7 | 326.0 ± 0.1 | 81.0 | 336.9 ± 0.2 | 83.7 | |
- aThe results are reported as BFA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and Phthalic acid concentrations in the analyzed solutions. bResults are expressed as the mean value ± s (n = 3). Standard deviation, s.
The method was, therefore, considered selective, robust, and reliable for a study on the migration of these micropollutants from meat packaging materials into food, together with the possibility of quantification at the legislated migration limits. Its applicability to some real meat packaging materials has been tested. One of the desired characteristics for the analytical methods is avoiding the use of highly toxic solvents like CCl4. The proposed method used acetonitrile, water, and TFA. Besides, the present is the first report determining the bisphenols and phthalates migration from Mexican meat packaging.
3.3. Bisphenols and Phthalates Migration from Meat Packaging Materials
High concentrations of BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA were found in all samples, except in C2, C3, D1, D2, D3, and E1, where the concentrations of DEP and BADGE were lower than the detection limit for these compounds. The mean concentrations (n = 13) found were 3.20 mg kg−1, 2.05 mg kg−1, 1.55 mg kg−1, 1.88 mg kg−1, 0.42 mg kg−1, 2.10 mg kg−1, 1.75 mg kg−1, and 5.24 mg kg−1 for BPA, DEP, BADGE, DBP, BisDMA, DHP, DOP, and PA, respectively.
The BPA and BADGE concentrations found in sausage packaging exceeded the SMLs established by the European Union (0.6 and 1 mg kg−1), while the DBP and DOP concentrations were also above the migration limit proposed by the European Committee for Food Contact Materials and Articles (0.3 mg kg−1 for both compounds).
It is important to note that previously, the migration of PAEs and bisphenols from the packaging of Mexican meat products (ham, Winnies, and sausages) had not been reported.
The results confirm the fact that these components belong to the packaging analyzed material, and they could be able to migrate into the meat. Adeyi and Cols. (2019) found a concentration of BPA of 4.28 × 10−3 mg kg−1 in meat products (hot dogs and ham) packed in plastic [11]. Sechecter and Cols. (2013) analyzed 13 meat products and found levels of 1.02 × 10−3 mg kg−1 and 1.3 28 × 10−3 mg kg−1 for DEP and DOP, respectively [6]. While García-Fábila et al. reported average concentrations of DEP and DBP (0.59 × 10−3 mg kg−1 and 0.0763 mg kg−1, respectively) in delicatessen and dairy products (ham, sausage, and chorizo) consumed by Mexican children, these concentrations were an estimate of the daily intake of these foodstuffs [9]. The bisphenol and PAE concentrations obtained in our work were greater than those reported by these authors because the analysis was realized on the packaging and not on the meat samples. Besides, these differences also could be explained if it is considered that the migration of these compounds is influenced by parameters, such as the types of components in packaging material, and more importantly, the type of foodstuff (fatty, acidic, or aqueous) [3]. Otherwise, Jarošová and Bogdanovičová found the DEP and DBP mean concentrations of 75–375 mg kg−1 and 75–325 mg kg−1, respectively, in 12 samples of meat products independent of the fat content. In this work, the concentrations of DEP (2.05 mg kg−1) and DBP (1.88 mg kg−1) found were lower than those mentioned in the above study [23].
Other studies related to BisDMA, DHP, and PA concentrations detected in packaging films of meat products were not found. Nevertheless, in this work, these three compounds were considered because of their reported toxicity [19, 32].
4. Conclusion
The migration of chemical compounds from packaging polymers into foods should be evaluated to ensure that the amount of migrating components meet the compliance standards set by regulatory agencies. In México, there is no sanitary regulation for this type of micropollutants. The issue is little-known and discussed between companies that thermally process meat products. The present one is the first report determining the bisphenols and phthalates migration from Mexican meat packaging into food simulant (water). The results showed that BPA, BADGE, DBP, and DOP concentrations found in meat packages exceeded the SMLs established by international agencies. The contribution of the current investigation was to provide information related to the presence of PAEs and bisphenols in the package of meat products, highlighting the health risks associated with their possible migration to the meat and the exposure of the consumers. The producers of this type of food can make improvements in quality control by choosing, for example, other types of packaging. Besides, the validation and application of a selective, robust, and reliable methodology for bisphenol and PAE identification and quantification using an HPLC-DAD technique was performed. Results achieved as linearity, accuracy, precision, LODs, and LOQs were statistically satisfactory for this research. This research could be useful to generate more reliable information for future developments.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Blanca G. Beltrán gratefully acknowledges the financial support for the research from the “Programa para el Desarrollo Profesional Docente [PRODEP UACH-PTC-367]” of the Autonomous University of Chihuahua.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request ([email protected]).