Construction of a New 3D Cu(II) Compound with Photocatalytic Activity and Therapeutic Effect on Ventriculitis
Abstract
A new Cu(II) compound, that is {[Cu(L)(ClO4)(H2O)(CH3CN)](ClO4)}n (1, L is 4-(furan-2-yl)-2,6-bis(pyridin-2-yl)pyridine), was solvothermally synthesized and structurally characterized. It features a 0D isolated unit, and these 0D isolated units are further hydrogen-bonded into a 3D supramolecular framework. The framework of 1 can be stable up to 284°C, and the optical band gap of 1 is 3.71 eV. Under the irradiation of ultraviolet light, complex 1 as a photocatalyst exhibits high activity for methylene blue (MB) degradation in the water solution. The above compound’s inhibitory activity on the inflammatory cytokines releasing was tested through exploiting ELISA detection. The real time RT-PCR was conducted to assess the compound’s suppression on the expression of bacterial survival gene.
1. Introduction
Medical-related ventriculitis and meningitis are common complications of neurosurgery, which seriously affect the prognosis and outcome of patients [1]. Although most patients have acute onset during hospitalization, there are still a small number of patients who develop delayed infections even several years after they are discharged from the hospital [2]. So far, the therapeutic effects of neurosurgery and neurosurgery on medical-related ventriculitis and meningitis are not satisfactory. Thus, new candidates need to be developed for the treatment of ventriculitis.
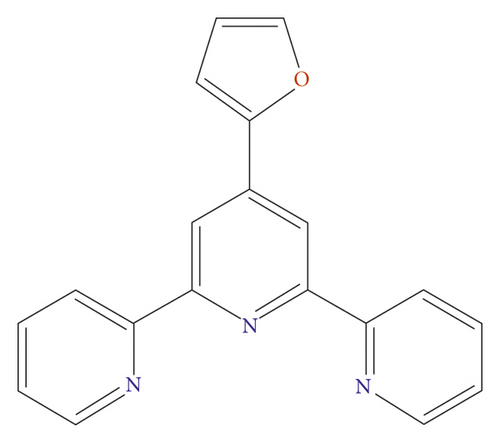
In the last several decades, much interest has been focused on designing and synthesizing the metal-organic frameworks (MOFs) owing to their underlying application values in magnetism, luminescence, heterogeneous catalysis, drug delivery, gas adsorption/separation, and so forth [3–6]. With the acceleration of industrialization, more and more toxic dye contaminants, which are hard to be biodegraded effectively, are discharged into the water system without any pretreatment, leading to sewer pollution that will cause lots of fatal diseases to human beings and other living things [7]. Recently, semiconductive MOFs, which not only feature a large surface area, high porosity, tunable pore structure, and coordinated unsaturated metal site but also can harvest solar energy effectively, have been successfully synthesized, and further utilized as photocatalysts in order to effectively degrade the abovementioned dyes into harmless small molecules under the irradiation of ultraviolet or visible light [8–12]. Therefore, the construction of more photoactive MOFs-based photocatalysts is urgent and indispensable. As we all know, the properties of MOFs are closely related with the structures of MOFs, organic ligands, and central metal ions [13, 14]. In order to obtain the photoactive MOFs, this requires us to choose an appropriate organic ligand with large conjugated groups that are favorable for the absorption of the solar energy [15, 16]. Considering that, in this work, we selected 4-(furan-2-yl)-2,6-bis(pyridin-2-yl)pyridine (L) (Scheme 1), which has large conjugated system and four potential coordination sites, as an organic building block for assembling with the Cu(ClO4)2·6H2O under conditions of solvothermal. In success, we gathered a novel Cu(II) complex showing a 0D isolated unit. These 0D isolated units arefinally combined into a 3-dimensional supramolecular framework via intermolecular H bonds. More importantly, this compound is highly thermostable and exhibits high photocatalytic effect in MB degradation in the water solution. A variety of biological researches were finished to assess the novel complex’s biological activity synthesized in this research.
2. Experimental
2.1. Materials and Instrumentation
All starting chemicals were commercially available in analytical grade and were utilized with no modifications. A Perkin-Elmer 240°C was employed for conducting the N, H together with C elemental analysis. A Rigaku D/Max-2500PC with 1.54056 Å Cu/Kα radiation with 0.05° step size was applied for collecting the patterns of PXRD. Thermostable experiment was implemented with the NETZSCH TG 209 with 2°C/min heating rate under the nitrogen flow. The UV-vis diffuse reflectance spectra data in solid state were gathered through employing Puxi TU-1901 ultraviolet-visible spectrophotometer, where BaSO4 plate was used as a standard.
2.2. Synthesis of {[Cu(L)(ClO4)(H2O)(CH3CN)](ClO4)}n(1)
The mixture of 0.1 mmol Cu(ClO4)2·6H2O, 0.1 mmol L, 3 mL CH3CN, and 4.0 mL CH3OH was placed into a stainless reactor (25 mL) lined by Teflon, which was heated under a temperature of 135°C for 3 days. The 1’s blue massive crystals were collected after cooling the above mixture to RT at 2°C per min decreasing rate, and with 38 percent yielding according to Cu(ClO4)2·6H2O. Elemental analysis calcd. for C21H18Cl2CuN4O10 (620.84 g/mol): N, 9.02, C, 40.59, and H, 2.90%. Found: N, 8.98, C, 40.57, and H, 2.92%.
2.3. X-Ray Structural Determination
On a Rigaku Mercury CCD diffractometer that with 0.71073 Å Mo-Kα radiation, the single-crystal architecture of compound 1 was detected at RT. The direct mean together with the full-matrix least squares was, respectively, applied for the generation and modification of crystal structure with SHELXTL embedded in OLEX2 [17]. All the nonhydrogen atoms were anisotropically refined, and the H atoms were fixed in the calculated sites. The complex 1’s crystallographic parameters together with its refinement parameters are listed in Table 1. The parameters of bond about central Cu(II) ion are summarized in Table S1.
| Formula | C21H18Cl2CuN4O10 |
|---|---|
| Fw | 620.84 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a (Å) | 8.1922 (4) |
| b (Å) | 10.7290 (7) |
| c (Å) | 14.2825 (10) |
| α (°) | 92.748 (6) |
| β (°) | 100.469 (5) |
| γ (°) | 96.407 (5) |
| Volume (Å3) | 1223.75 (13) |
| Z | 2 |
| Density (calculated) | 1.685 |
| Abs. coeff. (mm−1) | 1.175 |
| Total reflections | 8189 |
| Unique reflections | 4298 |
| Goodness of fit on F2 | 1.029 |
| Final R indices (I > 2sigma(I2)) | R = 0.0559, wR2 = 0.1494 |
| R (all data) | R = 0.0729, wR2 = 0.1639 |
| CCDC | 2164492 |
2.4. Inflammatory Cytokines Determination
The ELISA detection was implemented in the current work to investigate the compound’s inhibitory activity on the TNF-α and IL-1β content. This research was accomplished strictly following the instructions along with some changes. Shortly, forty BALB/c mice exploited in the current paper were provided by the Model Animal Research Center of Shanghai Jiao Tong University. All the mice were maintained between 20 and 25°C, with free food and free water. The animals were subsequently classified as five groups, namely, compound treatment group and model group as well as the control group. In compound treatment and model groups, the bacteria were injected into animal to create the model of ventriculitis. Next, the compound was applied for finishing the treatment at 1, 2, and 5 mg/kg concentration. After finishing specific treatment, the respiratory mucus was gathered and the content of TNF-α and IL-1β in brain was tested via ELISA detection.
2.5. Bacterial Survival Genes
The real time RT-PCR was employed for detecting the complex’s influence on the bacterial survival gene expression for the assessment. This study was implemented fully according to the protocols after mini change. Briefly, the bacteria were gathered and inoculated into the plates (96 well), the incubation was finished after adding compound with 10, 20, and 50 ng/ml. After specific treatment, the overall RNA in bacteria was extracted through utilizing the TRIZOL reagent. After testing its concentration, it was next reverse transcript into the cDNA. Eventually, the real-time RT-PCR was accomplished and the bacterial survival gene relative expression was detected.
3. Results and Discussion
3.1. Crystal Structure of Compound 1
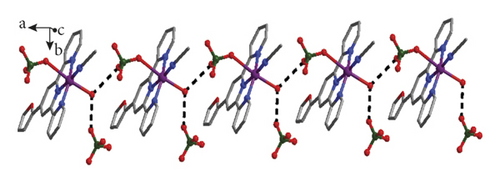
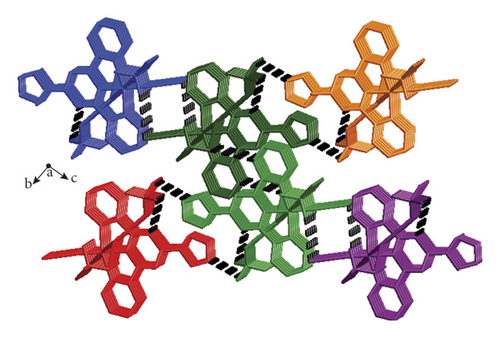
The crystallographic study of single crystal X-ray suggested that the complex 1’s fundamental unit contains a Cu(II) ion, a L ligand, a molecule of coordinated CH3CN, a coordinated H2O molecule, and a coordinated ClO4− anion, as well as one free ClO4− anion, and the structure of 1 shows a 0D isolated framework. According to Figure 1(a), Cu1 is 6-coordinated through four N atoms provided by a L ligand and one coordinated CH3CN molecule, and two O atoms come from a coordinated molecule of water and a ClO4− anion. Notably, two axial Cu-O distances (2.296(4)–2.584(4)Å) are longer than the Cu-N distances (1.920(4)–2.028(4)Å), thus the coordination geometry of Cu1 can be described as an elongated octahedron. Interestingly, the intermolecular H bonds between the ClO4− anions and coordinated molecules of water extend these 0D separated units into a 1-dimensional chain along direction b, as is revealed in Figure 1(b). In addition to O1w-H-O H bonds, there also are C-H-O H bonds between the L ligands and ClO4− anions from adjacent 1D supramolecular chain, and such type of hydrogen bonds finally connected adjacent 1D supramolecular chains together, leading to a 3-dimensional supramolecular structure (Figure 1(c)). The related hydrogen parameters are summarized in Table S2.

3.2. Powder X-Ray Diffraction Pattern (PXRD) and Thermogravimetric Analysis (TGA)
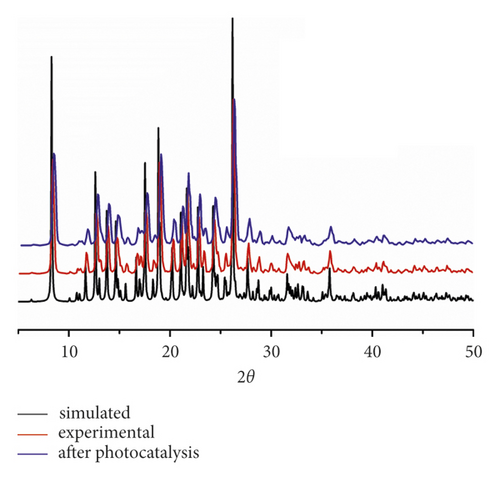
To prove whether the as-synthesized bulk solids are in single phase, the research of PXRD was finished under RT. As exhibited in Figure 2(a), there exist an excellent accordance between the patterns of simulation and experiment, suggesting the high purity of the obtained products.

The structural stability of 1 was also estimated by the TGA experiment under a nitrogen atmosphere, as is shown in Figure 2(b). The 1’s structure exhibited a two-step weightlessness between 30 and 800°C. The first occurred ranging from 80 to 114°C, which because of the loss of coordinated water and CH3CN molecules (obsd: 9.48%, calcd: 9.51%), and the second was observed occurring in the range of 284–467°C, which is owing to the organic ligands decomposition. The final residue with a weight of 10.19% corresponds well to the formation of metal copper (calcd: 10.23%).
3.3. UV-Vis Diffuse Reflectance Spectrum and Optical Band Gap of Compound 1
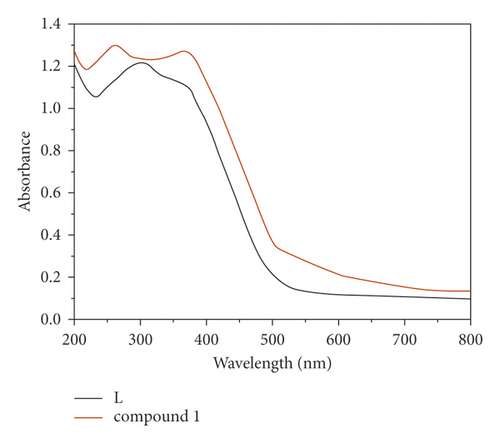
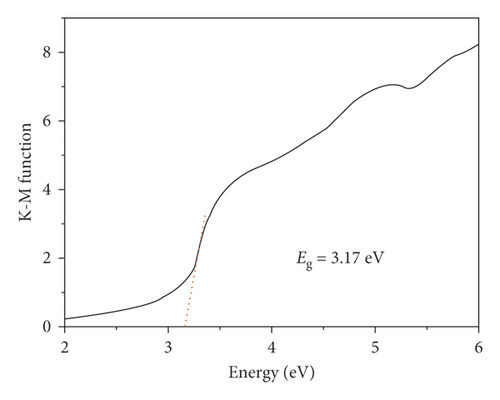
At RT, we determined the ultraviolet-visible diffuse reflectance spectra of free L ligand and compound 1. As reflected in Figure 3(a), the free ligand of L possess two major absorption bands at 355 nm and 302 nm, which could be assigned to π⟶π ∗ transitions, and the 1’s solid also exhibit two major absorption bands at 367 nm and 262 nm. Notably, the UV-vis absorption spectrum of 1 is similar to the ultraviolet-visible absorption spectrum of free L. Hence, the 1’s ultraviolet absorption bands are owing to intraligand π⟶π ∗ transitions [18]. In addition, its semiconductive property was also estimated by the optical band gap value. Through the calculation utilizing F = (1-R)2/2R, a Kubelka–Munk function (R: the absolute reflectance of the solid powders), the optical band gap (Eg) is 3.17 eV (Figure 3(b)), indicating compound 1 may be potential candidate as photocatalyst to photodegrade organic dye contaminants under the irradiation of UV light.
3.4. Photocatalytic Property of Compound 1
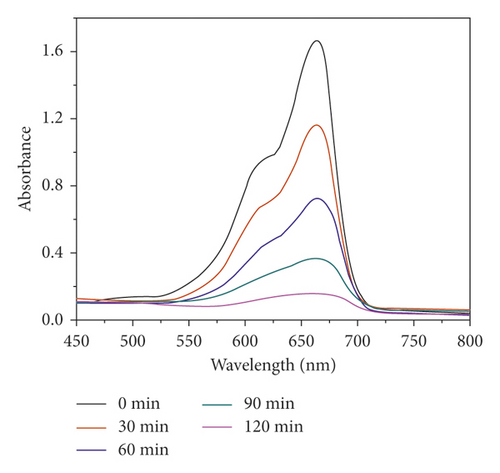
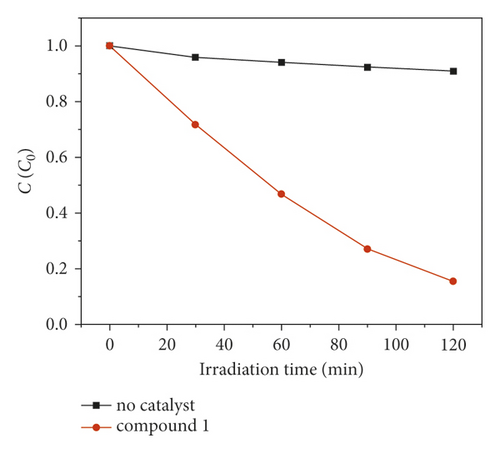
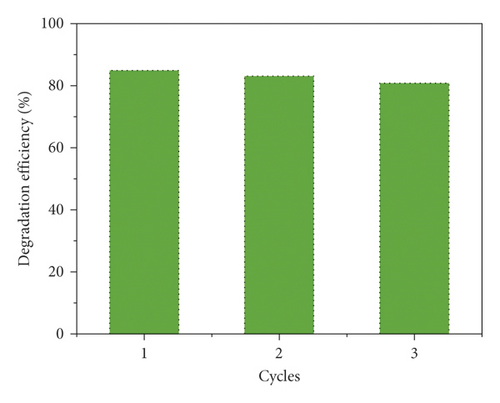
The compound 1’s photocatalytic property was analyzed through the choice of methyl blue (MB) as the model dye under irradiation of ultraviolet light. In the classical experiment, complex 1’s powder samples (50 mg) were ultrasonically dispersed into a MB water solution (100 mL) with 10 mg/L concentration in the dark for half an hour. After establishment of the absorption-desorption equilibrium, the suspension was exposed to the UV light under the condition of continuous stirring. At intervals of 30 min, we took out 3 mL reaction mixture, which was further separated by centrifugation. The clear solution was ultimately explored via the ultraviolet-visible spectrometer, and at 664 nm, the MB characteristic absorption peak was chosen for monitoring the process of photocatalysis. When using 1 as photocatalyst, the MB characteristic absorption peak sequentially reduced with the extending irradiation time (Figure 4(a)), and about 84.6%, MB was successfully photodegraded after 120 min UV irradiation (Figure 4(b)). While the result of comparison experiment without any photocatalyst shows that the degradation efficiency of MB is only 9.2% at the identical conditions (Figure 4(b)). The degradation efficiency in the presence of 1 is much higher than that without complex 1, demonstrating the 1’s excellent photocatalytic activity under UV light irradiation. After photocatalysis, the framework of 1 was not destroyed that was proved by the PXRD experiment (Figure 2(a)). In viewing of its good structural stability, the reusability was also investigated under the same conditions. Three cycled experiments were conducted using the recovered photocatalyst, and the degradation efficiency decreased a little from 84.6% to 80.5% (Figure 4(c)), indicating that compound 1 as photocatalyst has huge potential for reusability.
3.5. Compound Significantly Reduced the Inflammatory Cytokines Releasing into the Brain
In the course of ventriculitis, there was generally combined with an upregulated inflammatory cytokines releasing level. Hence, after treating through the abovementioned compound, the ELISA detection was implemented and the inflammatory cytokines content released into the brain was detected. Based on Figure 5, it reflects that the inflammatory cytokines content in the model group was much higher in comparison with the control group. After the compound’s treatment, the inflammatory cytokines levels released into the brain was significantly decreased. The biological activity of the new compound showed a dose-dependent manner.

3.6. Compound Obviously Inhibits Bacterial Survival Gene Expression
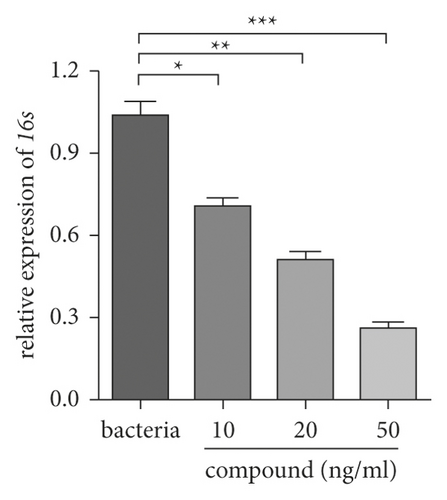
In the previous research, we demonstrated that this complex could be an outstanding candidate for treating ventriculitis by suppressing the inflammatory cytokines releasing. However, the novel compound’s influence on the expression of bacterial survival gene was required to be investigated. Hence, the real time RT-PCR was carried out and the bacterial survival gene relative expression was determined. From Figure 6, we can see this complex could evidently decrease the bacterial survival gene expression. In consistent with the above research, the suppression of this compound also exhibited a dose correlation.
4. Conclusion
To sum up, we have created a 3-dimensional supramolecular Cu(II) compound with high thermostability. The suitable optical band gap (3.17 eV) of 1 makes it reveal high photocatalytic effect for MB degradation under the irradiation of UV light. The detection of ELISA revealed that this compound could markedly decrease inflammatory cytokines releasing dose dependently. Moreover, the expression of bacterial survival gene was also suppressed through this complex. Ultimately, this compound could be a superb candidate for treating ventriculitis through suppressing inflammatory response and bacterial survival.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81571883).