Development of Duloxetine Hydrochloride Tablets for Delayed and Complete Release Using Eudragit L 100
Abstract
The purpose of the research was to optimize the preparation of duloxetine hydrochloride (duloxetine HCl) delayed release tablets. Duloxetine HCl produces a toxic substance called alpha-naphthol when duloxetine HCl is in contact with gastric fluid. Thus, duloxetine HCl when given orally needed a protective enteric coating that disable the delivery of duloxetine HCl in gastric fluid while enabling the drug delivery only in small intestine. Four different core tablets were prepared by direct compression technique, and the one which displayed quick disintegration and dissolution was chosen for enteric coating. The compressed tablets were enteric coated by dip coating technique. Since subcoating is required to safeguard the enteric coating, the core tablets were subcoated by using polymer HPMC K15M and then enteric coated with Eudragit L 100. The prepared tablets were assessed for the entire precompression and postcompression characteristics. FTIR study revealed the existence of all prominent peaks signifying its compatibility and authenticity. The in vitro studies showed that enteric-coated tablets were capable of restricting release in acidic media. The formulation F8 was optimised with 5% and 15% increase in weight of seal coat and enteric coat with good dissolution profile. Stability studies revealed that the optimized formulation was intact without any deterioration for 3 months. In conclusion, the optimized formulation could resist the drug release in acidic environment of gastrointestinal region and release the drug at a time once the tablet reaches the intestine.
1. Introduction
Oral drug delivery is the appropriate method of choice of therapeutic agents and applied to several drug products to improve safety and efficacy of drug profiles. In the point of revenue generation, oral drug delivery is the target for drug industries while developing new drug moieties and formulations due to its cost-effective manufacturing process, patient compliance, and convenience. The conventional oral medications that release drug immediately after oral administration suffer with drug degradation in hostile environment of stomach, inability to reach target site due to poor absorption properties, and hence, bioavailability-related problems [1, 2]. Such drawbacks of conventional oral therapy led to the expansion of modified release oral drug delivery that includes delayed release, sustained release, receptor targeting, and site-specific targeting dosage forms.
Delayed release (DR) dosage forms are intended to release the drug at a time rather than prompt after administration. The delayed drug release may be the impact of environmental settings like pH in GI tract (site specific) or time based [3, 4]. The drugs that degrade in the stomach due to acidic pH and digestive enzymes, identified to produce stomach distress, undergo intestinal site-specific absorption, and drugs meant to produce local effect at a specific intestinal site are appropriate to be designed as DR dosage forms [5, 6].
There are various DR dosage forms that are site-specific to attain drug target in GI region. For example, enteric-coated dosage forms release the drug after gastric emptying while dosage form reaches the small intestine. Several marketed products have been designed in such a way to release the drug in distal part of small intestine rather than in proximal part, e.g., mesalazine DR tablets [7], and this technology is also much useful for the drugs that degrade in gastric region [8, 9] and drugs that cause gastric irritation, e.g., nonsteroidal anti-inflammatory drugs [10]. Such techniques can be subjugated to increase positive clinical outcomes and to reduce nonbeneficiary side effects and adverse events [11, 12]. DR dosage forms also permit drug to be released at a prefixed time after administration by oral route.
The mechanism of enteric coating is to provide a surface that is unchanged and highly stable at acidic environment of stomach but disrupt rapidly at basic environment of intestine (>pH 5.5) [13–15]. Enteric coating tablets are commonly prepared using polymers such as cellulose acetate phthalate and its succinate, methacrylic acid copolymers, hydroxypropyl methyl cellulose phthalate, hydroxylpropyl ethyl cellulose phthalate, acrylic resin, and cellulose acetate tetrahydrophtalate [16].
This research was with the intension to prepare and evaluate DR tablets of Duloxetine HCl, a selective serotonin and norepinephrine reuptake inhibitor (SSNRI) and used in the treatment of depression, generalized anxiety disorder, and reliefs the pain of fibromyalgia and peripheral neuropathy [17]. It is chemically (+)-(S)-N-methyl-γ-(1-naphthyloxy)-2-thio phenypropyl amine hydrochloride, and its bioavailability is 50%. When conventional duloxetine HCl tablet dissolves in acidic environment of stomach (<pH 3.0; 0.1 N Hydrochloric acid) while administering through oral route, it produces a toxic product called alpha-naphthol. The gastric resistant tablets could be the right choice which is one of the best outcome of enteric coating technology to prevent the formation of alpha-naphthol and to improve the bioavailability of duloxetine HCl [18–20].
2. Materials and Methods
2.1. Materials
Duloxetine hydrochloride was obtained as a gift sample from Mylan laboratories Ltd. Magnesium stearate was purchased from S. D Fine Chemicals Ltd, Mumbai. Microcrystalline cellulose and sodium starch glycolate were obtained from Emco Industries Ltd Hyderabad, India. HPMC K15M and Eudragit L100 were obtained as gift samples from Dr. Reddy’s laboratories, Hyderabad.
2.2. Preparation of Duloxetine HCl Core Tablet
Four formulations of duloxetine HCl core tablets were prepared by direct compression technique by varying the superdisintegrant concentration. The required quantities of Duloxetine HCl, sodium starch glycolate, and microcrystalline cellulose (MCC) pH 102 were weighed and passed through sieve. The above shifted materials were transferred to a mortar and mixed thoroughly. Finally weighed quantity of magnesium stearate was added in the mortar, and the entire mixture was blended for 5 minutes, and then, the lubricated powder was compressed using 4 mm punch set of Rimek tablet punching machine, Minipress-I 12 station D tooling. The composition of duloxetine HCl core tablets is shown in Table 1.
| S. no | Ingredients (mg) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| 1 | Duloxetine HCl | 30 | 30 | 30 | 30 |
| 2 | Sodium starch glycolate | 2 | 5 | 10 | 15 |
| 3 | MCC pH 102 | 167 | 164 | 159 | 154 |
| 4 | Magnesium stearate | 1 | 1 | 1 | 1 |
All the quantities are in mg, and the total weight of the tablet is 200 mg.
2.3. Precompression Parameters
Vo = apparent unstirred volume
2.4. Postcompression Parameters
The prepared enteric-coated tablets were evaluated for hardness, friability, weight variation, thickness, and drug content.
2.4.1. Hardness
The tablet’s hardness in kg/cm2 was measured using Monsanto hardness tester for 10 tablets, and its mean and standard deviation were recorded.
2.4.2. Friability
2.4.3. Weight Variation Test
Weight variation test was performed by taking randomly 10 tablets from each batch and weighed individually. The average weight of 10 tablets was calculated along with its standard deviation. The tablets passes the weight variation test if not more than two of the individual tablet weights deviate from the average weight.
2.4.4. Thickness
Randomly selected 10 tablets were tested for thickness in mm using digital micrometer, and its mean value was recorded along with its standard deviation [23].
2.4.5. Drug Content
After calculating average weight of randomly selected 10 tablets, they were crumpled in a mortar. The crumpled powder weight equivalent to 100 mg of duloxetine HCl was taken and dissolved in 100 ml of pH 6.8 phosphate buffer. After filtering this solution, 5 ml of filtrate was diluted to 100 ml using pH 6.8 phosphate buffer. The resultant solution’s absorbance was measured against pH 6.8 phosphate buffer as blank at 292 nm in UV spectrophotometer [24].
2.5. Preparation of Coating Solution
The simple solution method was adopted to prepare enteric coating solution to coat core tablets. To prevent the dissolution of enteric coating due to the presence of alkaline substances in the core, intermediate layer (subcoating) was required between core tablet and enteric layer. First, the core tablets were subcoated with 3% w/v of HPMC K15 M solution in isopropyl alocohol and dichloromethane (6 : 4), and subcoated tablets were further used for enteric coating. The enteric coating solution was 10% w/v of Eudragit L 100 in isopropyl alcohol and dichloromethane (1 : 1) [25]. First, the solvents were taken in a beaker, and then, Eudragit L 100 was added and stirred until a homogenous solution was obtained. The tablets were coated with polymer solution by dip coating method. The tablet was dipped in to the coating solution repeatedly until desired weight gain is achieved.
2.6. Physico-Chemical Evaluation of Coating Films
The solution of enteric polymer was casted as dried polymeric film and tested for film solubility, weight, and thickness. The solution of enteric polymer was prepared by dissolving sufficient quantity of Eudragit L 100 in a solvent system of 1 : 1 ratio of isopropyl alcohol and dichloromethane and casted as film by solvent evaporation technique on a glass petriplate which is lidded with inverted funnel to provide controlled environment at room temperature. After allowing the film to dry for 24 h, the film size of 1 × 1 cm2 was cut, respectively, to determine film solubility, weight, and thickness. Ten films of the size 1 × 1 cm2 were cut, weighed using digital balance, and averaged. Digital micrometer was used to calculate average thickness. The film size of 1 × 1 cm2 was kept in a beaker holding 20 ml of specified pH medium and magnetically stirred at 37 ± 1°C for an hour and filtered. The filtrate was assessed for film content and solubility. This test was done in both pH 1.2 and pH 6.8 buffer medium.
2.7. Determination of Acid Uptake %
where Wt = weight of the tablet at time, t = 2 h. W0 = weight of the tablet at time, t = 0.
Enteric-coated tablets must not be disintegrated in acidic media; if so, the tablets are considered to have 100% acid uptake. A good enteric-coated tablet which maintains the coat integrity in acidic media should not become soft or swell after the test and also should show not more than 5% of acid uptake.
2.8. Dissolution Study
Dissolution testing of the enteric-coated tablets was carried out according to USP monograph “DR dosage forms dissolution method B” using USP type-I apparatus for 2 h with 0.1 N hydrochloric acid pH 1.2 followed by using USP type-II apparatus with pH 6.8 phosphate buffer for 10 min. A tablet was kept in basket of USP type-I apparatus containing 900 ml of 0.1 N hydrochloric acid pH 1.2 at 37 ± 0.5°C and was rotated at 100 rpm. Sample of 5 ml was withdrawn for every half an hour up to 2 h. Then, the tablet was taken out and kept in USP type II apparatus containing pH 6.8 phosphate buffer rotated at 50 rpm for 10 min at 37 ± 0.5°C. Sample of 5 ml was withdrawn for every 2 min for 10 min and analysed for the drug content at 292 nm using UV-Visible spectrophotometer against the respective buffers as blank and % cumulative drug release was computed [27].
2.9. Stability Studies
Stability of pharmaceutical product throughout its shelf life is much important. Stability studies are usually conducted by increasing the rate of physical or chemical degradation of a product by accelerating the storage conditions as per ICH guidelines. Stability studies are significant to prevent monetary impacts which may lead to extensive financial loss. From the safety point of view of patients, it is vital that the patient takes required dose of a drug throughout the product’s shelf life. To perform the test, the product was stored as per ICH guidelines for 3 months at 40°C ± 2°C/75% ± 5% RH. At the end of 3 months, the samples were taken and tested for drug content and dissolution time [28].
3. Results and Discussion
3.1. Drug-Excipient Compatibility
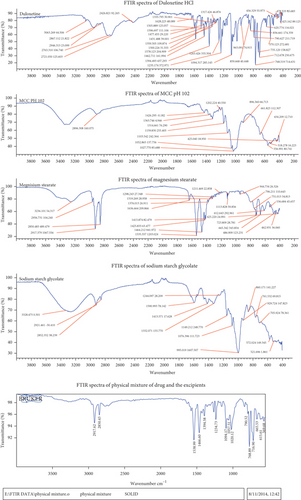
FTIR Spectroscopy study was carried out for drug, excipients, and physical mixture to find out the compatibility between drug and excipients. The FTIR spectra of the drug, excipients, and the physical mixture confirmed the absence of interaction between the selected drug and the excipients. The significant peaks of pure drug and drug with excipients peaks were interpreted and shown in Table 2 and Figure 1.
| Functional groups | FTIR significant peaks | |
|---|---|---|
| Pure drug (cm1) | Physical mixture (cm-1) | |
| C=O (1760-1605) | 1628 | 1631.62 |
| C-S (700-600) | 620 | 615 |
| CH3 (1450-1375) | 1389 | 1394.31 |
| N-H (1640-1550) | 1578 | 1596.99 |
3.2. Precompression Parameters
There are numerous technologies used in the development of enteric-coated tablets. In this research, it was utilized direct compression technique to prepare core tablets, and dip coating method was used to enteric coat the core tablets. Before compressing core tablet, flow properties of the powder blend were assessed. It was done first by measuring angle of repose. It provides quantitative and qualitative estimation on frictional force and internal cohesive force as the low level of external pressure is applied during mixing and compression. The angle of repose values were in the array of 25.7–28.2. The Carr’s compressibility index was found to be in the range of 11.92 to 15.54%, and Hausner’s ratio was found to be between 1.28 and 1.35. Hence, these results indicate that the prepared powder blends possess good flow characteristics. Table 3 shows the results of precompression characteristics of the powder blends from F1 to F4.
| Code | Angle of repose | Bulk density (gm/cm2) | Tapped density (gm/cm2) | Carr’s index (%) | Hausner’s ratio (HR) | Diameter (mm) | Thickness (mm) | Hardness (mg/cm2) | Friability (%) | Drug content (%) | Weight variation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 25.7 ± 0.05 | 0.37 ± 0.01 | 0.38 ± 0.01 | 11.92 ± 0.01 | 1.28 ± 0.03 | 4.1 ± 0.07 | 2.14 ± 0.01 | 4.31 ± 0.06 | 0.86 ± 0.02 | 98.45 | 200 ± 0.01 |
| F2 | 28.2 ± 0.03 | 0.42 ± 0.06 | 0.40 ± 0.02 | 13.91 ± 0.03 | 1.35 ± 0.03 | 3.9 ± 0.03 | 1.98 ± 0.07 | 4.42 ± 0.04 | 0.91 ± 0.02 | 97.62 | 202 ± 0.02 |
| F3 | 27.7 ± 0.02 | 0.39 ± 0.03 | 0.39 ± 0.01 | 12.85 ± 0.06 | 1.29 ± 0.01 | 4.3 ± 0.04 | 2.08 ± 0.06 | 4.27 ± 0.02 | 0.88 ± 0.01 | 98.75 | 198 ± 0.03 |
| F4 | 26.4 ± 0.03 | 0.43 ± 0.02 | 0.42 ± 0.03 | 13.57 ± 0.04 | 1.33 ± 0.01 | 4.1 ± 0.04 | 1.97 ± 0.01 | 4.53 ± 0.07 | 0.93 ± 0.02 | 97.54 | 201 ± 0.02 |
3.3. Postcompression Parameters
The thickness (mm) was found to be from 1.97 ± 0.07 to 2.14 ± 0.01 for the prepared tablets. According to the specifications of weight variation test in USP, the percentage deviation of the tablets weighing in the range of 130-324 mg is ±7.5%. The weight of all tablet formulations was perfectly found within limits of the official requirements. Drug content uniformity was much perfect for all the tablets compressed, and detected drug content was greater than 98%.The hardness of the prepared tablets was within the range of 4.2-4.5 kg/cm2. Not only tablet hardness is an out and out indicator of tablet’s strength but also its friability. Conventional compressed tablets that drop less than 1% of their weight are normally considered accepted. The results of friability test of less than 1% lose in weight of all the tablets indicated that the friability and hence the tablet’s strength is within the recommended limits. The postcompression properties of tablets were measured and reported in Table 3.
3.4. Physico-Chemical Evaluation of Coating Films
The enteric film made up of Eudragit L 100 was evaluated for various film properties such as film solubility, weight, and thickness. The enteric film was insoluble in pH 1.2 buffer solution and completely soluble in pH 6.8 buffer solution. Film thickness (mm) was found to be 0.39 ± 0.04, and film weight was found to be 0.102 ± 0.03.
3.5. Dissolution Profile of Duloxetine HCl Tablets
The in vitro dissolution studies were carried out for the duloxetine HCl core tablets using USP apparatus type I. The in vitro drug release profiles of Duloxetine hydrochloride from core are in Figure 2.
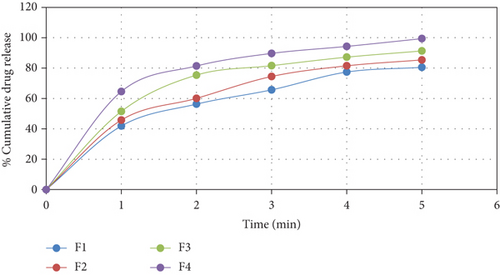
The analysis of the results of in vitro dissolution studies of uncoated core tablets in pH 6.8 phosphate buffer revealed that formulation F1 did not release the drug completely. This might be due to the excessive amount of diluent MCC. Formulations F2 and F3 showed considerable increase in drug release since the amount of diluent was consistently reduced in both the formulations. Formulation F1 released about 89.8% of drug within 10 min whereas formulations F2 and F3 released 92.3% and 94.5% of the drug, respectively, within 10 min. Formulation F4 showed acceptable release profile since it released about 99% of the drug in less than 10 min. Thus, the formulation F4 was further used for enteric coating studies.
3.6. Evaluation of Enteric-Coated Tablets
Polymeric solution of Eudragit L 100 was prepared in the solvent system of isopropyl alcohol and dichloromethane at 1 : 1 ratio for enteric coating the core tablets. Eudragit L 100 is an anionic copolymer-based methacrylic acid and methyl methacrylic acid. It is a solid powder with a faint characteristic odour. Molecular weight is 1,25,000 g/mol, acid value 315 mg KOH/g of polymer, and glass transition temperature is greater than 150°C. Targeted drug release area of the polymer is jejunum and dissolves at pH above 6. It is used for effective and stable coatings with fast dissolution in the upper bowel and site-specific drug delivery in intestine. Since it is needed to separate the alkaline drug core from the enteric coat, it is necessary to incorporate a subcoat/seal coat in between the core tablet and enteric layer. HPMC K15M was used for providing seal coat and polymeric solution was prepared using isopropyl alcohol and dichloromethane as solvents at 6 : 4 ratio. HPMC K15 M is a HPMC polymer with moderate hydroxypropyl substitution. HPMC K15M solution has excellent lubricity, easy to spread, and stable across a broad pH range. 3% w/v HPMC K 15 M solution was used for seal coat around the drug core and 10% w/v Eudragit L 100 solution was used for enteric coat. Four trials were carried out on the optimized core tablet F4, and those trial coating formulations were coded as F5, F6, F7, and F8.
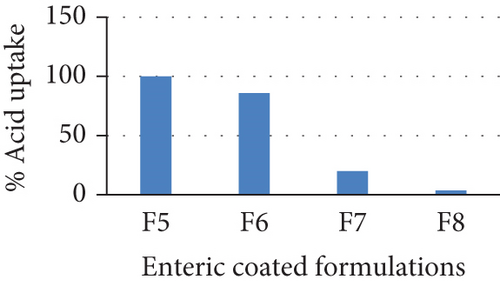
The % acid uptake was measured for all the trial formulations. Formulation F5 having weight gain of 2% after seal coat and weight gain of 5% after enteric coat showed 100% acid uptake. This might be due to insufficient thickness of either seal coat or enteric coat. Formulation F6 having weight gain of 2% after seal coat and 10% after enteric coat was showing 86% of acid uptake. So, the increasing thickness of enteric coat alone did not show any significant difference in acid uptake %. Formulation F7 having 5% increase in thickness of seal coat and 12% increase in thickness of enteric coat produced significant decrease in acid uptake of 20% but it is not to the optimum requirement of less than 5% of acid uptake. Thus, the formulation F8 was prepared using 5% increase in thickness of seal coat and 15% increase in enteric coat thickness that showed 3.6% of acid uptake (Figure 3 and Table 4).
| Parameters | Formulations | |||
|---|---|---|---|---|
| F5 | F6 | F7 | F8 | |
| Average weight (mg) | 212 ± 0.02 | 225 ± 0.03 | 236 ± 0.01 | 242 ± 0.03 |
| Thickness (mm) | 2.19 ± 0.02 | 2.21 ± 0.05 | 2.22 ± 0.01 | 2.24 ± 0.03 |
| Hardness kg/cm2 | 4.9 ± 0.3 | 4.9 ± 0.5 | 5.1 ± 0.2 | 5.3 ± 0.5 |
| Acid uptake % | 100 | 85 | 20 | 3.6 |
| Disintegration time (min) (0.1 N HCl) | 10-15 | 30-40 | 60-90 | Stable for 120 |
| Disintegration time (min) (pH 6.8 phosphate buffer) | NA | NA | NA | 10 |
| Dissolution (0.1 N HCl) | NA | NA | NA | 1.5% |
| Dissolution (pH 6.8 phosphate buffer) | NA | NA | NA | 99% |
- NA: not applicable.
In F5, seal coat was given up to 2% increase in total weight of tablet and enteric coat with 5% increase of total weight, but due to insufficient seal coat and enteric coat, the drug got released in acidic pH (0.1 N HCl). The USP 29 Drug Release Standard for Enteric-Coated Articles requires a drug release of less than 10% after 2 h dissolution testing in 0.1 N HCl. F6 trial coated formulations with seal coat of 2% and enteric coat of 10% increase in weight of core tablet was stable for half an hour in acidic media, but due to insufficient enteric coat, the coat got removed; thus, trial II was failed. In trial III (F7), seal coat and enteric coat were given to an increase in weight of 5% and 12%, tablet was stable for 1 1/2 h in acidic media, and later coat got removed due to insufficient coating; thus, trial III was failed. In trial IV (F8), seal coat and enteric coat were given to an increase in weight of 5% and 15%, In vitro release studies revealed that the enteric-coated tablets with 15% increase in weight of polymer Eudragit L100 did not release the drug for 2 h in acidic buffer 0.1 N HCl (Table 4 and Figure 4).
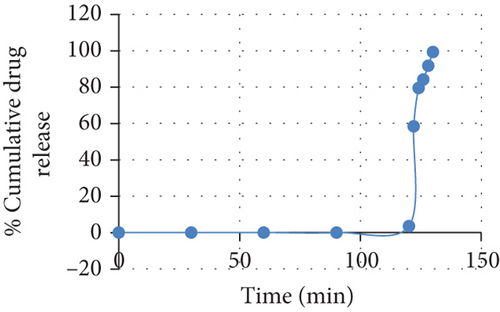
Thus, the Eudragit L 100 film on the seal-coated core tablets at 15% weight increase might have had optimum thickness and uniform enough to shelter the core tablet and prevent the drug release in acidic medium. Also, this might be due to the decrease of drug dissolution when the polymer coating level increased to 15% of core tablet weight. It is well-known phenomenon in coated tablets that dissolution decreases while coating amount increases due to an increase in time taken to dissolve coating layer if polymer is water-soluble. On the other side, it increases time taken by the dissolution medium to transport through coated layer if polymer is water insoluble [29]. When the dissolution medium was changed to intestinal pH, enteric coat ruptured, seal coat dissolved, and almost complete drug dissolution occurred within 10 min. The results are depicted in Table 4 and Figure 4. Higher solubility of Eudragit L100 in phosphate buffer pH 6.8 might resulted a faster rupture due to an increased number of pore formations on the surface of the coated tablet. This might increase the transport of the dissolution medium to the core tablets and thus resulted in faster dissolution [29].
3.7. Comparison with Marketed Product
The optimized enteric-coated formulation F8 release profile was compared with marketed product, and similarity factor was calculated. The characteristics of marketed product is shown in Table 5.
| S. no | Evaluation parameter | Observations |
|---|---|---|
| 1 | ∗Thickness (mm) | 1.52 ± 0.03 |
| 2 | ∗Hardness (kg) | 4.5 ± 0.05 |
| 3 | Weight variation (mg) | 120 ± 0.05 |
| 4 | Disintegration time | 50 sec |
| 5 | % drug content∗ | 99.8 ± 0.02 |
| 6 | % cumulative drug release ∗ | 99.7 ± 0.04 |
- ∗Each value is an average of 3 determinations.
3.8. Calculation of Similarity Factor (f2)
| pH media | Time (min) | % cumulative drug release | |
|---|---|---|---|
| F8 | Marketed product | ||
| PH 1.2 (2 H) | 30 | 0 | 0.3 |
| 60 | 0 | 0.3 | |
| 90 | 0.9 | 1.1 | |
| 120 | 1.5 | 1.6 | |
| pH 6.8 phosphate buffer (10 min) | 122 | 58.4 | 51.5 |
| 124 | 75.3 | 68.3 | |
| 126 | 84.2 | 81.4 | |
| 128 | 91.8 | 92.7 | |
| 130 | 99.8 | 98.5 | |
| F2 | 88.4 | ||
where n = number of full point
Rt = the reference profile at the time point t
Tt = the test profile at the same point
The similarity factor (f2) was calculated as 88.4 for the formulation F8 in comparison with innovator product. It indicates that formulation F8 has similar in vitro release profile with marketed product (Table 6).
3.9. Stability Studies
The stability studies were conducted as per ICH guidelines for the formulation F8. Results of stability studies dictated that there were no significant changes in physical appearance, hardness, and drug content of the tablets tested (Table 7).
| Formulation F8 | 0 month | 1st month | 2nd month | 3rd month |
|---|---|---|---|---|
| Hardness (kg) | 5.3 ± 0.5 | 5.1 ± 0.8 | 5.2 ± 0.6 | 5.2 ± 0.5 |
| Drug content (%) | 99.54 | 99.65 | 99.63 | 99.56 |
| Average weight of tablet (mg) | 255 | 254 | 254 | 253 |
4. Conclusion
Efforts were made in this research to make a stable composition of DR tablets of Duloxetine HCl. The results conclude that the optimized formulation is consistent and provides excellent resistance in acidic environment and releases the drug only in alkaline location of small intestine. Seal coating trials and enteric coating trials were done on optimized tablet core and confirmed that 5% increase in weight of seal coat and 15% increase in weight of enteric coat on tablet could be an optimum to resist the drug release in acidic environment of stomach and also allow rapid release of the drug in intestinal environment. Accelerated stability testing results also confirmed the stability of selected product throughout the study period of three months. Thus, it was concluded that the simple and effective enteric coating of duloxetine HCl tablets with optimum release profile as delayed drug delivery system could be the effective alternative to the conventional duloxetine oral capsules and tablets.
Ethical Approval
This research does not contain any studies with human participants or animals performed by any of the authors.
Consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Contributions
We declare that this work was done by the authors named in this article, and all liabilities pertaining to claims relating to the content of this article will be borne by the authors.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Program (Project number 314-42). This research was funded by the Deanship of Scientific Research through General program (Project number 314-42) of King Khalid University, Abha-61421, Saudi Arabia.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.