NiO-Based Gas Sensors for Ethanol Detection: Recent Progress
Abstract
In this review, we summarized the state-of-the-art progress on the ethanol performance of NiO by means of morphology, doping, loading noble metal particles, and forming heterojunctions. We first introduced the effect of modulating NiO morphology on ethanol performance that has been reported in recent years. The morphology with large specific surface area and high porosity was considered to be the one that can bring high gas response. Then, we discussed the enhanced effect of the doping of metal cations and noble metal particle loading on the ethanol-sensitive properties of NiO. Doping ions increased the ground-state resistance and increased the oxygen defect concentration of NiO. The effects of noble metal particles on the performance of NiO included chemical sensitization and electronic sensitization. Finally, the related contents of NiO forming complexes with metal oxides and bimetallic oxides were discussed. In this section, the specific improvement mechanism was discussed first, and then, the related work of researchers in recent years was summarized. At the same time, we presented a reasonable outlook for NiO-based ethanol sensors, imagining future directions.
1. Introduction
Volatile organic compounds (VOCs) often appear in our lives, such as the smell of gasoline, paint, and nail polish. These VOC gases degrade air quality and even destroy the ozone layer. At the same time, these gases threaten human body [1–3]. For example, ethanol exists in laboratories, medical industries, and industrial production. When exposed to high concentrations of ethanol, humans are prone to adverse symptoms such as skin irritation and headache [4–6]. In order to prevent problems before they occur, it is essential to develop ethanol sensors to monitor ethanol concentration. In addition, ethanol sensors also play an important role in detecting drunk driving [7]. Currently, gas detection technology includes gas chromatography [8], quartz crystal microbalance [9], and TDLAS detection technology [10]. However, real-time monitoring of ethanol is achieved by chemiresistive sensors with the advantages of easy preparation, low cost, and portability [11, 12].
Metal oxide semiconductors (MOS) have special electrical conductivity, good stability, and high gas response [13–15]. ZnO [16–18], SnO2 [19–21], NiO [22, 23], WO3 [24–26], In2O3 [27–29], etc. are widely used in ethanol gas sensors. For instance, Jiang et al. [16] synthesized an ethanol sensor of mesoporous ZnO nanospheres, which had abundant pore structures and large specific surface area. From the ethanol gas sensing test results, it could be seen that the ZnO sensors achieved a response of 58.4 to 100 ppm ethanol at 250°C. Furthermore, this sensor could detect ppb levels of ethanol, getting a response of 1.17 at 500 ppb ethanol. For the MOS gas sensor, the resistance is used as the measured physical signal to detect the gas. When the MOS is in the air, oxygen molecules adsorb on the surface of the MOS and take electrons out. After the electrons are extracted, the carriers (electrons) of the n-type semiconductor decrease to form an electron depletion layer (EDL), while the carriers (holes) of the p-type semiconductor increase to form a hole accumulation layer (HAL). When the material comes into contact with the reducing ethanol molecules, the electrons captured by oxygen are released back into the sensitive material. The carriers of the n-type semiconductor increase, so the resistance becomes larger; the carriers of the p-type semiconductor decrease, so the resistance becomes smaller [30–32]. In addition to single metal oxides, bimetallic metal oxides have also been used as gas sensing materials in recent years [33], including NiCo2O4 [34], ZnCo2O4 [35], and LaFeO3 [36]. Even though MOS possess the above advantages, high operating temperature and low selectivity are still their bottlenecks as gas sensors [37]. Among them, we often say that there is no selectivity, which means that pure MOS has only a weak gas response to the gas. It is not easy to distinguish it after mixing with other gases. Therefore, if the selectivity is not outstanding, it is considered to be nonselective. Scholars have done a lot of research to overcome these shortcomings, including doping, compounding with other materials, morphology manipulation, and UV activation [38–40]. For example, extensive experiments showed that the loading of Pd particles was important for the H2 selectivity. Because H2 adsorbs on Pd to form PdHx, which not only increased the electrical resistance but also caused the volume expansion of the Pd host. The volume expansion of Pd enabled rapid and highly selective detection of H2. Polyaniline had high selectivity to NH3 due to its carbon structure and special protonation/deprotonation process [41, 42]. At the same time, these modification methods can also reduce the temperature of the MOS-based gas sensors, hoping that it can make sensors work at low temperature or even room temperature. For example, ultraviolet light gives the sensor an external energy from the outside, and the energy required for the reaction is not necessarily obtained from the temperature. At the same time, the UV makes the sensitive material generate photogenerated carriers for the gas sensing reaction, which further reduces the temperature and increases the response [43]. The morphology manipulation is often used, such as the hierarchical structures. These hierarchical structures have a large number of pore structures that provide efficient gas diffusion paths, which can enhance gas response and significantly reduce operating temperature [44].
The p-type semiconductor NiO has strong oxidizing properties, excellent catalytic activity, and good electrical conductivity, so it has attracted attention in gas sensors [45, 46]. So far, more than 1,000 reports on NiO materials used in gas sensors have been published, and these reports are mainly concentrated in the past five years. For example, Li et al.’s team [47] fabricated ultrathin Ni(OH)2 nanosheets directly on the substrate without using additional surfactants. The obtained NiO nanosheets had a porous structure, which was favorable for the diffusion of gas molecules. At the same time, the nanosheets provided a large surface area and crystal planes with an affinity for acetone gas. Due to these advantages, the NiO nanosheet sensor had a response value as high as 60% when detecting 100 ppb acetone gas. In addition, the NiO nanosheets exhibited a low LOD of 0.8 ppb for acetone with good stability. However, according to reports, the response of pristine NiO to ethanol was low, so the researchers modified NiO using various methods. These modifications include morphology control, doping, loading of noble metal particles, and formation of composites with n-type semiconductors. According to the survey, there have been many reports on the detection of ethanol by NiO sensors in recent years. However, the rare review on the detection of ethanol by NiO is summarized in the existing reports. Therefore, a review article is needed to systematically introduce the research basis of NiO ethanol sensors, which will help scholars to quickly clarify their ideas and understand the entire research field. In addition, scholars can discover unresolved questions and think about new research directions from this review.
In this review, we will describe the influence of different modification methods on the ethanol sensitivity of NiO and summarize the development level of NiO for ethanol detection in recent years in a tabular form. We first introduced the effect of modified NiO morphology on ethanol performance that has been reported in recent years. The morphology with large specific surface area and high porosity was considered to be the structure that could bring high gas response. Then, we discussed the enhanced effect of the doping of metal cations and noble metal particle loading on the ethanol-sensitive properties of NiO. Doping ions increased the ground-state resistance and the oxygen defect concentration of NiO. The effects of noble metal particles on the performance of NiO included chemical sensitization and electronic sensitization. Finally, the related contents of NiO forming composites with metal oxides and bimetallic oxides were discussed. In this section, the specific improvement mechanism was discussed first, and then, the related work of researchers in recent years was summarized. At the same time, we presented a reasonable outlook for NiO-based ethanol sensors, imagining future directions.
2. Morphology
The gas response goes hand in hand with the specific surface area and porosity of the material [48, 49]. Therefore, researchers’ research on topography is based on high specific surface area and porosity. During the study of the morphology, the researchers found that the grain size of the material is an important factor. When the grain size is equal to or less than twice the Debye length (2λD), the conductance is highly influenced by each grain. So the gas response is strongly influenced by the grain size. When the grain size is larger than 2λD, the relationship between the gas response and grain size gradually weakens, and the response becomes independent. But in this case, the conductivity is affected by the Schottky barrier at the grain boundaries [50]. However, compared with studies on grains, recent reports on NiO morphologies have focused on obtaining large specific surface areas and high porosity, such as NiO nanosheets [51–53] and NiO nanoflowers [54–58]. Next, the progress of NiO nanomaterials with different morphologies in ethanol sensing will be introduced.
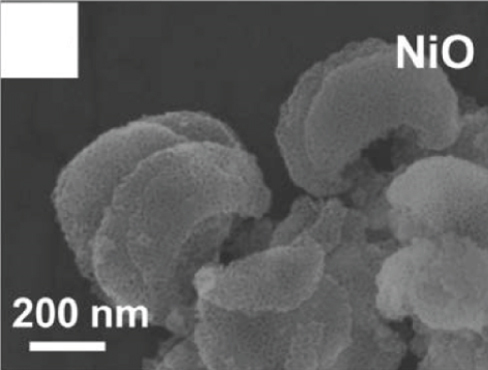
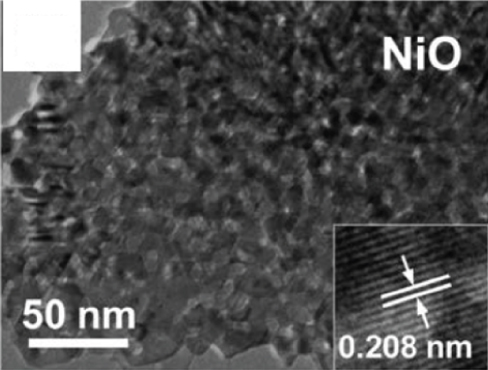
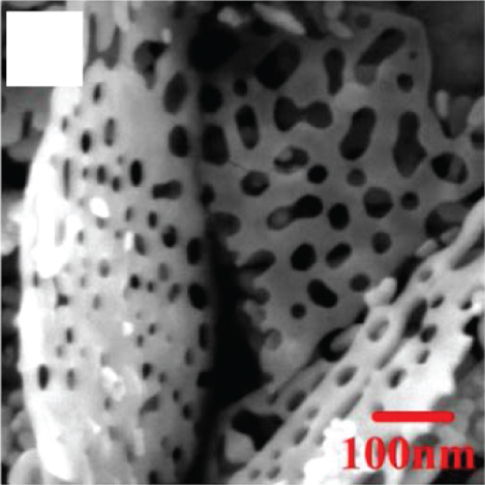
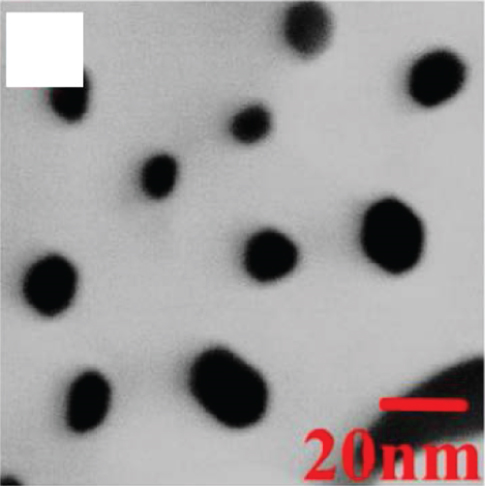
The 2D structure of nanosheets has remarkably high specific surface area and abundant gas channels, while the spatial confinement endows 2D sensitive materials with unique properties [59–61]. The more target gas molecules attached to the metal oxides surface, the greater the resistance change of the material, that is, the higher the gas response [62]. Besides, the flexibility of 2D metal oxides enables for high compatible integration with flexible substrates, which can be applied in the field of flexible wearable sensors. Therefore, nanosheets with satisfactory sensitive properties are favored by scholars in resistive sensors. Many reports on metal oxide nanosheets have pointed out that fabricating porous surfaces on nanosheets could effectively improve their gas sensing properties [63]. Su et al. [53] prepared a new and unique crescent-shaped NiO nanosheet. During the calcination process, the water and gas from the NiO nanosheets were get rid of, resulting in a porous structure (as shown in Figures 1(a) and 1(b)). The steady crescent-shaped structure alleviated the accumulation of nanoparticles, and the mesopores uniformly distributed on the nanosheets improved the gas diffusion channel, making ethanol molecules easier to diffuse into NiO. The gas sensors based on crescent NiO nanosheets could rapidly detect 100 ppm ethanol at 130°C (response time and recovery time was 5 s and 20 s, respectively), with a response as high as 68.1%. At the same time, the LOD showed a low value of 200 ppb by calculation. The authors believed that the excellent ethanol sensitivity gave the credit to its steady porosity structure with tiny particle size and abundant reaction sites. Cao et al. [52] prepared a lotus root-like NiO nanosheet (as shown in Figures 1(c) and 1(d)). The average pore diameter of this lotus root structure was 15-50 nm. The specific surface area of lotus root NiO was 156.485 m2/g, which was 13 times that of ordinary NiO. Due to this unique morphology, lotus root NiO had a response of 1.51 to 80 ppm ethanol at 300°C.
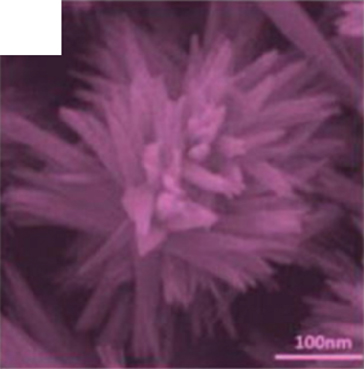
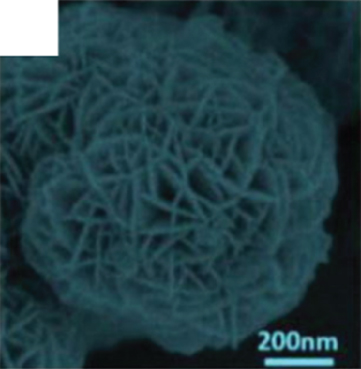
The hierarchically nanoflowers structured are assembled from nanoneedles and nanosheets. This hierarchical structure is beneficial for gas adsorption and electron transport, which improves the surface reaction efficiency. At the same time, the 3D structure increases the number of its adsorption sites. This novel flower-like structure has been demonstrated to have excellent functions in photoluminescence and photocatalysis [64–66]. Zhang and Zeng [55] investigated the sensitivity of nanoflower gas sensors assembled by nanoneedles and nanosheets to ethanol (Figures 2(a) and 2(b)). Both of them showed excellent gas response to ethanol. Due to the larger specific surface area of nanosheets, which could attract more gas molecules to react, the nanoflower sensors based on nanosheets obtained a larger gas response (the gas response of nanoneedles was 25 and nanosheets was 55). In particular, the authors mentioned that the nanoneedle-based NiO flowers could detect ethanol faster due to the lower potential energy and higher conductivity of the nanoneedles (the response/recovery time of nanoneedles was 1.7/2.2 s and nanosheets was 4.8/7 s). Nanoflowers composed of nanoneedles and nanosheets had their unique advantages, which meant that different morphologies can be synthesized according to specific needs in the future. If the sensor needed a higher response, the nanosheet flower might be a good choice; if the sensor needed to detect the target gas quickly, then the nanoneedle flower was an effective way. In addition to nanoflowers assembled from nanoneedles and nanosheets, Carbone and Tagliatesta [56] reported a granular flower assembled from particles. This granular flower was an aggregate of particles with a diameter of 10 nm (Figure 2(c)), whose surface had a great quantity of electrons that favor surface reactions. At the same time, the sturdy interparticle distance provided a stable surface for gas adsorption. NiO nanoparticles had charge/hole accumulation in short dimensions, which contained more accumulation of defects and traps. Therefore, these several factors led to the excellent ethanol sensing performance. The NiO granular flower sensor had a 35% response to 150 ppm ethanol at 200°C with a response/recovery time of 3/6 s.
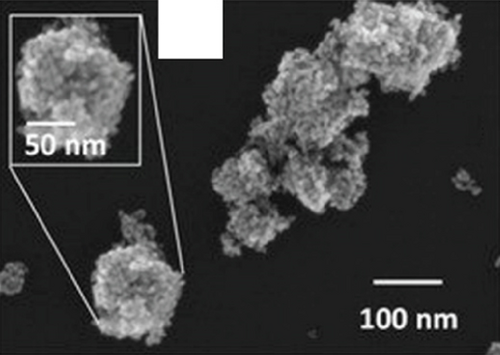
It is worth noting that a lot of research have found that the hollow structure will bring a higher specific surface area and more adsorption active sites [67–69]. The interior of the hollow structure can also serve as a microreaction chamber, providing a place for gas molecules to react with sensitive materials, and a short distance for carrier transport. For the hollow structure of NiO, NiO hollow nanofibers [70] and hollow spheres [71–75] have been reported, and they have obtained excellent performance in gas sensing with large specific surface area. In recent years, only NiO hollow spheres have been reported for ethanol gas sensing. Chu et al.’s team [73] used the Kirkendall effect to prepare NiO hollow nanospheres. At 240°C, the NiO hollow sphere-based sensor obtained a response of 3.1 to 800 ppm ethanol. The authors exhibited that the hollow nanostructures had enhanced surface activity, while the hollow shell layer enabled rapid diffusion of ethanol molecules and easy penetration into the interior of sensor. These properties made the hollow structure a new route to realize highly sensitive NiO ethanol sensors.
The exposed surfaces of semiconductor nanomaterials are also of interest to scholars, because different exposed surfaces lead to different surface atomic structures and properties. It has been confirmed by numerous experiments that NiO with exposed {111} facets had excellent sensitive characteristics, which owned to the 3-coordinated unsaturated Ni atoms of the {111} facets. Due to the lack of oxygen and great reducibility, the 3-coordinated Ni atoms can adsorb O2, bring free electrons, and promote the chemical reaction between oxygen species and ethanol gas [76, 77]. Furthermore, the {111} planes of NiO have a greater number of unsaturated O atoms and a higher surface energy than the usually exposed {220} planes, which is beneficial for sensitive performance [78, 79]. Liu et al. [77] prepared NiO foam structures with exposed {111} and {220} planes, and NiO with exposed {111} planes had fewer surface single-bond H groups and had higher 3-coordinated Ni atomic density. In addition, NiO with exposed {111} planes had a high Ni3+/Ni2+ ratio, which was favorable for electron transport. Combining with these advantages, the NiO gas sensor with an exposed {111} surface could detect ethanol as low as 20 ppm (response value was 1.57).
In conclusion, the morphology has a significant impact on the ethanol-sensitive performance of NiO. The morphology with large specific surface area and high porosity facilitates the adsorption and transport of gas molecules. The special advantages of the hollow structure are beneficial to the detection of ethanol gas by NiO. Meanwhile, there are a still few reports on NiO with exposed {111} facets. Therefore, the synthesis of porous hollow nanofibers and hollow spheres, as well as NiO sensors with exposed {111} planes, can be considered in future topography work. Table 1 summarizes gas performance of NiO in different morphology. However, the effect of morphology adjustment on gas response is still weak and needs to be combined with other modification methods.
| Material | Morphology | Conc. (ppm) | Operating temp (°C) | Response | Response/recovery time (s/s) | LOD (ppm) | References |
|---|---|---|---|---|---|---|---|
| NiO | Nanosheets | 100 | 130 | 68.1% | 5/20 | 0.2 | [53] |
| NiO | Nanosheets | 80 | 300 | 1.51 | -/- | — | [52] |
| NiO | Nanosheets | 50 | 240 | 11.15 | 4/7 | 1 | [51] |
| NiO | Nanoflower | 200 | 300 | 25 | 1.7/2.2 | — | [55] |
| NiO | Nanoflower | 200 | 300 | 55 | 4.8/7 | — | [55] |
| NiO | Nanoflower | 150 | 200 | 35 | 3/6 | 2.6 | [56] |
| NiO | Nanoflower | 100 | 190 | 46 | -/- | — | [58] |
| NiO | Hollow sphere | 800 | 240 | 3.1 | -/- | — | [73] |
3. Cation Doping
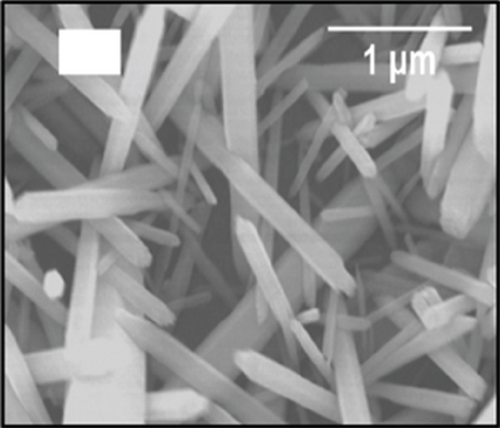
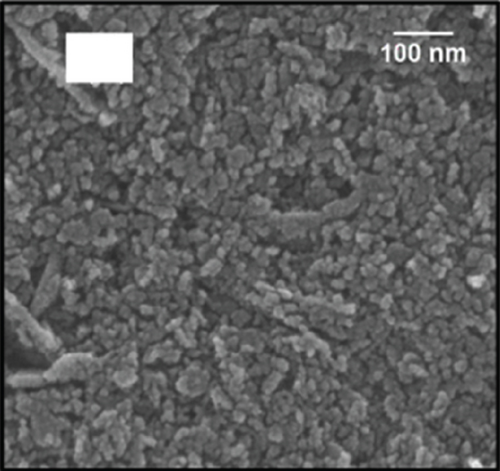
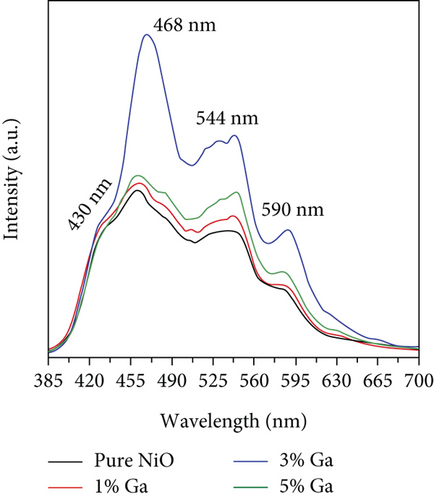
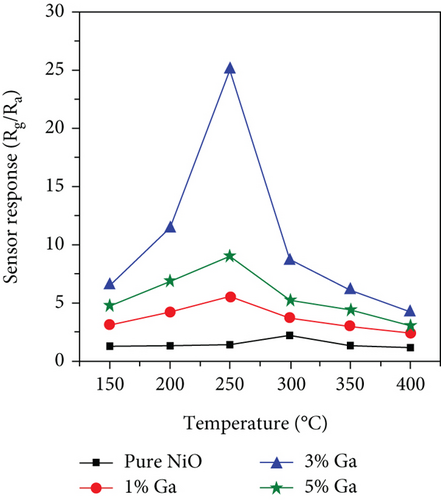
Doping is an essential way to enhance performance. Whether it is adding metal elements to alloys to improve their mechanical properties or adding donor and acceptor elements to dielectric ceramics to improve dielectric properties, doping is very effective [80, 81]. Doping ions are divided into high-valence ions and low-valence ions, that is, the donor ions and acceptor ions mentioned above. In improving the ethanol sensing performance of NiO, ions with a higher valence state than Ni2+ are often introduced. It was found that the introduction of high-valence cations always enhanced the response by changing the ground-state resistance of NiO. As the high-valence ions replace the Ni2+ sites, those generate electrons to compensate for the substitution. At the same time, the extra electrons provided by the high-valence ions recombine with the holes in the NiO valence band. These two factors cause the hole concentration decrease in NiO and the ground-state resistance increase, resulting in an enhanced response to the target gas [82–84]. Furthermore, doping induces lattice distortion and introduces more oxygen vacancy defects. When detecting ethanol, ethanol molecules are easily trapped by oxygen vacancy defects, allowing more target molecules to react with NiO, resulting in a larger gas response [85–87]. Shailja et al. [88] introduced Ga3+ into NiO. The introduction of Ga3+ transformed the structure of NiO from rod to a particle (Figures 3(a) and 3(b)). The BET test showed that this morphological transformation gave Ga-NiO a higher specific surface area (135.72 m2/g). The intensity of the photoluminescence peaks of pure NiO was lower than that of Ga-NiO (Figure 3(c)), which meant that the introduction of Ga brought more defects to NiO. The sensor was tested for its ethanol-sensitive properties, and it was found that Ga-NiO had lower operating temperature and higher gas response (300°C for pure NiO and 250°C for Ga-NiO, respectively) (Figure 3(d)). When the Ga doping level was 3%, the gas response to 50 ppm ethanol at 250°C was 25, and the response/recovery time was 8/13 s. In addition, Ga-NiO could detect ethanol down to 10 ppm.
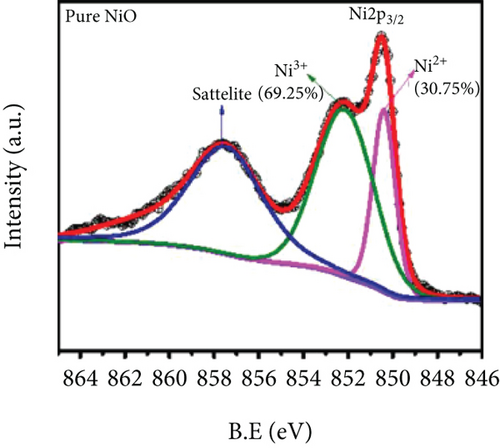
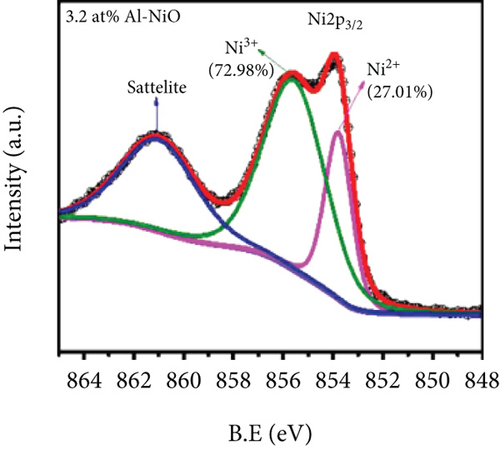
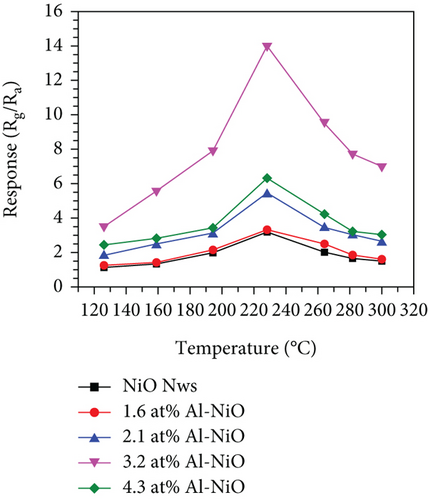
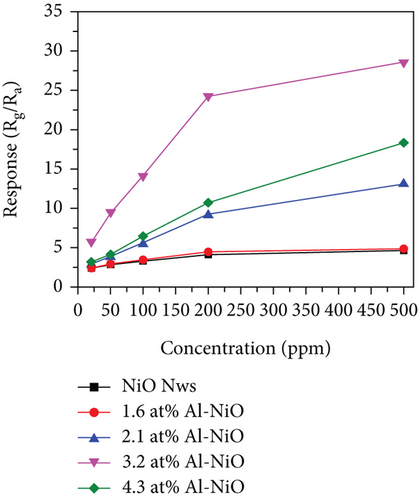
Mahmood et al. [89] studied the gas sensing performance of mesoporous Al-doped NiO ultralong nanowires to ethanol. Al3+ made NiO nanowires generate many pores, which brought a larger specific surface area. Owing to the smaller atomic radius of Al3+ (0.54 Å for Al3+ and 0.69 Å for Ni2+), Al3+ was easily substituted by Ni2+, resulting in a greatly increased Ni3+/Ni2+ ratio (Figures 4(a) and 4(b)). Larger Ni3+/Ni2+ meant higher electronic performance, leading to better gas sensing performance. At 228°C, the sensitivity efficiency of 3.2 at% Al-NiO to 100 ppm ethanol showed 14.01, which was higher than that of undoped NiO (Figures 4(c) and 4(d)). Meanwhile, 3.2 at% Al-NiO enabled rapid detection of ethanol (response/recovery time of 73/76 s). For the enhanced sensitive performance, the authors believed that it was related to the changes in pores, oxygen species content, and carrier concentration brought about by the incorporation of Al3+.
Besides doping high-valence ions, Chen et al. [90] also introduced Li+ and Zn2+ into NiO. The study found that the incorporation of low-valence ions Li+ and equivalent ions Zn2+ accelerated the detection of ethanol molecules by NiO. The response times of Li-NiO and Zn-NiO were 11 s and 17 s, respectively, while the response time of pure NiO was 33 s. However, Zn2+ hardly improved the response to ethanol, and Li+ even degraded the sensitivity of NiO. The authors believed that the Li+ extracted electrons from the valence band after forming the Li-O bond, leading the original resistance to decrease. Zn2+ in the same valence state hardly influenced the hole concentration, but the radius of Zn2+ was quite different from that of Ni2+, so more defects were induced, which led to a slight improvement in the response of Zn2+ to ethanol.
The ethanol gas sensing performance of NiO will be worsened by doping low-valence Li+ alone. But there was a study reported that the gas sensing performance of NiO may be ameliorated by adding high-valence ions at the same time as adding low-valence ions. Chang et al. [91] reported the simultaneous incorporation of Sc3+ and Li+ into NiO nanoflowers. In addition to resulting in higher specific surface area, codoping could stabilize metastable surface oxygen vacancies (OV) in metal oxides due to Li+ active sites, so Sc and Li codoping increased the OV ratio. Besides, Li+ could make the bonding strength of surface oxygen weak, thereby promoting the surface redox reaction. When the doping amount was 2 at.% Sc and 5 at.% Li, the sensitivity to 100 ppm ethanol at 200°C was 118.4, which was 105 times higher than that of pristine NiO. Meanwhile, the response time and recovery time of the Sc/Li-NiO sensor were 86 s and 31 s. The LOD was as low as 10 ppb. According to defect characterization and first-principle calculations, the codoping of Sc3+ and Li+ significantly increased the stable OV and Ni3+, which greatly promoted the adsorption of ethanol molecules, resulting in an excellent gas response.
In this section, we discussed the mechanism by which cation doping enhances the performance of NiO gas sensors. NiO is mainly doped with high-valence cations, and the changes in hole concentration and defect concentration caused by high-valence cations make NiO have better ethanol sensing performance. In particular, low-valence cations do not seem to be a method to enhance the sensitive performance of NiO. However, by doping with high-valence ions and low-valence ions, the unique properties of low-valence ions can be exerted, while high-valence ions improve the sensitivity. In the future, for the development of doping methods, we can pay more attention to high-valence/low-valence codoping. Table 2 exhibits gas performance of NiO doping by metal cation. However, the amount of ion doping is less controllable, and whether the ions successfully enter the lattice is difficult to control, while doping noble metal particles is relatively easy to control.
4. Precious Metal Particle Loading
Precious metal particle loading is a common way to improve sensitive properties. The improved performance of precious metal particles can be attributed to two reasons. First is chemical sensitization. Chemical sensitization exploits the catalytic properties of precious metal particles. Noble metal particles can catalyze O2 molecules to capture electrons from the valence band of NiO and dissociated into ionic species, resulting in more holes in the valence band [94]. A lower baseline resistance can result in a larger change in resistance, meaning better sensitivity. This results in a higher sensing response to ethanol gas. Second is electron sensitization. Electron sensitization utilizes a change in the Fermi level. After the precious metal particles are in contact with NiO, the Fermi levels of the two are not at the same level. To reach electronic equilibrium, the Fermi level of NiO will shift, creating additional depletion layers and reducing the HAL thickness [68].
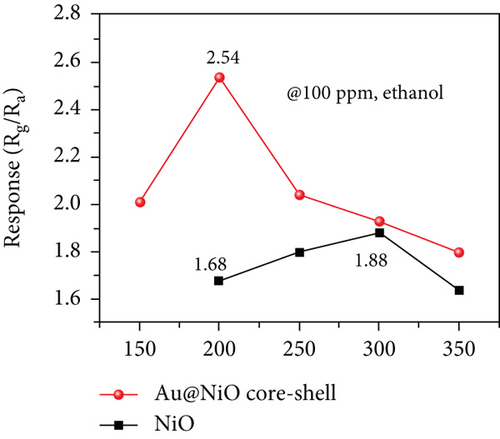
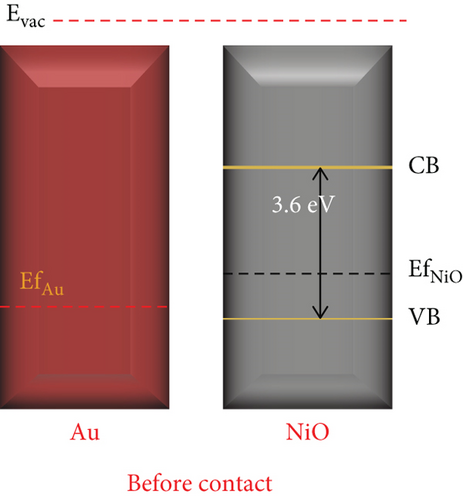
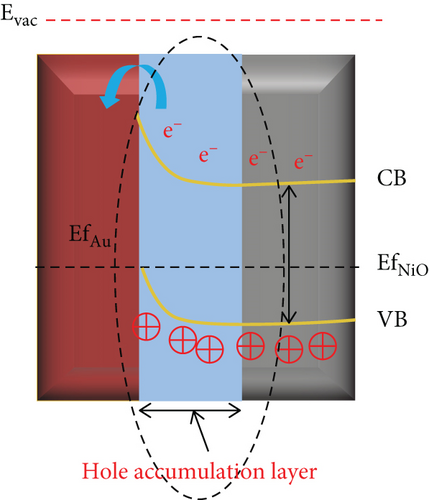
Enhanced sensitive properties are usually caused by a combination of the two. For example, Majhi et al. [95] utilized the electronic and chemical sensitization effects of Au to obtain an Au-NiO gas sensor with excellent gas sensing performance. Au particles were encapsulated inside NiO to form a core-shell structure (Figure 5(a)). The optimal operating temperature of Au-NiO was 200°C, which was 100°C lower than that of NiO (Figure 5(b)). Au-NiO obtained a 2.54 response to 100 ppm ethanol at 200°C, and the response time and recovery time were 250 s and 420 s. Compared with pristine NiO, Au-NiO had a larger response and more rapid response/recovery speed. Since the Fermi levels of Au and NiO were not at the same level, Au and NiO contacted to form a Schottky junction (Figures 5(c) and 5(d)). At the same time, additional HAL was formed near the Au-NiO interface, which increased the quantity of holes in Au-NiO, resulting in a larger resistance. Fu et al. [96] synthesized Pt-NiO nanotubes by electrospinning, and the NiO nanotubes functionalized by Pt were dendriform. Due to the activation and catalytic effect of Pt on NiO, the response of 0.7% Pt-NiO nanotubes at 100 ppm ethanol and 200°C was 20.85, while pure NiO got 2.06 under the equal environment. Park et al. [97] studied the sensing performance of pristine NiO and Au-modified NiO for ethanol. The responses of NiO and Au-NiO sensors to 1000 ppm ethanol at 325°C were 273% and 442%, respectively. The author attributed the enhanced sensitive performance to the additional depletion layer and Schottky barrier formed by the Au particles, high catalytic activity, and smaller diameter of Au-NiO.

Although the chemical catalysis and electronic catalysis of precious metal particles bring excellent ethanol sensing properties to NiO, there are few reports on the modification of NiO by those in recent years. The main reason is that precious metal particles are expensive. Considering the cost, it is difficult to apply them to actual production. Table 3 is gas performance of NiO functional by precious metal particles in recent years. The Schottky barrier generated by the combination of noble metal particles and NiO is similar to PN heterojunctions, so scholars are keen to form heterojunctions to improve the ethanol-sensitive properties of NiO.
5. Composites
NiO, p-type semiconductor, is often combined with n-type semiconductors to form a PN heterojunction. The Fermi level of the p-type semiconductor is lower than that of the n-type semiconductor. When the two are in contact, electrons move to the p-type semiconductor and holes move to the n-type semiconductor, resulting in band bending. An EDL is then formed in the hetero area and strongly inhibits the conduction channel. When the heterojunction contacts the target gas, electron release greatly reduces the potential barrier at the heterojunction, causing a bigger change in resistivity and a higher sensitivity [46, 98, 99].
5.1. Metal Oxides
In recent years, NiO has been reported to recombine with n-type semiconductors such as ZnO [63, 100–110], SnO2 [67, 111–118], WO3 [119–121], Fe2O3 [122–124], and In2O3 [125, 126].
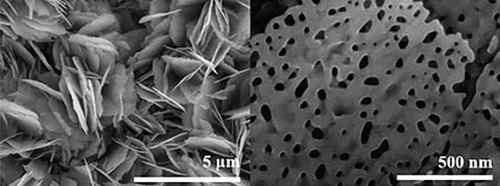
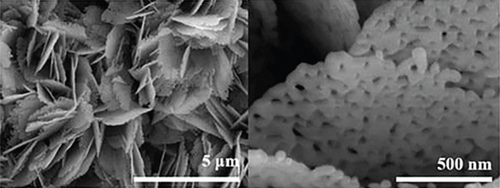
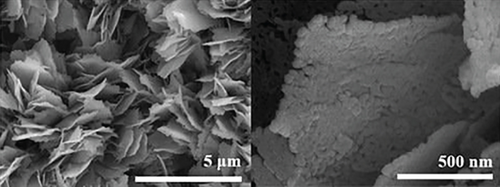
The n-type semiconductor ZnO has received attention due to its low cost and fast response speed, which can detect a variety of gases [17, 18, 127]. Among the recent reports, NiO- and ZnO-combined sensors have the most detection of ethanol. Bai et al. [108] used electrospinning and atomic layer deposition (ALD) to design a NiO/ZnO core-shell nanotube (CSNT), in which NiO was the core and ZnO was the shell (Figure 6). There were many pores and some hollow structures in such nanotubes, which helped gas molecules to be fixed in the pore channels. NiO/ZnO CSNTs obtained a gas response of 16 for the detection of 100 ppm ethanol at 325°C, and it was much bigger than that of pristine ZnO (8.8) and pure NiO (2.5). In addition, NiO/ZnO had a shorter response time (13 s) but a longer recovery time (498 s). The nice gas sensing performance thanked to the shell thickness of NiO/ZnO close to the Debye length (λD). In this case, a fully electron-depleted state was established and the most effective resistance modulation occurred, causing the highest gas sensing performance of NiO/ZnO. When the shell of the core-shell structure was thicker, the resistance modulation through the shell would be significantly reduced, owing to the depletion of some electron in the thick shell, thus resulting in a reduced gas response. Liang et al.’s team [63] sputter-deposited NiO onto porous ZnO nanosheets. NiO was uniformly attached to ZnO, and the addition of NiO narrowed the pore size on ZnO nanosheets (Figure 7). Due to the different work functions, a built-in electric field was formed at the NiO/ZnO heterojunction interface, which also induced band bending. The formation of the built-in electric field leads to a higher barrier height and a higher initial resistance. After exposure to ethanol gas, electrons were released, reducing the barrier height of the depletion layer. Besides, NiO/ZnO had a distinctive porous nanosheet structure, which provided abundant surface active sites and a lot of gas diffusion channels for ethanol molecules.
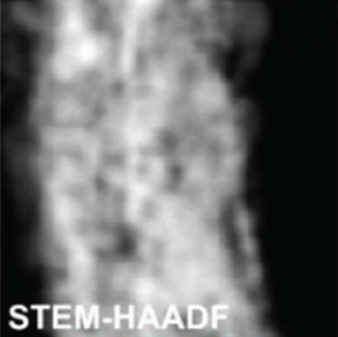
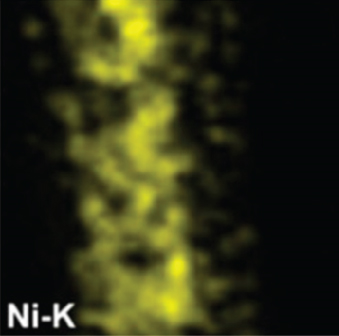
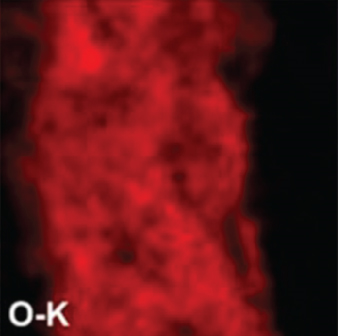
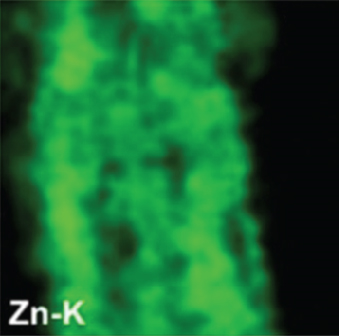
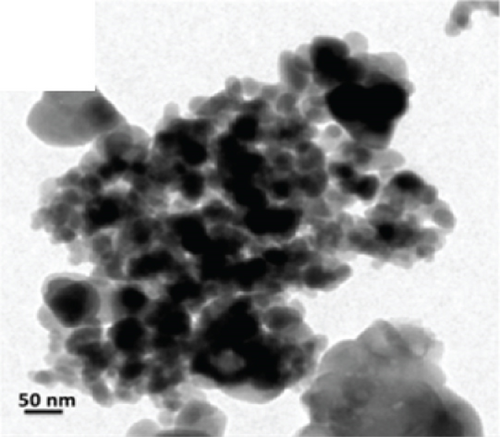
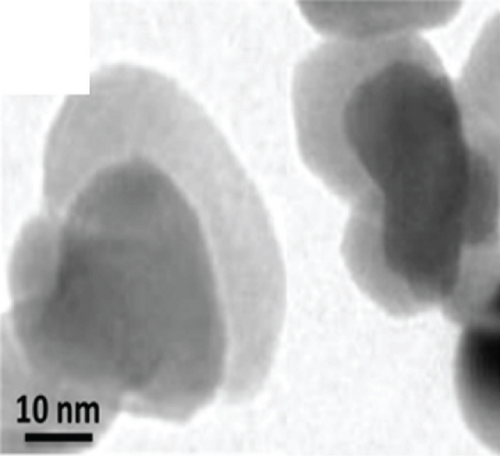
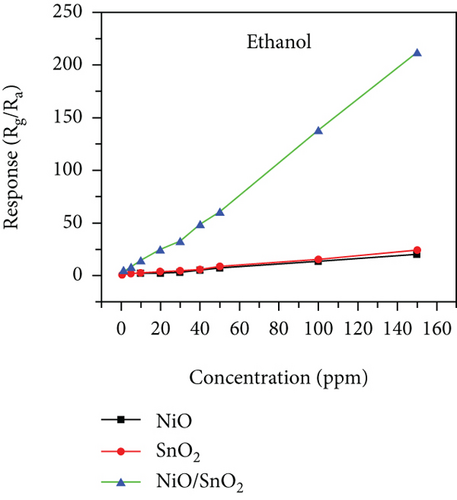
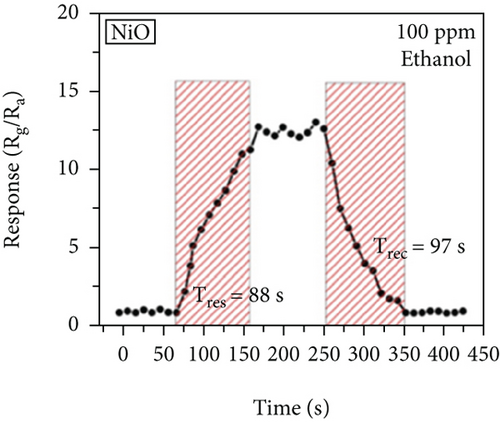
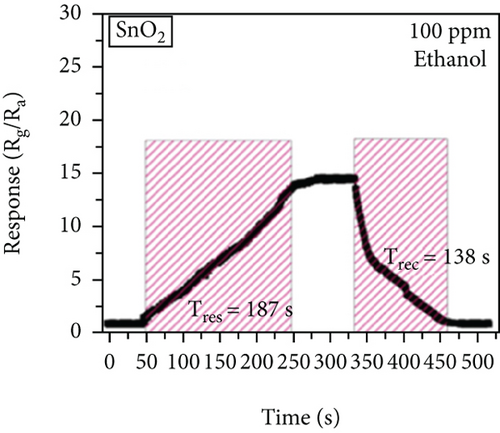
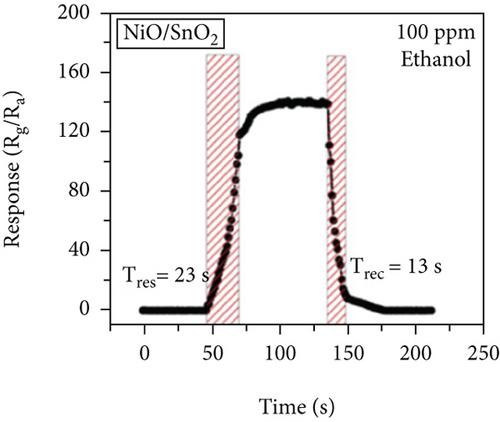
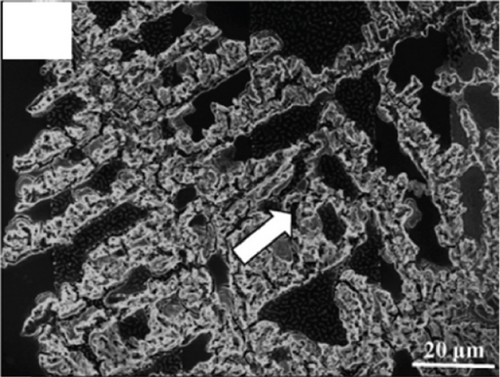
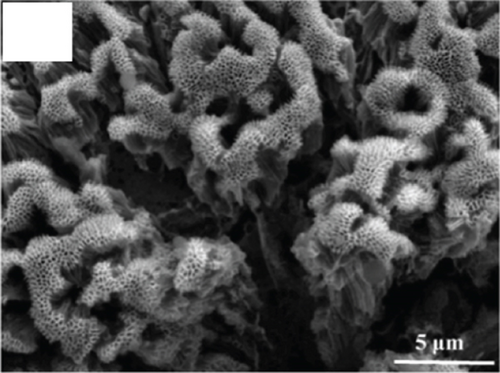
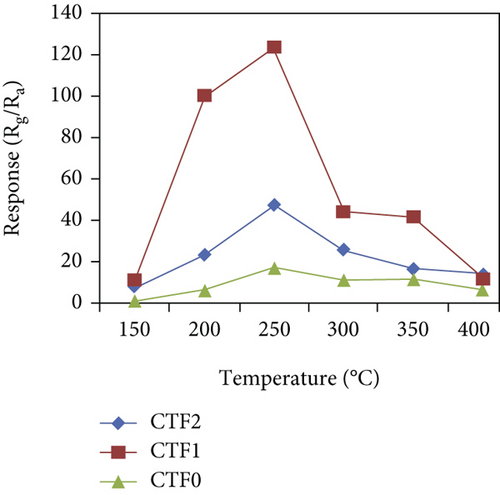
There have been many reports that SnO2 is easily composited with other materials to form heterostructures, which is a perfect sensitive material and is often used in ethanol gas sensors [13, 15, 128]. Jayababu et al. [111] composited NiO with SnO2 to form an ethanol sensor. NiO had a semishielding effect on SnO2 nanoparticles by HRTEM; that is, NiO partially covered SnO2 (as shown in Figures 8(a) and 8(b)). Due to the PN heterostructure and catalytic activity brought by NiO, the NiO/SnO2 sensors exhibited excellent sensing performance for ethanol. At RT, NiO/SnO2 achieved a response as high as 140 for ethanol, much higher than SnO2 and NiO (seen in Figure 8(c)). Beyond that, NiO/SnO2 showed fast response and recovery speed, with response and recovery times of 23 s and 13 s, respectively (as shown in Figures 8(d)–8(f)). Zhang et al.’s group [115] synthesized a novel NiO/SnO2 vertical nanotube composite film, which consisted of 20 nm diameter NiO nanosheets attached to one-dimensional SnO2 nanotubes (Figures 9(a) and 9(b)). When the molar ratio of Ni2+/Sn4+ was 5%, NiO/SnO2 had high adsorbed oxygen. At 250°C, it had the highest gas response to ethanol (123.7 for 1000 ppm ethanol, as shown in Figure 9(c)). Figure 9(d) is the gas response curve for five gases. NiO/SnO2 has high selectivity for ethanol, which was beneficial to realize the efficient detection of ethanol. Since SnO2 nanotubes could transport electrons quickly, NiO/SnO2 could rapidly detect ethanol compounds within 10 s. After the heterojunction of SnO2 and NiO was formed, the band bending led to the narrowing of the electron transport channel, which increased the ground-state resistance. The higher ground-state resistance was beneficial in enhancing the gas sensing performance, so the respond value of NiO/SnO2 was higher than NiO.
α-Fe2O3, a n-type semiconductor, has been demonstrated as an ethanol sensor due to its low cost, high stability, and environmental friendliness [129–131]. Tan et al. [124] employed microwave-assisted liquid-phase synthesis to support Fe2O3 on NiO nanosheets. Owing to the enhanced sensitivity of the heterojunction pair of Fe2O3 and NiO, the 1.5% Fe2O3/NiO sensor got a high response of 170.7 and an ultrafast response/recovery speed (0.5/14.6 s) to 100 ppm ethanol. In2O3 has received attention in gas sensors because of its stable electrical conductivity, good chemical stability, and low cost [28, 132]. Yan et al. [125] successfully synthesized In2O3/NiO composites through electrospinning technology and ion exchange reaction. The NiO nanosheets were arranged in one dimension, and the In2O3 nanoparticles were uniformly attached to them. During the detection of ethanol, the charge carriers of In2O3/NiO were conducted through the continuous NiO. The formation of the PN junction created a thicker hole depletion layer under In2O3. Therefore, a small change in carrier concentration resulted in a larger gas response. WO3 is favored in ethanol gas sensors because of its special physicochemical and electrical properties [133, 134]. Juang and Wang’s team [120] found that the composite of porous NiO spheres and WO3 nanoplates had a response of 155% to ethanol, which was 1.4 times that of pure WO3 nanoplates. The enhanced ethanol sensitivity was attributed to the formation of the PN heterojunction. Table 4 shows gas performance of NiO/metal oxide-based composites.
| Material | Conc. (ppm) | Operating temp (°C) | Response | Response/recovery time (s/s) | LOD (ppm) | References |
|---|---|---|---|---|---|---|
| NiO/SnO2 | 100 | 160 | 13.4 | -/- | — | [112] |
| NiO/SnO2 | 100 | RT | 140 | 23/13 | 1 | [111] |
| NiO/SnO2 | 100 | 160 | 23.87 | 57/66 | — | [113] |
| NiO/SnO2 | 50 | 300 | 9 | -/- | 5 | [114] |
| NiO/SnO2 | 1000 | 250 | 123.7 | 10/58 | — | [115] |
| NiO/SnO2 | 1000 | 150 | 84.7 | 5/15 | — | [116] |
| NiO/SnO2 | 100 | 260 | 153 | -/- | 5 | [117] |
| NiO/SnO2 | 1000 | 320 | 576.5 | 9/34 | — | [118] |
| NiO/SnO2 | 100 | 75 | 248 | -/- | — | [67] |
| NiO/ZnO | 100 | 260 | 61 | -/- | — | [101] |
| NiO/ZnO | 100 | 200 | 11.3 | 4/78 | — | [63] |
| NiO/ZnO | 100 | 350 | 54 | -/- | — | [102] |
| NiO/ZnO | 200 | 300 | 33 | -/- | — | [103] |
| NiO/ZnO | 250 | RT | 32.48% | 2.7/3.6 | — | [105] |
| NiO/ZnO | 500 | 400 | 37.5 | 2.1/4.1 | — | [106] |
| NiO/ZnO | 100 | 325 | 16 | 13/498 | — | [108] |
| NiO/ZnO | 20 | 200 | 49 | 4/28 | 3 | [110] |
| NiO/WO3 | 100 | 300 | 58.2 | -/- | — | [119] |
| NiO/WO3 | 200 | 175 | 155% | 19/20 | — | [120] |
| NiO/WO3 | 200 | 320 | 10 | -/- | — | [121] |
| NiO/Fe2O3 | 100 | 255 | 170.7 | 0.5/14.6 | 0.2 | [124] |
| NiO/In2O3 | 100 | 280 | 4.61 | -/27 | — | [125] |
| NiO/In2O3 | 5 | 350 | 9.76 | -/23 | 2 | [126] |
| NiO/Co2O3 | 100 | 250 | 4.26 | -/- | 0.073 | [135] |
5.2. Bimetallic Oxides
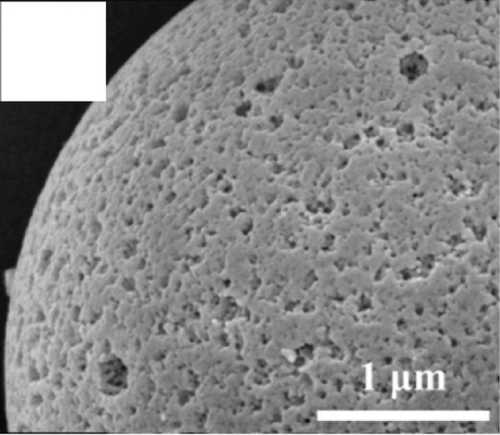
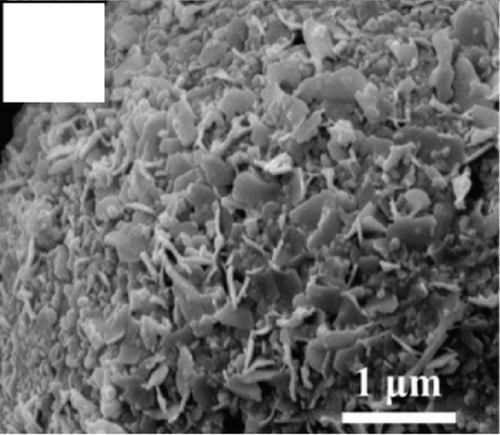
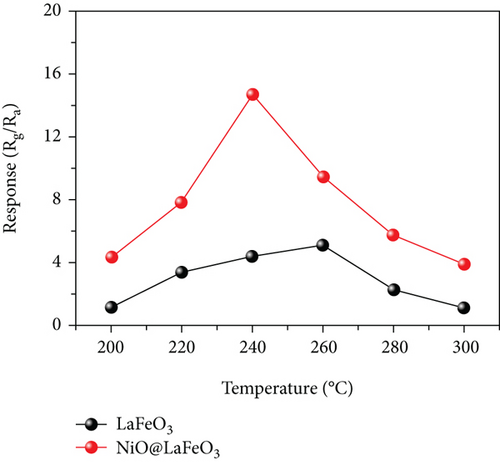
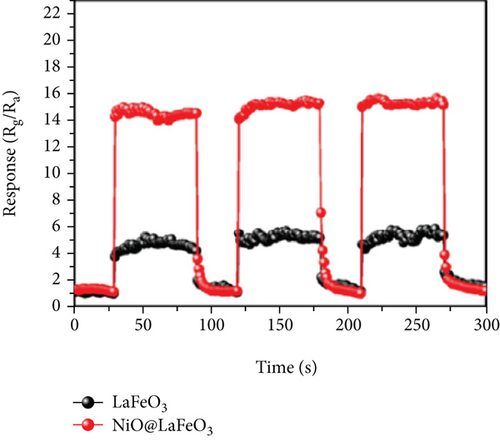
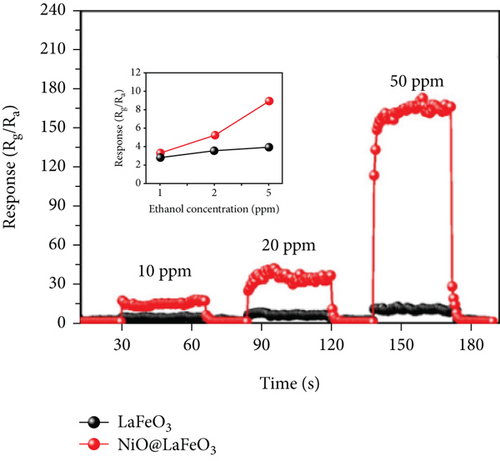
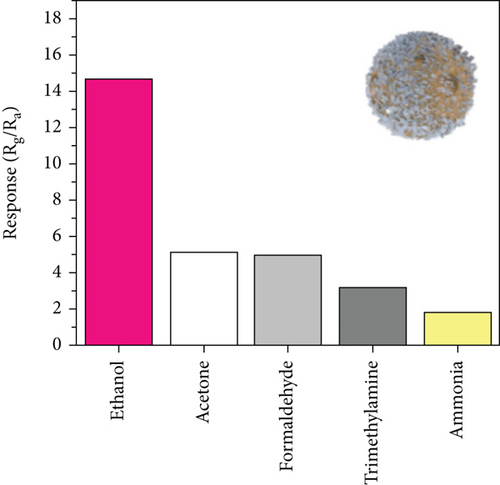
Apart from the above single metal oxides, bimetallic oxides have also been used to improve NiO materials. LaFeO3 has been studied in gas sensors owing to its high electrical conductivity and good redox properties [136, 137]. Hao et al.’s team [138] modified NiO nanosheets onto porous LaFeO3 microspheres and studied their ethanol sensing properties. Figures 10(a) and 10(b) are the SEM images of LaFeO3 and NiO/LaFeO3. The surface of LaFeO3 had abundant pore structure, and NiO nanosheets were uniformly distributed on the LaFeO3 microspheres. This morphology effectively increased the contact area between the composite and ethanol. Figure 10(c) is the gas response of NiO/LaFeO3 and LaFeO3 under different temperatures at 10 ppm ethanol. The optimum operating temperature of NiO/LaFeO3 was 240°C, while LaFeO3 was 260°C. It meant that NiO/LaFeO3 could lower the operating temperature, which was beneficial for low temperature sensing. The response recovery curves of 10 ppm ethanol at 240°C are shown in Figure 10(d). Both NiO/LaFeO3 and LaFeO3 had good reproducibility, and the response of NiO/LaFeO3 was much higher than that of LaFeO3. In addition, NiO/LaFeO3 had fast response and recovery speed (response time and recovery time were 2 s and 9 s, respectively). The response of NiO/LaFeO3 also increased with increasing ethanol concentration and was consistently higher than that of pristine LaFeO3 (Figure 10(e)). The NiO/LaFeO3-based sensors detected five different gases, as shown in Figure 10(f). The NiO/LaFeO3 sensors exhibited a good selectivity to ethanol, which provided a new way to fabricate high-performance ethanol sensors. The enhanced sensing performance was ascribed to the special structure, the catalytic performance of NiO, and the electronic modulation of the Fermi level.
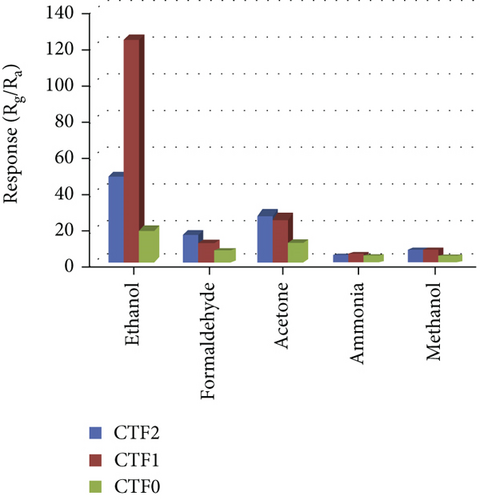
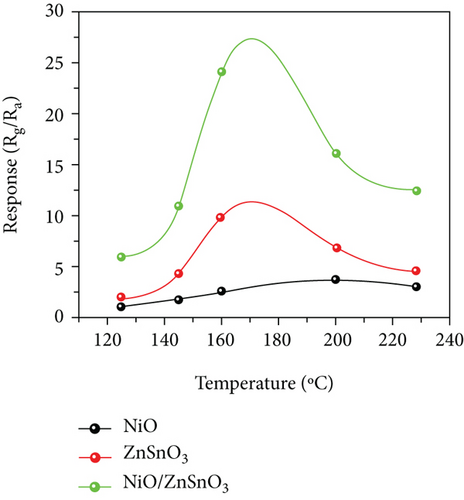
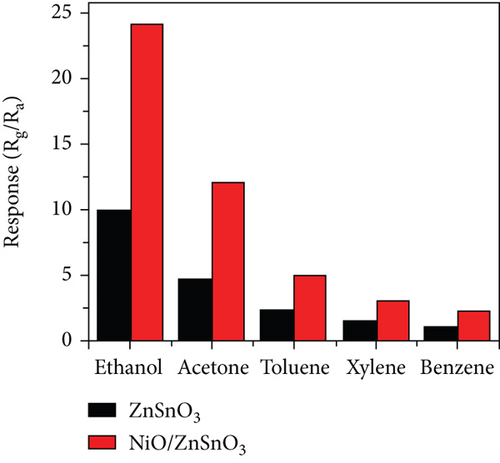
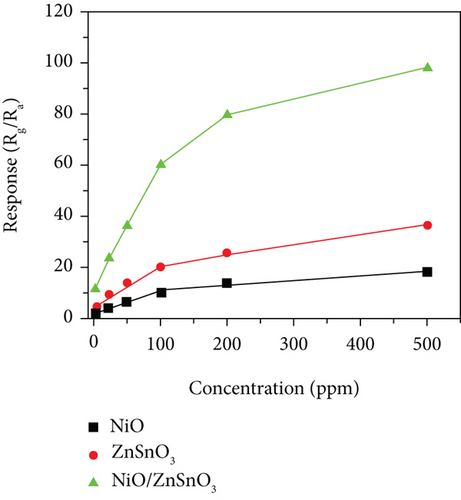
Haq et al. [139] used a hydrothermal method to decorate NiO nanoparticles on ZnSnO3 fibers (Figure 11(a)). The gas responses of all samples at different temperatures were volcanic shape (Figure 11(b)). It was worth noting that NiO/ZnSnO3 obtained the highest response at 160°C, while NiO obtained the highest response at 200°C. It meant that NiO/ZnSnO3 obtained the highest response at 160°C. The ZnSnO3 composite could detect ethanol at lower temperatures. As in Figure 11(c), ethanol, acetone, toluene, xylene, and benzene were detected. The NiO/ZnSnO3 sensors possessed a much higher response to ethanol than other gases, with better selectivity. Figure 11(d) is the gas response of NiO/ZnSnO3, NiO, and ZnSnO3 to different ethanol concentrations at 160°C. As the ethanol concentration increased, the gas response also increased gradually. And NiO/ZnSnO3 obtained the best ethanol sensing performance. The response of NiO/ZnSnO3 to 20 ppm ethanol gas at 160°C was 23.95. The enhanced sensitivity was attributed to the more oxygen content of ZnSnO3 after NiO decoration. At the same time, ZnSnO3 was an n-type semiconductor. The modulation effect brought by the PN heterojunction also made the resistance change of NiO/ZnSnO3 larger, resulting in a higher gas response. Table 5 shows gas performance of NiO/bimetallic oxide-based composites.

In this section, we discussed the reported composites of NiO combined with MOS such as ZnO, SnO2, Fe2O3, In2O3, WO3, and bimetallic oxides LaFeO3 and ZnSnO3 in ethanol sensing. The combination of NiO with these oxides mainly relies on the electronic modulation brought about by the formation of PN heterojunctions. Although NiO composites have been reported, most of them are about oxides. For the future, other potential gas sensing materials, such as conducting polymers, MXene, and carbon-based materials, can also be compounded with NiO to improve the sensitive properties.
6. Outlook
Although there have been many reports on the ethanol-sensitive performance of NiO in recent years, reports on the in-depth study of the selective mechanism of ethanol are still rare. Most of the literature addresses the fact that there is selectivity but does not discuss the ultimate mechanism of selectivity. Highly selective gas sensors are necessary in complex gas environments, where they need to accurately identify target gases among interfering gases. The study of the mechanism of selectivity is helpful for the further development of highly selective gas sensors. In future work, researchers can further study the mechanism of ethanol selectivity to facilitate the efficient detection of ethanol in environments with interfering gases. At the same time, molecular imprinting technology is expected to be applied to improve selectivity. Molecular imprinting is a lock-and-key technology that enables specific identification of specific gases. Combining molecular imprinting technology with resistive sensors is believed to further address the selectivity challenges of gas sensors.
7. Conclusion
In conclusion, this review presented an overview of NiO-based ethanol gas sensors. We first introduced the effect of modified NiO morphology on ethanol performance that has been reported in recent years. The morphology with large specific surface area and high porosity was considered to be the structure that can bring high gas response. Then, we discussed the enhanced effect of the doping of metal cations and precious metal particle loading on the ethanol-sensitive properties of NiO. Doping ions increased the ground-state resistance and increased the oxygen defect concentration of NiO. The effects of precious metal particles on the performance of NiO included chemical sensitization and electronic sensitization. Finally, the related contents of NiO forming composites with metal oxides and bimetallic oxides were discussed. In this section, the specific improvement mechanism was discussed first, and then, the related work of researchers in recent years was summarized.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the Graduate Research and Innovation Foundation of Chongqing, China (Grant No. CYS22003).
Open Research
Data Availability
All data can be obtained from corresponding author Yanqiong Li.