Mixture of Toxic Metals and Volatile Organic Compounds in a River Induces Cytotoxicity
Abstract
Pollution of aquatic ecosystems due to toxic mixtures is a worldwide problem associated with the increase of wastewater discharges that causes problems to human health and biodiversity. This study aims to evaluate the cytotoxic potential of water from the Atoyac River. Meristems of Allium cepa L. were exposed to water samples from the Atoyac River with different concentrations for 120 hours. Pearson correlation was used to investigate the relationship between contaminants and cytotoxicity. The results corroborated the cytotoxic effect of the mixture of agents such as toxic metals and volatile organic compounds found in all river sampling sites. The Allium cepa test showed decreased mitotic alterations in prophase and metaphase indices. There was a strong negative association between the concentration of toxic metals and volatile organic compounds and the cytotoxic effect. The observations of cytotoxic effects show that the contaminant mixture contains aneugenic agents which prevent the synthesis and fixation of fibers of the mitotic spindle to the kinetochore, which prevents the displacement of the chromosomes. This study shows the need to study the effects at the cellular and molecular level in heavily polluted rivers to prevent negative effects on exposed ecosystems and populations.
1. Introduction
Rivers are one of the main sources of drinking water for humanity [1]; however, its pollution has been increasing in developed countries and has become a global environmental problem. It is estimated that, of the 500 major rivers in the world, 50% are polluted [2]. The main sources that have contributed to river pollution are agricultural runoff and industrial and urban wastewater discharges [3], which contain great amounts of waste such as fertilizers, pesticides, toxic metals, and organic and inorganic compounds [4], which upon reaching the aquatic ecosystem produce toxic pollutant mixtures [5] with high cytotoxic potential capable of inducing biodiversity changes and people mutations and diseases such as cancer [4–7].
Few studies have been conducted globally to determine the cytotoxic potential of complex mixtures of toxic metals (TM) and volatile organic compounds (VOCs) in aquatic ecosystems such as rivers [8, 9], and most have focused on evaluating the cytotoxic effects of mixtures of TM and VOCs in occupational [10, 11] and urban environments [12]. Therefore, there is a need to conduct cytotoxic assessment studies using biomodels that explain the short- and long-term health risks of organisms exposed to aquatic environments influenced by contaminant mixtures of TM and VOCs that are potentially carcinogenic.
The objective of this research was to evaluate the effect of the contaminant mixture of TM and VOCs present in the water of the Atoyac River and its correlation on the mitotic index (MI) and phase index (PI) in the cell population with respect to the concentration of contaminants in different sampling zones using the biomodel Allium cepa L., which is a model recognized as one of the best indicators to evaluate the cytotoxic potential of environmental pollutants due to its high sensitivity, good correlation with other test systems, easy handling, low cost, and large chromosomes [13]. The study will serve as a proposal to consider cytotoxic evaluation as part of the integral characterization of water quality in contaminated water bodies and will help provide information to complement the Official Standards to avoid health risks to which the inhabitants of river areas with strong environmental degradation could potentially be exposed.
2. Materials and Methods
2.1. Sample Collection
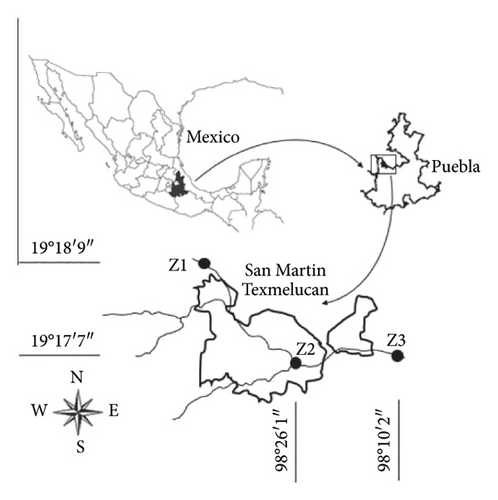
Surface water samples were collected in 3 zones along the Atoyac River through the municipality of San Martin Texmelucan, Puebla (Figure 1). San Martin Texmelucan (geographical coordinates: 19°17′07″N 98°26′01″W), which is in the western part of the Puebla-Tlaxcala Valley, has a population of 152,052 inhabitants which are distributed in a territorial extension of 71.45 km2 [14]. The collection areas were chosen according to the anthropogenic characteristics of the site (Table 1). Samples were collected during the rainy season (April 2019), which begins in the municipality of San Martín Texmelucan in April and ends in October with a rainfall range of 700–800 mm [15]. Therefore, the area is classified as torrentially rainy according to the criteria of State Weather Agency [16].
| Zone | Locality | Coordinates | Characteristics |
|---|---|---|---|
| 1 | San Cristobal Tepatlaxco | 19°17′54.6″ N | Agroforestry area (corn and wheat crops), livestock activities (bovine cattle), 12,045 inhabitants, 3 small industries (processing of dairy products), and 290 commercial establishments |
| 98°27′23.0″ W | |||
| 2 | San Martin Texmelucan Labastida | 19°16′59.1″ N | Urban area with high population density (60,040 inhabitants), 36 medium-sized companies, and 3265 commercial establishments |
| 98°25′31.4″ W | |||
| 3 | Villa Alta | 19°17′75.8″ N | Rural area with industrial manufacturing activity, agricultural production of grains and vegetables, an industrial park and petrochemical complex, and 5974 inhabitants. |
| 98°10′1.6″ W |
For taking the samples, the guidelines of the Mexican Standard NMX-AA-121/1-SCFI-2008 [17] were followed. A volume of 2 L was collected from each area in properly sterilized wide-mouth polyethylene containers. The containers with the samples were stored under refrigeration at approximately 5°C in a cooler and transported immediately to the laboratory to be used the same day for physicochemical analysis and bioassays.
2.2. Allium cepa Test
2.3. Physicochemical Analysis
Determining some physicochemical parameters of water samples helps us to better understand the relationship with the results, since water quality can affect metabolism in biological systems. In the present investigation, pH parameters were determined in situ by using a pH meter (pHep® model HI 98107), electrical conductivity was determined by using a portable meter (CONDUCTRONIC model PC18), dissolved oxygen was calculated using the Winkler method with a Hanna® HI 3810 Dissolved Oxygen Test Kit, and temperature was determined by using a glass thermometer with a mercury column scale from −10°C to 110°C (Celsius brand).
Water quality characterization was complemented with laboratory tests to determine the following parameters: chemical oxygen demand (COD), which was determined with a Spectroquant kit in the 25–1500 mgL−1 range. The test is based on the method for determining chromium III and chromium sulfuric acid oxidation, biochemical oxygen demand (BOD5), turbidity, volatile organic compounds, and toxic metals. Turbidity measurement was carried out with the Nova 60 Spectroquant photometer using a 10 ml volume of the sample, without any treatment or filtering, with a 50 mm thick rectangular measuring cell. The determination of VOCs was carried out by gas chromatography coupled to a mass spectrometer, under the guidelines of NMX-AA-103-SCFI-2006 [19], for which a gas chromatograph with a capillary injection port and mass selective detector was used. The measurement of TM was carried out in accordance with NMX-AA-051-SCFI-2006 [20] using a flame atomization atomic absorption spectrophotometer with a double beam monochromator, light source, and photomultiplier detector adjustable to the wavelength intervals of the analytes to be quantified.
2.4. Statistical Analysis
Comparison was made between treatments and their respective control groups in each study area. Quantitative data were analysed by the ANOVA method to compare several groups in a quantitative variable. The results were plotted as the mean ± standard error of the mean (SEM). Post hoc analysis was done by Tukey’s test, p ≤ 0.05. The correlation between the concentration of arsenic, cadmium, chromium, copper, mercury, benzene, chloroform, and tetrachloroethylene with mitotic index was determined by calculating Pearson correlation coefficients with p ≤ 0.05 significance. Statistical treatments of the data were carried out using Minitab 19 software.
3. Results
Table 2 presents the analysis of variance of 14 physicochemical parameters evaluated in the 3 sampling zones of the Atoyac River. The analyses showed significant differences in 35.7% and 85.7% of the parameters evaluated in zones 2 and 3, respectively, in relation to zone 1. The values of BOD5 and COD increased 209% and 752%, respectively, in zone 3 in relation to zone 1, and the amount of DO decreased 11 times in relation to zone 1. A significant increase in the concentration of TM was observed in in zone 3.. For example, mercury levels were 600 times higher in zone 3 than in zone 1. Regarding VOCs, the results showed that 100% of the parameters quantified in zone 3 were significantly higher than in zones 1 and 2. Benzene and trichloroethylene were 25.5 and 23.3 times higher, respectively, than in zone 1. These results indicate that river pollution is generalized in the municipality, but it is not the same at all points, and zone 3 presents the highest degree of water pollution due to the strong influence of anthropic activities in the area, most of which are of industrial origin.
| Parameters | Sampling area | ||
|---|---|---|---|
| Z1 | Z2 | Z3 | |
| pH (UpH) | 6.5 ± 0.2a | 4.9 ± 0.5a | 5.2 ± 0.4 |
| Ec (mS) | 0.143 ± 0.02a | 0.246 ± 0.13a | 0.310 ± 0.23b |
| TUB (NTU) | 145.6 ± 2a | 208 ± 3b | 237.6 ± 3b |
| COD (mgL−1) | 62 ± 3a | 189 ± 4b | 192 ± 6b |
| BOD5 (mgL−1) | 6.95 ± 0.28a | 34.24 ± 0.94b | 58.79 ± 0.52b |
| DO (mgL−1) | 5.5 ± 0.6a | 1.1 ± 0.3b | 0.5 ± 0.2b |
| As (mgL−1) | 0.4a | 0.7a | 1.7b |
| Cd (mgL−1) | 0.3a | 0.4a | 0.9b |
| Cu (mgL−1) | 3.8a | 4.8a | 8.8b |
| Cr (mgL−1) | 1.3a | 1.5a | 7.5b |
| Hg (mgL−1) | 0.002a | 0.2b | 1.2c |
| C6H6 (µgL−1) | 0.02a | 0.3a | 0.5b |
| CHCl3 (µgL−1) | 0.05a | 0.05a | 0.30b |
| C2HCl3 (µgL−1) | 0.03a | 0.03a | 0.70b |
- pH: hydrogen potential; Ec: electrical conductivity; TUB: turbidity; COD: chemical oxygen demand; BOD5: biochemical oxygen demand; DO: dissolved oxygen; As: arsenic; Cd: cadmium; Cr: chromium; Hg: mercury; C6H6: benzene; CHCl3: chloroform; C2HCl3: trichloroethylene; Z1: study area 1; Z2: study area 2; Z3: study area 3. Different literals (a, b, and c) indicate a significant difference between study zones (p < 0.05).
Table 3 shows the analysis of variance of the mitotic index and cell population phases in the apical meristems of Allium cepa L. exposed for 120 hours to different concentrations of water from the Atoyac River in the 3 study zones. The mitotic index decreased in the treatments of zones 2 and 3. The most significant decreases occurred in the treatments of 50% and 100% of zone 3 with a decrease of 23.1% and 100%, respectively, in relation to the control group. These results show that there is a concentration-dependent antimitotic effect of the pollutants present in the different study zones.
| Zone | Group | MI (M ± SD) | % Phase index (M ± SD) | |||
|---|---|---|---|---|---|---|
| PI | MeI | AI | TI | |||
| Z1 | C | 54.3 ± 0.5a | 91.5 ± 1.4a | 3.5 ± 0.4a | 3.1 ± 0.4a | 2.0 ± 0.6a |
| 25% | 53.6 ± 0.9a | 91.1 ± 0.5a | 3.9 ± 0.2a | 3.4 ± 0.4a | 1.7 ± 0.3a | |
| 50% | 51.6 ± 0.9a | 91.5 ± 1.3a | 4.1 ± 0.2a | 2.7 ± 0.8a | 1.8 ± 0.4a | |
| 100% | 54.3 ± 1.1a | 93.1 ± 2.1a | 2.7 ± 0.7a | 2.6 ± 0.7a | 1.5 ± 0.8a | |
| Z2 | C | 53.8 ± 0.5a | 92.2 ± 1.5a | 3.1 ± 0.3a | 2.9 ± 0.7a | 1.8 ± 0.6a |
| 25% | 54.3 ± 0.2a | 91.7 ± 1.7a | 3.4 ± 0.1a | 2.3 ± 0.4a | 1.9 ± 0.3a | |
| 50% | 46.5 ± 1.4b | 94.2 ± 1.3a | 2.2 ± 0.2b | 2.1 ± 0.7a | 1.5 ± 0.4a | |
| 100% | 42.0 ± 0.7c | 96.3 ± 0.5b | 1.5 ± 0.3b | 1.6 ± 0.2a | 0.6 ± 0.4a | |
| Z3 | C | 54.1 ± 0.3a | 93.6 ± 0.4a | 3.1 ± 0.3a | 1.9 ± 0.2a | 1.4 ± 0.2a |
| 25% | 43.8 ± 0.7b | 95.0 ± 0.6a | 2.3 ± 0.2a | 1.7 ± 0.3a | 1.1 ± 0.2a | |
| 50% | 41.6 ± 0.8c | 96.0 ± 1.2b | 1.4 ± 0.4b | 1.7 ± 0.2a | 1.0 ± 0.6a | |
| 100% | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | |
- C: concentration; MI: mitotic index; PI: prophase index; MeI: metaphase index; AI: anaphase index; TI: telophase index; M: mean value; SD: standard deviation; Z1: study zone 1; Z2: study zone 2; Z3: study zone 3. Different literals (a, b, and c) indicate there is a significant difference between study zones (p < 0.05). The 100% treatment in Z3 did not show root development.
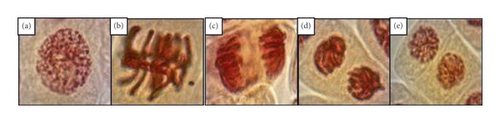
In relation to the PI, a tendency to increase was observed in the 3 sampling zones as the concentration of contaminants increased. The greatest increase was observed in the 100% treatment in zone 2 with 4.4% with respect to the control. The MeI showed a tendency to decrease in each treatment of the sampling zones with respect to the concentration of the treatments. The greatest decrease was observed in the 50% treatment in zone 3 with a decrease of 54.8% with respect to the control group. Regarding the anaphase and telophase indices, no significant variations were observed in any of the experimental groups in the sampling zones. This indicates that the cytotoxic effect of toxic metals and volatile organic compounds does not affect all metaphase stages in the Allium cepa L. meristem cell population.
The correlation between the concentration of 8 parameters of TM and VOCs in the 100% water samples of the study area with respect to the cytotoxic effect of Allium cepa L. meristems (Table 4) showed that there is a high negative correlation between the concentration of TM and VOCs in relation to the frequency of cells in the mitosis stage. As the concentration of TM and VOCs increased, the mitotic index of the apical meristem cell population decreased. Copper was the element that presented the greatest cytotoxic effect with respect to its concentration. For example, it had a 22% and 11% greater antimitotic effect with respect to trichloroethylene and chromium. It is clearly observed that the greatest antimitotic effect is induced by TM and not by VOCs.
| As | Cd | Cr | Cu | Hg | C6H6 | CHCl3 | C2HCl3 | |
|---|---|---|---|---|---|---|---|---|
| Cd | 0.994 | |||||||
| Cr | 0.975 | 0.963 | ||||||
| Cu | 0.973 | 0.990 | 0.917 | |||||
| Hg | 0.960 | 0.931 | 0.987 | 0.872 | ||||
| C6H6 | 0.937 | 0.913 | 0.989 | 0.848 | 0.992 | |||
| CHCl3 | 0.967 | 0.954 | 0.999 | 0.905 | 0.986 | 0.992 | ||
| C2HCl3 | 0.933 | 0.908 | 0.987 | 0.843 | 0.989 | 1.000 | 0.991 | |
| MI | −0.963 | −0.984 | −0.899 | −0.999 | −0.851 | −0.824 | −0886 | −0.819 |
- Correlation matrix: Pearson’s coefficient. Correlation significance: p < 0.05.
4. Discussion
Worldwide, rivers are exposed to enormous discharges of urban and domestic wastewater, as well as agricultural runoff, which upon reaching the ecosystem form complex mixtures of pollutants with toxic metals, volatile organic compounds, dyes, acids, bases, etc. This contamination in rivers and bodies of water is directly influenced by the type of anthropogenic activities that take place in the area where they are located and conditions their contaminant profile [21]. Seventy-two percent of the industries that operate in the localities of the study area belong to the metalworking and textile sectors. Because of this, the largest proportion of pollutants dumped on the site are toxic metals, dyes, acids, bases, chlorine, and peroxides. In addition, other types of industries present in the area to a lesser extent, representing 12%, such as printing, chemical, and petroleum derivatives producers [22] are primary sources of VOCs, which are organic chemical substances whose base is carbon and evaporate at ambient temperature and pressure in the rivers, generating vapors that enter the air, reaching the inhabited areas near the river [9]. The water samples that were collected come from 3 areas with different anthropogenic activities. Zone 1 presents a contribution mainly from agricultural and urban runoff, zone 2 includes urban wastewater, and zone 3 includes urban, industrial, and agricultural wastewater. All the samples induced cytotoxicity that was verified by the inhibition in the cell division process and consequently in the growth of roots. Because of this, most of the population is exposed to a high variety and quantity of substances that put their health at risk of cytotoxicity and that is dependent on the concentration of pollutants emitted in the area.
Our results are consistent with the theory that the contamination of the Atoyac River in the 3 selected sampling points is generalized because the physicochemical evaluation indicated the presence of organic matter, turbidity, TM, and VOCs exceeding the maximum limits allowed by the NOM. Additionally, the results partially agree with what was reported by Mokarram et al. [23], Chamarra and Koichi [24], Brito et al. [25], and Peng et al. [26] who determined that changes in water quality due to higher concentrations of pollutants such as organic matter and TM are associated with the increase in anthropogenic activities due to rapid urbanization and increased industrial activity, since the discharges of these effluents do not receive adequate treatment before being discharged and therefore do not comply with local official environmental standards. Few works at a global level have carried out the evaluation of VOCs in aquatic ecosystems. Our results partially agree with those of Montero et al. [9] and Sandoval et al. [27] who identified chloroform, benzene, xylene, and toluene in the Atoyac River, and their results were below those recorded in the present investigation.
This behaviour in water quality in rivers globally is mainly due to the intense industrialization that began in the 1970s with the imposition of the neoliberal model [28] and continues to this day, which has allowed the limitation of the state and public spending in favor of the private sector [29], coupled with lax laws, and permissibility of authorities has resulted in high pollution with toxic agents in the world’s water bodies. It is important to clarify that the results of this study have contributed to determine pollutants such as VOCs that have been little evaluated; however, the presence of certain types of toxic agents will depend on the socioeconomic environment and the spatial and temporal distribution of pollutant sources. The evaluation of the physicochemical quality of water resources is a subject that has been addressed since the last century; for this purpose, several studies have been carried out in the world, such as those conducted by Vega et al. in Spain [30], Apsite and Klavins in Latvia [31], and Ouyang et al. in the United States [32], which have quantified main parameters to determine the water quality, mainly the quantity of organic matter such as BOD, COD, TOC, nitrates, nitrites, phosphates, salinity, minerals (Mg and Ca), and toxic metals. Even more studies are currently being conducted to evaluate new pollutants such as the so-called emerging pollutants, among which are pharmaceutical waste [33], microplastics [26, 34], and cosmetics [35]. Unfortunately, worldwide and particularly in Mexico, there are few research works that include the determination of VOCs in the physicochemical analysis when determining water quality in rivers. The importance of evaluating the presence and quantity of VOCs in river water quality analysis is because some VOCs such as benzene, chloroform, and ethyl tetrachloride have been classified by the WHO as type I carcinogens. It is therefore necessary to carry out more quantitative assessments of pollutant mixtures containing these types of compounds to help authorities examine the potential health hazard to avoid or at least reduce it.
The pollutant mixture of TM and VOCs in the waters of the Atoyac River induces antimitotic effects and alterations in the phase index in the cells of organisms exposed to this mixture and can cause mutations in their genetic material and lead to diseases such as cancer. Our results agree with the theory because the analysis of Allium cepa L. meristem cells exposed to water samples from the Atoyac River showed inhibition in the mitosis process of the cell population. This could be attributed to VOCs and TM, which promote the synthesis of nuclear protein kinase (WEE1) responsible for phosphorylating and inactivating the Cdk1 enzyme preventing its association with cyclin B protein and thus inhibiting the activity of mitosis-promoting factor (MPF) [36, 37]. The results of this research agree with those of other investigations [38, 39] that observed a decrease of MI dependent on the increase of the dose of their treatments with respect to time by the increase of nanoparticles and glyphosate, respectively, in the medium. The prophase index (PI) also showed alterations in the cell population of exposed Allium cepa meristems because increases were observed in the groups with higher concentrations of TM and VOCs. These results demonstrate the cytotoxicity produced by TM and VOCs that probably block the Cdk and cyclin A-B complexes, which are responsible for initiating the condensation of genetic material as well as activating proteins called condensins that are responsible for carrying out the polymerization of microtubules for the formation of the achromatic spindle, which enables the normal transition of dividing cells from the prophase stage to the metaphase and anaphase stages [40]. The metaphase index (MI) showed significant differences in the higher concentration treatments in sampling zones 2 and 3. This decrease is related to the concentrations of TM and VOCs, which exceed the maximum limits allowed by the Mexican Official Standards and would be acting directly on the center of microtubule organization (COMT) that is responsible for inducing the assembly of the mitotic spindle, so that when this process is affected, the passage from the prophase stage to the metaphase stage is prevented [41, 42]; this coincides with the results obtained in relation to the decrease in the number of cells in metaphase. From the results obtained from the different treatments for the anaphase index (AI) and telophase index (TI), nonsignificant concentration-dependent decreases were observed for the treatments in the 3 sampling zones. This indicates that the concentrations of TM and VOCs moderately interfere with the synthesis of the anaphase promoter complex (APC), allowing the activity of proteins called cohesins and catastrophins, which are responsible for carrying out the chromatid separation process so that cells can transit from anaphase to telophase. With respect to the nonsignificant decrease in TI, this is due to the imbalance observed in the PI, since the increase in the number of cells in the prophase stage means that the mitosis process decreases and is ultimately reflected in the decrease in TI [36, 43, 44]. The results obtained from the AI of this research partially agree with those reported by Liman et al. [45] who evaluated the cytogenetic and genotoxic effects of rosmaniric acid on Allium cepa L. roots at different times and concentrations and did not observe a pattern that could explain the increase or decrease of cells in anaphase relative to his control group. Additionally, these results agree with those reported by Quispe et al. [46] who evaluated the effects of potassium sorbate at different concentrations and exposure time on the cell cycle in root meristems of Allium cepa L. and observed that TI percentages decreased as concentration and exposure time increased. The cytotoxic evaluation of contaminant mixtures in aquatic ecosystems is complicated due to the quantity and variety of compounds that may be present; however, they are not limited to a single type of zone or contaminant. The use of plants as biomodels to assess the cytotoxic potential of contaminated water bodies is relatively new, and most cytotoxic research has focused on assessing the damage of individually toxic agents such as aluminium [47], herbicides [48], pesticides [49], and hypochlorite [50], among others. Unfortunately, in nature, organisms are not exposed to only one type of contaminant, so it is essential to carry out evaluations of the cytotoxic effects of mixtures of contaminants in natural environments, for which different types of plants have been used as biomodels such as Vicia faba L., Pseudokirchneriella subcapitata, and Lactuca sativa L. [51]. Allium cepa L. has been used to evaluate the cytotoxicity of medicinal plant extracts, xenobiotics, herbicides, nanoparticulates, fertilizers, preservatives, etc. Few ecotoxicological investigations of water bodies such as rivers have used Allium cepa L. to determine its cytotoxic potential, such as those carried out in Malaysia [52], Brazil [53], and Colombia [54]. In Mexico, research to evaluate the cytotoxic potential of contaminated water in rivers is very scarce. Therefore, it is important to perform this type of evaluation of contaminated river water with biomodels to correlate the potential health damage that this type of contamination can induce in biological systems.
According to the dose-response theory of the kinetic toxic effect, we infer that the higher concentration of contaminants is a factor that directly influences the cytotoxic effects of the cell population in the roots of Allium cepa. Our findings corroborate that the antimitotic effect depends on the concentration of TM and VOCs. The results of this research agree with those reported by Batista et al. [5], Bianchi et al. [6], and Hilario et al. [53], who demonstrated that there is a direct correlation between the concentration of pollutants in rivers and mitotic index parameters. TM and VOCs upon entering the cells of the organism have a great capacity to bind to organic molecules [55, 56], and their effects depend on the reactions with ligands such as sulfhydryl groups, amino radicals, phosphate, carboxyl, and hydroxyl. Then, if the proportion of pollutants is greater, there will be a greater amount of ligands that would react resulting in greater response to cell damage. Although it is difficult to evaluate the cytotoxic effect of contaminant mixtures due to the synergism or antagonism effects that may occur with contaminants, it is advisable to perform this type of study in natural environments because that is how living beings are exposed in real life to real environmental contaminant mixtures [57].
5. Conclusions
This research demonstrated that the contaminant mixture of toxic metals and volatile organic compounds in a river has the capacity to induce aneugenic effects in the apical meristem cells of Allium cepa L. and may have a strong negative correlation because as the concentration of contaminants increases, the mitotic index decreases. Therefore, additional research is needed to determine the damage caused by each pollutant, and based on this, governmental decisions should be made to mitigate the environmental impacts on rivers caused by different anthropogenic activities.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
Andrés Estrada Rivera would like to thank Consejo Nacional de Ciencia y Tecnología (CONACYT) for the scholarship (601562). The costs to publish in open access were covered by Programa para el Desarrollo Profesional Docente (PRODEP) of the Secretaria de Educación Pública de México (SEP). This research was funded by VIEP_BUAP.
Open Research
Data Availability
The experimental data used to support the findings of this study are available upon request from the corresponding author.