Paeoniflorin Protects against ANIT-Induced Cholestatic Liver Injury in Rats via the Activation of SIRT1-FXR Signaling Pathway
Abstract
Paeoniflorin (PF), a water-soluble monoterpene glycoside, is initially isolated from the dried roots of Paeonia lactiflora Pall., which has effects on ameliorating cholestasis in our previous study. However, comprehensive approaches for understanding the protective effects and mechanisms underlying cholestatic liver injury from the regulating of bile acid metabolism have not been sufficiently elucidated. This study was aimed to explore the effectiveness as well as potential mechanism of PF on alpha-naphthylisothiocyanate (ANIT)-induced cholestatic liver injury. Rats with cholestasis induced by ANIT was used to evaluate the protective effects and mechanism of PF by regulating SIRT1/FXR and NF-κB/NLRP3 signaling pathway. Rats were intragastrically administrated with ANIT to establish cholestatic liver injury model. Serum levels of ALT, AST, TBA, TBIL, ALP, γ-GT and ALB in rats were detected. The histopathology of the liver of rats was analyzed in vivo. The relative mRNA expression and protein expression levels of IL-18, IL-1β, TNF-α, HO-1, Nrf2, TLR4, NLRP3, Caspase-1, ASC, NF-κB, FXR, and SIRT1 in liver of rats were investigated. The results showed that the serum indexes and the liver histopathology were significantly improved by PF. The overexpression of IL-18, IL-1β, TNF-α, NLRP3, ASC, and Caspase-1 in liver was markedly reduced by PF. Furthermore, PF dramatically increased the mRNA and protein expressions of SIRT1, FXR, HO-1, and Nrf2, but decreased NF-κB p65 and TLR4 levels in liver of rats. Taken together, the protective effects of PF on cholestatic liver injury were possibly related to the activation of the SIRT1/FXR and inhibition of NF-κB/NLRP3 inflammasome signaling pathway. These findings might provide a potential protection for cholestatic liver injury.
1. Introduction
Cholestasis, characterized by bile secretion disorder and excessive bile acid (BA) accumulation in the liver, is clinically associated with a variety of liver diseases, such as progressive familial intrahepatic cholestasis, primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), pregnancy and drug-induced liver injury [1–4]. There are various factors which can lead to cholestasis, such as malnutrition, drug abuse, viral infection and metabolic diseases. The persistent cholestasis can lead to liver fibrosis, cirrhosis, and even liver failure [5–7]. Current opinions believe that oxidative stress, inflammatory damage, and transporter disorders are potential pathological mechanisms related to the development of cholestasis [1, 8]. At present, the clinical treatment of cholestasis is very limited. Both obeticholic acid (OCA) and ursodeoxycholic acid (UDCA) are the therapeutic drugs approved by Food and Drug Administration (FDA), which can be used in the treatment of cholestatic liver diseases. However, the clinical effect is not satisfactory, and approximately 50% of patients show no response to UDCA treatment. In addition, OCA has serious side effects such as abdominal pain, aggravating itching and fatigue [9, 10]. Therefore, it is urgent to find new targets and develop related new candidate drugs to treat cholestatic liver injury.
In recent years, with the vigorous development of complementary and alternative medicine, the enthusiasm for exploring new natural plants for the treatment of cholestatic liver injury has increased exponentially. These agents might provide a complementary therapy and alternative method to enhance the effectiveness of cholestatic liver injury [11]. Paeoniflorin (PF), a water-soluble monoterpene glycoside, is extracted from the dried roots of Paeonia lactiflora Pall.. Studies have shown that PF has a wide range of pharmacological effects, such as anti-inflammation [12], anti-oxidation [13], anti-depressant [14], and anti-apoptosis [15]. Our previous studies have shown that PF significantly improve cholestatic liver injury [13, 16]. However, the underlying molecular mechanism in regulating cholestasis and anti-inflammatory has not been fully revealed.
Previous studies have reported the key pathological mechanisms of cholestatic liver injury, providing the possibility for the discovery of new drug candidates for the treatment of cholestasis [17, 18]. Sirtuin 1 (SIRT1) and FXR have been proved to play a central role in protecting cholestatic liver injury [8]. SIRT1, an evolutionarily conserved NAD+-dependent histone III deacetylase, is a member of the silent information regulator 2 (Sir2) families of proteins and participates in a wide range of metabolic process including regulating glucose, bile acid, and lipid metabolism as well as reducing oxidative stress and inflammation [19, 20]. SIRT1 directly or indirectly regulates multifarious nuclear receptors and cofactors, such as FXR, LXR, NF-κB, which is considered to be a sensor for various metabolic processes [8, 21, 22]. FXR has been considered as an important nuclear receptor in bile acid metabolism, which plays an important regulatory role in inhibiting BA synthase, restraining liver uptake transporters, inducing bile efflux transporters, and promoting BA metabolism in the liver [17], and currently represents a promising target for new treatments for human cholestatic diseases. Some studies have shown that liver-specific SIRT1 deletion can lead to BA metabolic dysfunction by downregulating the FXR signal, which can be reversed by overexpression of SIRT1 [20].
In this study, based on an assessment of the protective effect of PF on cholestatic liver injury, the effect of PF on SIRT1/FXR and NF-κB/NLRP3 inflammasome signaling pathway was further investigated, which might provide a deeper comprehension of PF for cholestasis.
2. Materials and Methods
2.1. Materials
Paeoniflorin (PF), with purity higher than 98% determined by ultraperformance liquid chromatography (UPLC) analysis, was purchased from the Chengdu Pufei De Biotech Co., Ltd. (Chengdu, China). ANIT (dissolved in olive oil) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). As the positive control, ursodeoxycholic acid (UDCA) was supplied by Losan Pharma GmbH (Germany). Biochemical indicator kits for alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), total bile acid (TBA), alkaline phosphatase (ALP), γ-glutamyltranspeptidase (γ-GT), and albumin (ALB) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All the other experimental supplies were purchased from commercial sources.
2.2. Animals and Drug Treatments
Male Sprague-Dawley (SD) rats weighing 200 ± 10 g were purchased from Sibeifu (Beijing) Biotechnology Co., Ltd. (Beijing, China, Permission No. SCXK (jing) 2019-0010). All animals were housed under standard laboratory conditions of temperature (25 ± 2°C) and lighting (12 : 12 h light: dark cycle). Rats were provided with free access to water and chow diet. All animal experiments were approved by the Ethics Committee of the Ethics of Animal Experiments of the Fifth Medical Center of PLA General Hospital (Approval ID: IACUC−2019−004).
All the animals were acclimated for 1 week prior to the experiment. Sixty rats were randomly divided into five groups (12 rats per group), including control group, ANIT group, positive drug group (UDCA, 60 mg/kg), PF low dose group (PFL, 50 mg/kg), and PF high dose group (PFH, 200 mg/kg) [23]. PF and UDCA were dissolved in normal saline and intragastrically given to experimental groups for consecutive five days. At the same time, the control group and ANIT group were intragastrically administrated with the same volume of normal saline. During administration, the control group was administrated with olive oil alone, while the other groups were intragastrically given 60 mg/kg ANIT (dissolved in the olive oil) on the third day to induce cholestatic liver injury. Forty-eight hours after ANIT treatment, all the rats were sacrificed to collect the blood and livers. Blood samples were centrifuged at 3000 ×g for 10 min to obtain serum and stored at −80°C. Liver samples were immediately collected and divided into two parts: one part of liver tissue was excised and fixed in 10% neutral-buffered formalin for HE staining, and another part was snap-frozen in liquid nitrogen and stored at −80°C for RT-PCR, western blotting and immunohistochemistry analysis.
2.3. Serum Biochemical Analyses
Synergy H1 Hybrid Reader (Biotech, USA) was used for the detection of serum biochemical indices. The serum levels of ALT, AST, TBIL, TBA, γ-GT, ALP as well as ALB were measured using commercial kits in accordance with the manufacturer’s instructions.
2.4. Histopathological Assessment
After rats were sacrificed, the liver tissues of the same leaf of each rat were immediately collected and fixed in formalin, then embedded in paraffin and sectioned to 5 μm slices. All the slices were stained with hematoxylin and eosin (H&E) following a standard protocol. Histological assessment was carried out independently by two researchers unaware of the different groups. Any differences arising in the process of were settled through discussion and negotiation with another pathologist. Then the pathological changes in the liver tissues were captured with a Nikon microscope (Nikon Instruments Inc., Japan), and the microscope analysis was performed by 200x and 400x.
2.5. Quantitative Real-Time PCR
The total RNA rat liver tissue of each group was extracted using Trizol reagent following the manufacturer’s instructions. The concentration and purity of the total RNA were determined at 260 nm and 280 nm on a spectrophotometer. Then, cDNA was obtained by reverse transcribed 2 μg of total RNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, MA, USA). The cDNA synthesized was stored at −20°C for subsequent PCR reactions. The amplification reaction of RNA was performed by QuantStudio™ Real-Time PCR System version 1.3 (Applied Biosystems by Thermo Fisher Scientific). The quantity of mRNA was normalized with the GAPDH expression and all the data were calculated for comparison through 2−∆∆CT method. The list of primers used in our study is listed in Table 1.
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| NLRP3 | GCAGCGATCAACAGGCGAGAC | TCCCAGCAAACCTATCCACTCCTC |
| Caspase-1 | AAACACCCACTCGTACACGTCTTG | AGGTCAACATCAGCTCCGACTCTC |
| ASC | TGGTTTGCTGGATGCTCTGTATGG | ACAAGTTCTTGCAGGTCAGGTTCC |
| NF-κB | GGGATGGCTTCTATGAGGCTGAAC | CTTGCTCCAGGTCTCGCTTCTTC |
| TLR4 | TTGCTGCCAACATCATCCAGGAAG | CAGAGCGGCTACTCAGAAACTGC |
| HO-1 | CAGACAGAGTTTCTTCGCCAGAGG | TGTGAGGACCCATCGCAGGAG |
| Nrf2 | CAAACATTCAAGCCGATTAGAGG | CGGCAACTTTATTCTTCCCTCT |
| IL-18 | CGACCGAACAGCCAACGAATCC | TCACAGATAGGGTCACAGCCAGTC |
| IL-1β | CTCACAGCAGCATCTCGACAAGAG | TCCACGGGCAAGACATAGGTAGC |
| TNF-α | ATGGGCTCCCTCTCATCAGTTCC | GCTCCTCCGCTTGGTGGTTTG |
| GAPDH | TTCCAGGAGCGAGATCCCGCTAAC | CATGAGCCCTTCCACGATGCCAAAG |
2.6. Western Blotting Analysis
Rat liver tissues (about 80 mg) were homogenized and then lysed in the prepared ice-cold lysis buffer with 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor mixture. Subsequently, tissue debris was removed by centrifugation at 12, 000 ×g and 4°C for 10 min. After centrifugation, the supernatant was aliquoted and stored at −80°C for the subsequent western blotting assay. The concentrations of total protein in supernatants were quantified using a BCA protein assay reagent kit (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China). The samples with the same amount of protein (10 μL) per lane were separated by 10% SDS-PAGE of gel at 80 V for 30 min and 120 V for 1 h. After electrophoresis, the gels were transferred onto polyvinylidene difluoride (PVDF) membranes. All the membranes were blocked with 5% fat-free milk at room temperature for two hours, then incubated overnight at 4°C with antibodies against anti-FXR rabbit polyclonal antibody (bs-12867R, Bioss, dilution: 1 : 1000), anti-SIRT1 rabbit monoclonal antibody (ab189494, Abcam, dilution: 1 : 1000), anit-NLRP3 rabbit monoclonal antibody (ab263899, Abcam, dilution: 1 : 1000), anit-Caspase-1 rabbit polyclonal antibody (342947, ZEN BIO, dilution: 1 : 1000), anit-ASC rabbit polyclonal antibody (340097, ZEN BIO, dilution: 1 : 1000), anit-NF-κB p65 rabbit polyclonal antibody (380172, ZEN BIO, dilution: 1 : 1000), anit-HO-1 rabbit polyclonal antibody (43966, Cell signaling technology, dilution: 1 : 1000), anit-GAPDH rabbit polyclonal antibody (10494-1-AP, proteintech, dilution: 1 : 1000). After washes 5 × 5 min in TBST (Tris-buffered saline with Tween 20), the membranes were incubated with horseradish peroxidase conjugated secondary antibodies (ab6728, abcam, dilution: 1 : 10,000) at room temperature for 1 hour, and subsequently the protein bands were measured using an enhanced chemiluminescence detection system. Samples were assessed for GAPDH content as an internal control.
2.7. Immunohistochemical Analysis
The protein levels of NF-κB p65 (380172, ZEN BIO) and Nrf2 (380773, ZEN BIO) were analyzed by immunohistochemistry as previously described [24]. In brief, the liver tissues sections were incubated with primary antibodies directed against NF-κB p65 and Nrf2 overnight at 4°C, then treated with corresponding peroxidase-coupled secondary antibodies for 50 min at room temperature and then developed by diaminobenzidine (DAB). Next, the sections were stained with hematoxylin for 3 minutes. Last, images were captured with a digital camera system under 200x magnification.
2.8. Statistical Analysis
All data were presented as the mean ± standard deviation (). The differences between the group means were calculated by one-way ANOVA analysis and Duncan’s multirange test with the SPSS computer program (version 24.0). GraphPad Prism software (version 8.2.0) was used to visualize the results. The differences were considered to be statistically significant when P < 0.05 and highly significant when P < 0.01.
3. Results
3.1. Protective Effect of Paeoniflorin against ANIT-Induced Liver Injury
3.1.1. Effect of PF on Liver Function Indexes
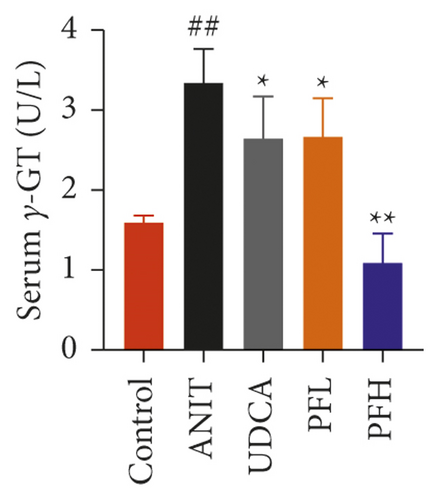
As shown in Figures 1(a) and 1(b), serum AST and ALT were increased in the ANIT-treated rats and were significantly reduced by PF pretreatment (P < 0.01). Similarly, PF pretreatment also alleviated ANIT-induced cholestatic liver injury, as evidenced by preventing the ANIT-induced the elevation of serum TBIL, ALP, TBA, and γ-GT (P < 0.01) (Figures 1(c)–1(f)). The content of ALB decreased in ANIT-induced cholestatic rats, compared with the control group. Conversely, PF substantially increased the serum level of ALB (P < 0.01) (Figure 1(g)). Taken together, the results indicated that the protective effects of PF on cholestatic liver injury are related to ameliorating liver function, and PF provided remarkable protection against ANIT-induced hepatotoxicity and cholestasis.
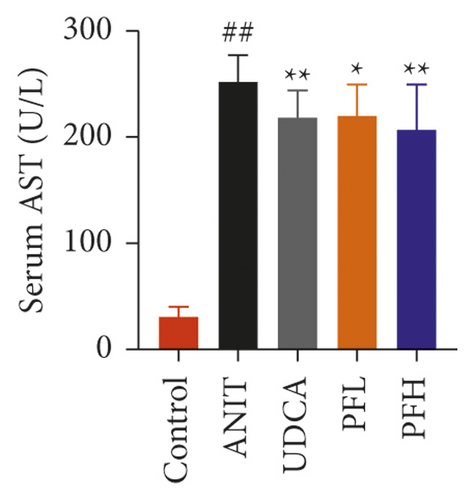
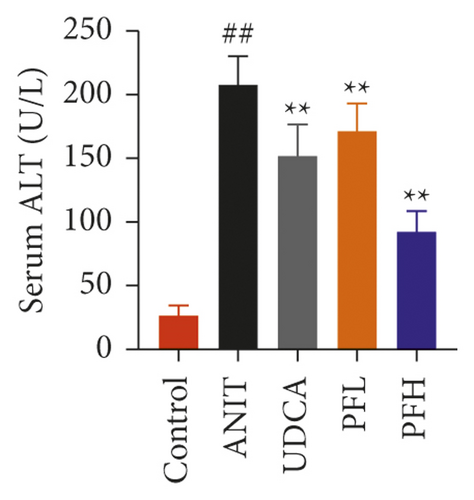
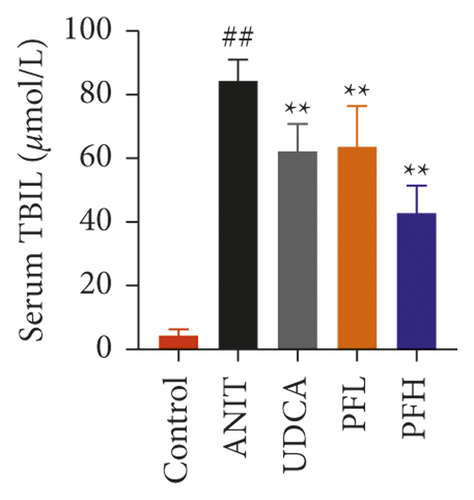

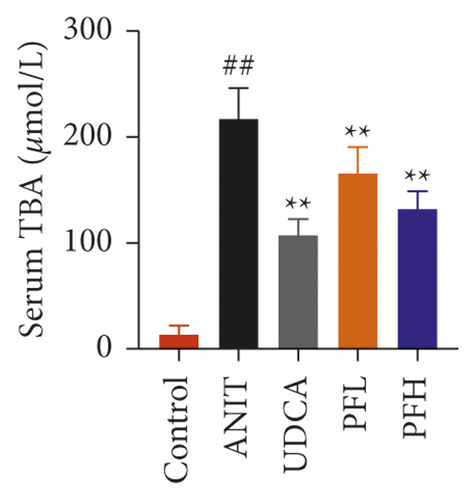

3.1.2. Effect of PF on Histopathology
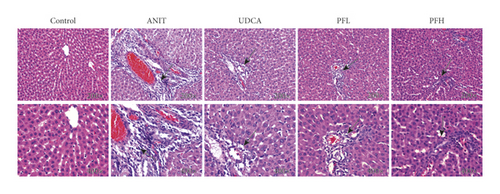
H&E staining of liver sections showed that the control group exhibited the normal structure without abnormal morphological changes, liver cell cord in order, sound hepatic cell with uniform stain, and no evidence of infiltration of neutrophilic granulocyte. In contrast, the ANIT-induced group displayed acute infiltration by polymorphonuclear neutrophils, cellular edema, sinusoid congestion, hepatic lobules destruction, and association with hepatic necrosis. However, the liver tissue damage severity significantly relieved in the groups of pretreatment with PF. In the PFL and PFH groups, there was a certain degree of bile duct epithelial damage and defined hepatocyte hydropic degeneration, accompanied by less hepatic neutrophil with infiltration the degree of hepatic necrosis was significantly attenuated and the inflammatory cell infiltration was ameliorated in a dose-dependent manner. Additionally, UDCA, as positive control drug, had an ameliorative effect with liver injury. These results above revealed a significant improvement with cholestatic liver injury in rats pre-treated with PF (Figure 2).
3.1.3. Effect of PF on Inflammatory Factors in the Liver Tissue
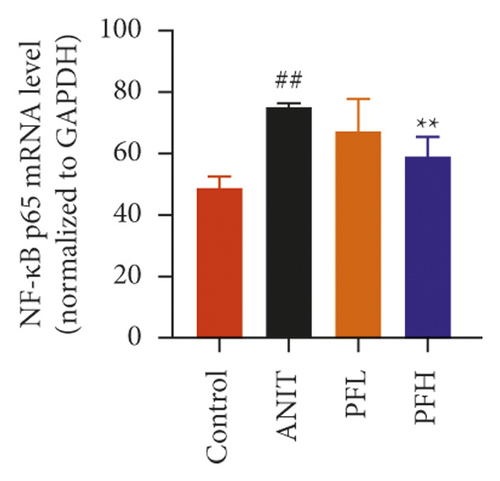
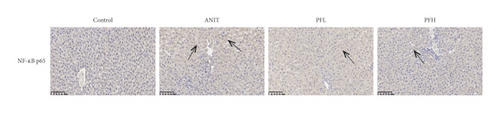
Inflammation is one of the characteristics of cholestatic liver injury. Therefore, the mRNA expression level of hepatic inflammation-related factors, including TNF-α, IL-1β, and IL-18 were determined. As illustrated in Figures 3(a)–3(c) and Table 2, the results indicated that the expression level of TNF-α, IL-1β, and IL-18 were remarkably increased by ANIT-treated groups (P < 0.01). In contrast, PF pretreatment could attenuate the expression of these indicators. To further explore the anti-inflammatory mechanism of PF in cholestasis rats, the mRNA and protein expression level of NF-κB p65, which could modulate various inflammatory factors including TNF-α, IL-1β, and IL-18 was detected. In this study, the results indicated that the relative mRNA and protein expression of NF-κB p65 were significantly increased in the ANIT-treated group. However, PF significantly inhibited the mRNA and protein expression of NF-κB p65 (Figures 3(d), 4(a), and 4(d)) (P < 0.01). In addition, the immunohistochemical analysis was consistent with the result of western blotting analysis (Figure 3(e)). These findings suggest that PF is responsible for inhibiting inflammation in ANIT-induced cholestasis via the NF-κB pathway.
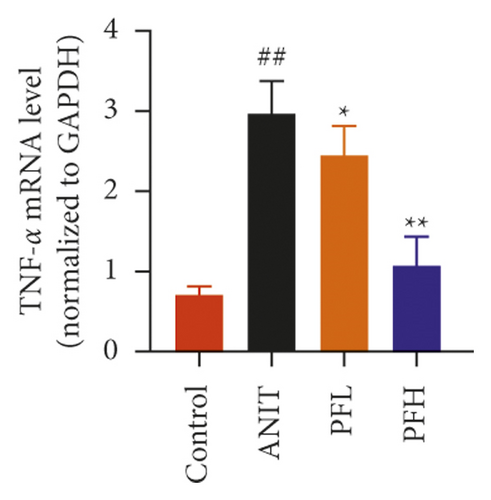
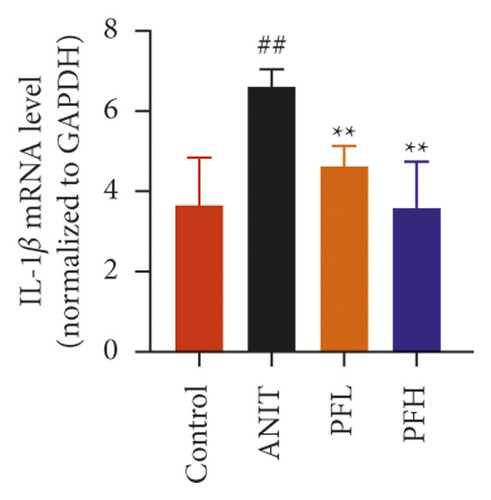
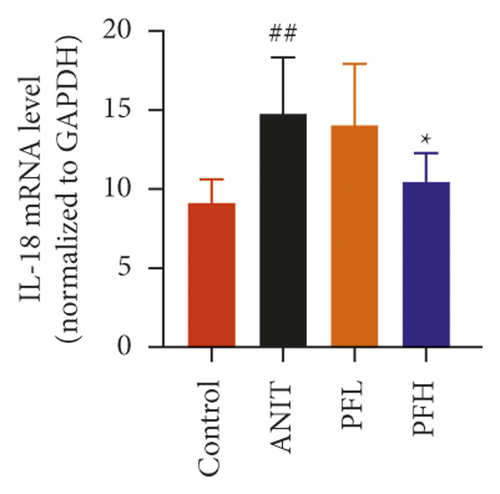
| Group | TNF-α | IL-1β | IL-18 |
|---|---|---|---|
| Control | 0.71 ± 0.11 | 3.65 ± 1.20 | 9.11 ± 1.51 |
| ANIT | 3.00 ± 0.41## | 6.60 ± 0.44## | 14.75 ± 3.60## |
| PFL | 2.44 ± 0.37∗ | 4.63 ± 0.51∗∗ | 14.02 ± 3.88 |
| PFH | 1.07 ± 0.36∗∗ | 3.58 ± 1.15∗∗ | 10.46 ± 1.83∗ |
- Data were expressed as mean ± SD. ##P < 0.01 compared with control group; ∗P < 0.05 and ∗∗P < 0.01 compared with the ANIT group (n = 6).
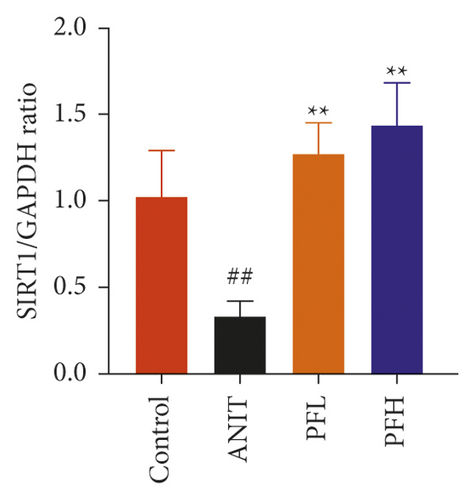
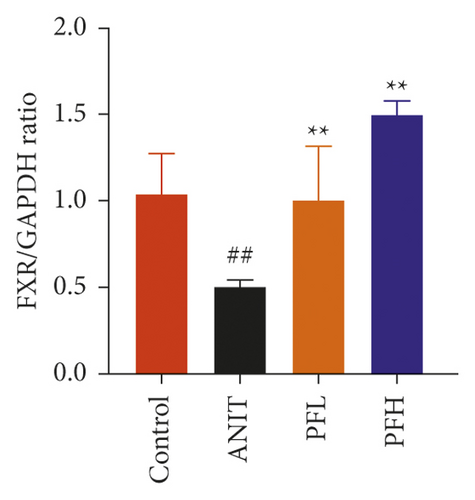

3.2. PF Activated SIRT1/FXR Signaling Pathway in Cholestasis Rats
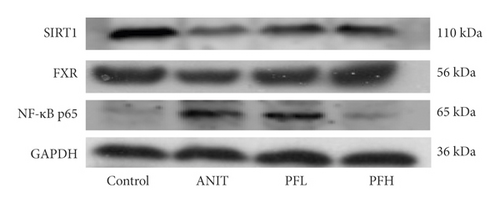
To dissect the potential mechanism of PF for the inhibition of NF-κB pathway, several protein expressions associated with BA homeostasis were investigated. As an important sensor that is critical for bile acid metabolism, the expression of FXR is tightly controlled by an intricate regulatory network in response to various complex environments. Thus, the protein expression of SIRT1, which is the upstream target of FXR, was determined. In comparison to the control group, the protein expression of SIRT1 and FXR decreased markedly in rats treated with ANIT, while their expression in PF-treated rats were restored (Figures 4(a)–4(c)) (P < 0.01). These findings suggested that the protective effect of PF against cholestasis might be a result of suppressing NF-κB, which is mediated via SIRT1 and FXR activation.
3.3. PF Suppressed the Expression of NF-κB by Activating FXR/Nrf2 Signaling Pathway
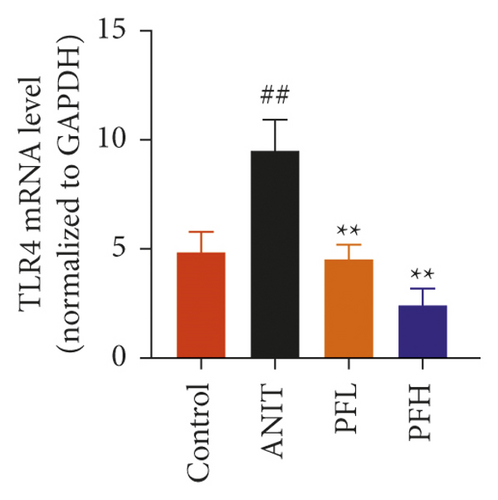
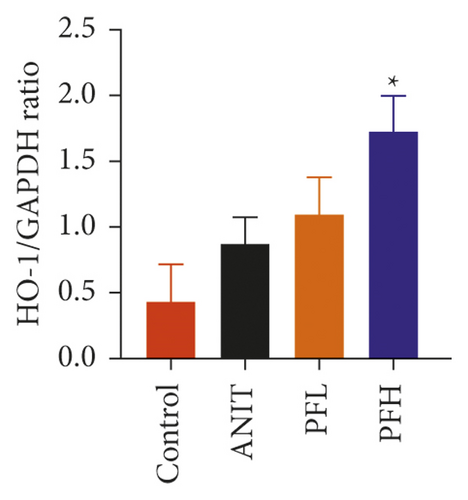
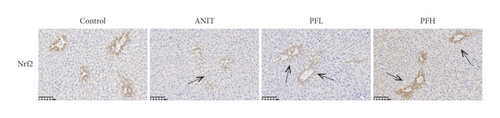
To further explore the mechanism underlying protection of PF against ANIT-induced inflammation, the relative mRNA expressions of Nrf2, HO-1, and TLR4 were determined. The results indicated that ANIT treatment decreased Nrf2 mRNA level (P < 0.01), but not significantly altered the relative mRNA expressions of HO-1 (P > 0.05). Furthermore, the relative mRNA expressions of TLR4 remarkably increased in ANIT-induced cholestasis rats (P < 0.01). As illustrated in Figures 5(a)–5(c), PF pretreatment dramatically upregulated the mRNA expression of HO-1, Nrf2, and downregulated the level of TLR4 (P < 0.01). In addition, immunohistochemical staining demonstrated that the increased expression of Nrf2 in ANIT-induced rats after PF pretreatment (Figure 5(f)). Next, the relative protein expression of HO-1 was determined by western blotting analysis. As shown in Figures 5(d) and 5(e), HO-1 was not significantly changed in the ANIT administration group (P > 0.05). Conversely, PF therapy increased the relative protein expression of HO-1, especially in high dose of PF (Figures 5(d) and 5(e)) (P < 0.01). Overall, the results suggested that PF could exert an anti-inflammatory action on ANIT-induced hepatotoxicity and cholestasis via the FXR/Nrf2 signaling pathway.
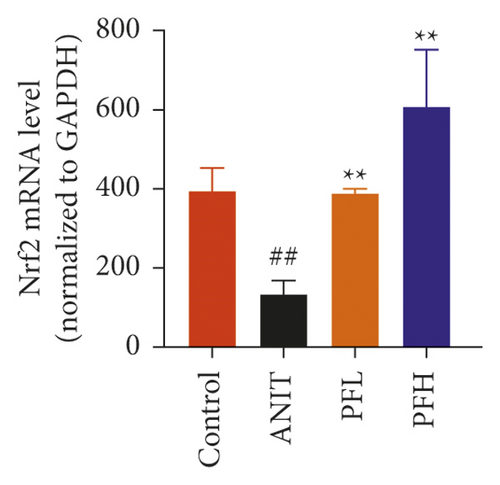
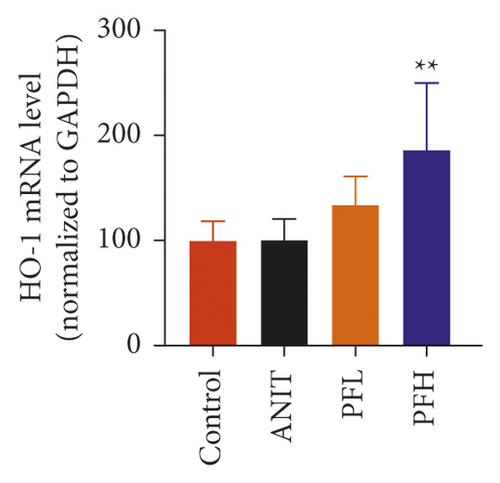

3.4. PF Inhibited the NF-κB/NLRP3 Inflammasome Pathway in Cholestasis Rats
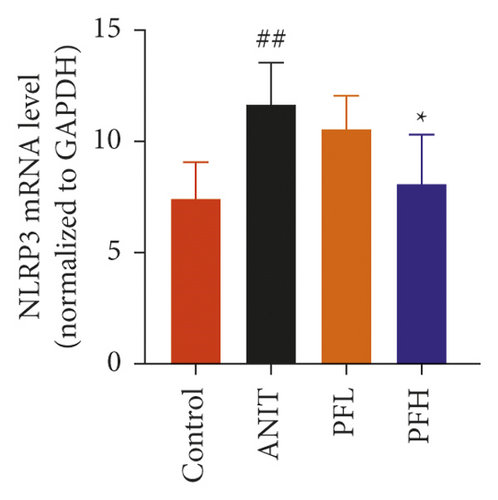
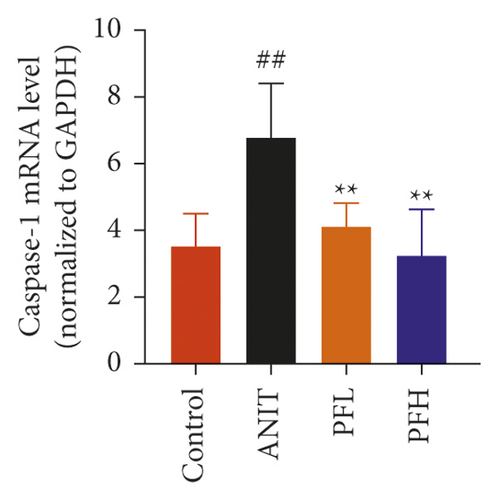
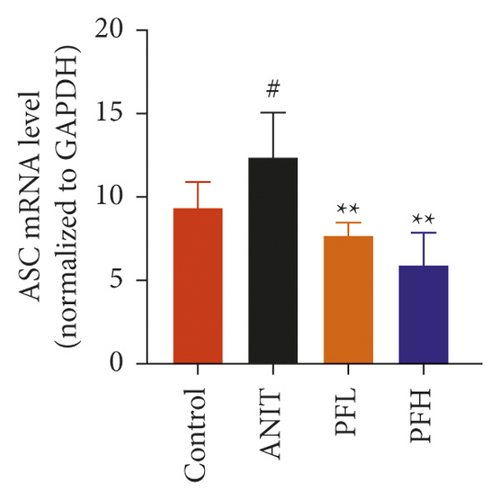
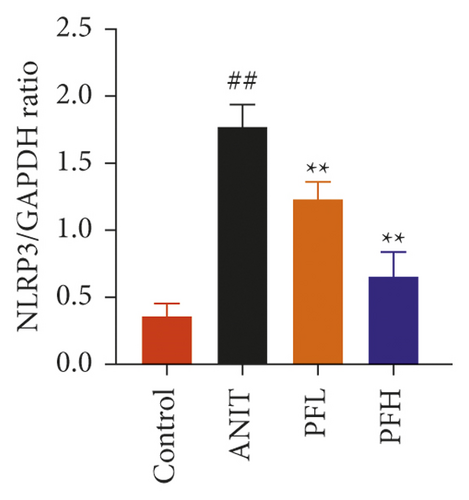
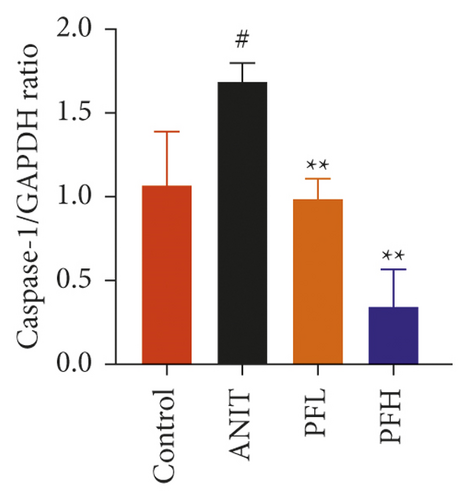
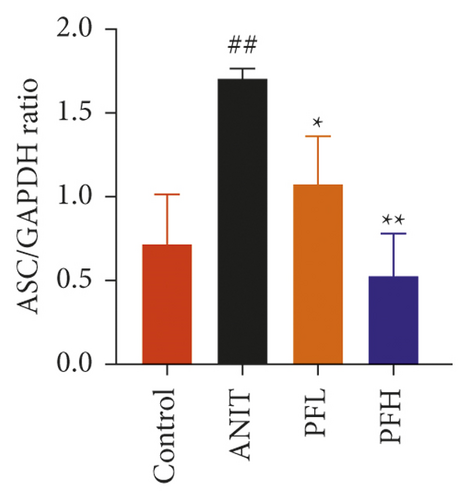
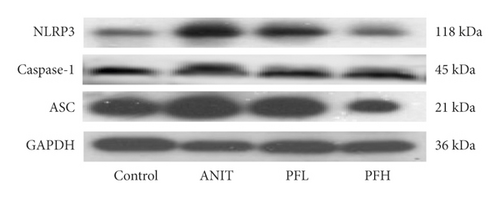
To further evaluate the mechanism underlying protection of PF against ANIT-induced cholestatic liver injury, NF-κB/NLRP3 inflammasome pathway was determined. As shown in Figures 6(a)–6(c), the effect of PF on NLRP3 inflammasome mRNA expression, including NLRP3, Caspase-1, and ASC was further detected. As expected, after ANIT stimulation, the relative mRNA expression of NLRP3, Caspase-1, and ASC were increased in the liver tissues compared with that in the control group (P < 0.05 or P < 0.01). However, PF at the dose of 50 and 200 mg/kg significantly reduced the relative expression of NLRP3, Caspase-1, and ASC in ANIT-induced cholestatic liver injury (P < 0.05 or P < 0.01). To verify the accuracy of the mRNA results on the induction of these indices by PF, the relative protein levels of NLRP3, Caspase-1, and ASC were measured using western blotting analysis. The results were in consistent with the RT-PCR results (Figures 6(d)–6(g)) (P < 0.01). Taken together, these observations showed that PF ameliorated ANIT-induced cholestasis by restraining NF-κB/NLRP3 inflammasome pathway.
4. Discussion
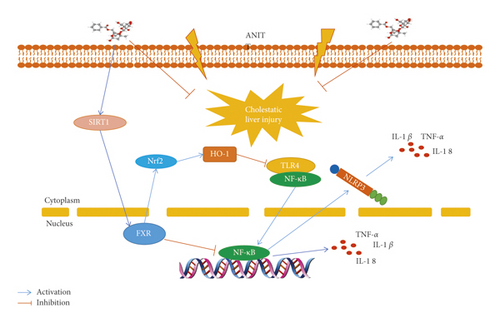
The present study showed that PF had a good protective effect on cholestatic liver injury induced by ANIT, and the main role of PF on improving cholestatic liver injury were to attenuate inflammation and regulate bile acid metabolism. Further research showed that the protective effect of PF against ANIT-induced cholestatic liver injury by upregulating the expression of SIRT1/FXR and inhibiting NF-κB/NLRP3 inflammasome pathway (Figure 7).
Cholestasis is characterized by intrahepatic accumulation of toxic bile acids due to defective secretion of hepatocellular or cholangio cellular and bile ducts obstruction. Bile acid metabolism disorder and inflammation are known to be the common feature of cholestatic liver injury. The accumulation of bile acids may cause hepatocyte toxicity and liver injury-induced inflammatory response [24–26]. It is believed that inflammation and bile acid metabolism disorder are the decisive generating factors in the pathogenesis of cholestatic liver injury, and anti-inflammatory and regulating bile acid metabolism therapy will be the recommended therapeutic strategy. Up to now, UDCA and OCA are commonly used in the treatment of cholestatic liver diseases, however, the effect are not satisfactory. PF has been proved to have liver protective effect [23]. In our study, ALB, like AST and ALT, is a commonly used clinical indicator of liver function. This study showed that the content of ALB decreased significantly in cholestatic rats, which was in agreement with the previous finding [27]. Pre-administration of PF significantly increased the content of ALB, which provided protection for the liver. In addition, PF significantly decreased ANIT-induced elevation of serum ALT, AST, ALP, TBA, and TBIL levels. Moreover, the pathological injuries were relieved after PF pretreatment at the dose of 50 and 200 mg/kg, which were coincident with the previous report [28].
SIRT1/FXR signaling pathway plays a key role in inflammation and BA metabolism in cholestatic liver injury [29, 30]. One of the interesting findings from the previous study was the significant reduction of SIRT1 in human and mouse cholestasis [31]. In addition, studies have shown that the expression of FXR is suppressed in liver injury, and the activation of FXR has been proved to improve liver injury [8, 32]. Interestingly, it has been found that SIRT1 and FXR can together form an interactive regulatory network [33]. Previous studies have demonstrated that SIRT1 was a critical transcriptional and transactivation regulator of FXR and regulated its activity by deacetylating proteins and histones. More importantly, loss of liver-specific SIRT1 can lead to BA metabolic dysfunction by downregulating FXR signaling, while it can be reversed by SIRT1 overexpression [20, 34]. In addition, activation of the SIRT1/FXR signaling pathway has been confirmed to have a protective effect on cholestatic liver injury [35]. In the current study, our data indicated that SIRT1 and FXR were greatly reduced by ANIT treatment, which were in line with previous studies [25, 36]. While after pretreatment with PF at the dose of 50 and 200 mg/kg, the expression of SIRT1 and FXR were substantially attenuated. Our results suggest that the protective effects of PF against ANIT-induced cholestatic liver injury may be dependent on the activation of the SIRT1/FXR signal.
Interestingly, it has also been shown that activation of FXR exerts anti-inflammatory effects in liver diseases. Accumulating studies have reported that NF-κB is a downstream gene of FXR, and activating FXR signaling pathway had an effective protective effect on liver injury by reducing inflammatory responses [36–39]. In addition to the direct regulation of NF-κB, FXR can also indirectly regulate the expression of NF-κB through Nrf2 [40, 41]. With the discovery of new target genes, it has been found that Nrf2 not only plays a key role in the dynamic balance of redox, but also affects the inflammatory response [42]. Previous studies have shown that Nrf2/HO-1 pathway is considered to be one of the upstream molecules against inflammation induced by NF-κB [42, 43]. Wu et al. recently reported that the lack of Nrf2 effectively prevents PF-mediated inhibition of LPS-induced NF-κB translocation and inflammatory mediator expression [44]. Furthermore, toll-like receptors are important pattern recognition receptors that mediate innate immunity, and their mediating signal pathways play an important role in the occurrence and development of inflammation. The activation of TLR4 can cause the activation of NF-κB into the nucleus. It has previously been reported that the high expression of HO-1 significantly inhibited the expression of TLR4/NF-κB [45]. In the current research, ANIT treatment displayed significant inhibition on Nrf2 expression, but had little effect on the expression of HO-1, along with induction on TLR4 and NF-κB. Moreover, PF pretreatment exhibited an advantageous regulation pattern, which caused the increase of Nrf2 and HO-1, and the decrease of TLR4 and NF-κB. The protective effect of PF in ANIT-induced cholestasis liver injury has been indicated to be due to decreased the levels of NF-κB/TLR4, which is closely related to Nrf2-mediated HO-1 upregulation. Taken together, the above data demonstrate an anti-inflammatory role for PF during cholestatic liver injury, and shed new insights into the significance of the FXR signaling pathway in mediating the protective effect.
The NF-κB/NLRP3 inflammasome pathway is an important pathway for regulating inflammatory factors. Recent studies have shown that NLRP3 may cause liver injury through the activation of NF-κB-related pathways, and NLRP3 inflammasome is of great significance in regulating the occurrence and development of inflammatory response in liver injury [46, 47]. When stimulated by external factors, NF-κB is activated to regulate the expression of NLRP3, IL-18, TNF-α, and other genes. Then, macrophages activate the NLRP3-ASC-Caspase-1 inflammasome complex, and the expression of NLRP3 protein regulated by NF-κB provides raw materials for the assembly of the complex. NLRP3 inflammasome activates Caspase-1 via the adaptor protein of ASC, and activated Caspase-1 mediates the maturation of IL-1β precursor. Mature IL-1β can lead to the aggregation and activation of macrophages in the liver, further amplifying inflammation reaction [48]. With the increase of IL-1β and other pro-inflammatory factors, NF-κB is further activated, and the cascade of inflammation is gradually amplified. Eventually, the inflammatory response cannot be controlled and causes severe liver tissue injury. In the current study, the inflammatory factors, including TNF-α, IL-1β, and IL-18 were significantly elevated in ANIT-treated rats. However, PF reversed the change of inflammatory factors. Furthermore, we found that PF dramatically inhibited the relative protein and mRNA expressions of NLRP3, ASC, and Caspase-1 in ANIT-induced cholestatic liver injury in rats. All the evidence suggest that PF has the ability to alleviate ANIT-induced cholestatic liver injury by negatively regulating inflammation via the NF-κB/NLRP3 signaling pathway.
5. Conclusion
In summary, this study demonstrated PF protects against ANIT-induced cholestatic liver injury in rats, and the potential mechanism is related to upregulating the expression of SIRT1/FXR, and inhibiting NF-κB/NLRP3 inflammasome pathway. Thus, PF may be a promising therapeutic agent for the treatment of cholestatic liver disease. These findings may provide evidence why PF has potential protective effects on cholestatic liver injury in the clinic.
Abbreviations
-
- PF:
-
- Paeoniflorin
-
- ANIT:
-
- Alpha-naphthylisothiocyanate
-
- ALT:
-
- Alanine aminotransferase
-
- AST:
-
- Aspartate aminotransferase
-
- TBIL:
-
- Total bilirubin
-
- TBA:
-
- Total bile acid
-
- ALP:
-
- Alkaline phosphatase
-
- γ-GT:
-
- γ-glutamyltranspeptidase
-
- ALB:
-
- Albumin
-
- UDCA:
-
- Ursodeoxycholic acid
-
- OCA:
-
- Obeticholic acid
-
- BA:
-
- Bile acid
-
- PBC:
-
- Primary biliary cirrhosis
-
- UPLC:
-
- Ultraperformance liquid chromatography
-
- PSC:
-
- Primary sclerosing cholangitis
-
- SIRT1:
-
- Sirtuin 1
-
- H&E:
-
- Hematoxylin and eosin
-
- PVDF:
-
- Polyvinylidene difluoride
-
- TBST:
-
- Tris-buffered saline with Tween 20.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors’ Contributions
Lisheng Chen conceived the study and wrote the manuscript. Shizhang Wei conducted methodological investigation and verification. Honghong Liu and Jianyu Li managed the data. Mani Jing curated and checked data. Yuling Tong collected and prepared the samples. Ruisheng Li conducted animal experiments. Jianxia Wen revised the manuscript. Hanqiu Zhan supervised the whole process. Yanling Zhao designed the study and amended the paper. All the data were generated in-house, and no paper mill was used. All the authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (81874365).
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.




