Time to Develop and Predictors for Incidence of Tuberculosis among Children Receiving Antiretroviral Therapy
Abstract
Infection by the human immune deficiency virus (HIV) is the strongest risk factor for latent or new infection of tuberculosis (TB) through reduction of CD4 T-lymphocytes and cellular immune function. Almost one-third of deaths among people living with HIV are attributed to tuberculosis. Despite this evidence, in Ethiopia, there is a scarcity of information regarding the incidence of tuberculosis for children living with HIV. Thus, this study assessed time to develop and predictors for incidence of tuberculosis in children attending HIV/AIDS care in public hospitals: North West Ethiopia 2021. Methods. A facility-based retrospective cohort study was conducted among 421 seropositive children on antiretroviral therapy in two hospitals between January 1, 2011 and December 31, 2020. EPI-DATA version 3.2 and STATA/14 software were used for data entry and analysis, respectively. Tuberculosis-free survival time was estimated using the Kaplan-Meier survival curve. Bivariate and multivariable Cox regression model was fitted to identify predictors at a P value <0.05 within 95% CI. Results. In the final analysis, a total of 421 seropositive children were included, of whom, 64 (15.2%) developed tuberculosis at the time of follow-up. The mean (±SD) age of the children was 10.62 ± 3.32 years, with a median (IQR) time to develop TB that was 23.5 (IQR = ±19) months. This study found that the incidence of tuberculosis was 5.9 (95% CI: 4.7; 7.6) per 100 person-years (PY) risk of observation. Cases at baseline not taking cotrimoxazol preventive therapy (CPT) (AHR = 2.5; 95% CI, 1.4-4.7, P < 0.021), being severely stunted (AHR = 2.9: 95% CI, 1.2-7.8, P < 0.03), and having low hemoglobin level (AHR = 4.0; 95% CI, 2.1-8.1, P < 0.001) were found to be predictors of tuberculosis. Conclusion. A higher rate of tuberculosis incidence was reported in our study as compared with previous studies in Ethiopia. Cases at baseline not taking cotrimoxazol preventive therapy (CPT), being severely stunted, and having low hemoglobin (≤10 mg/dl) levels were found to be at higher risk to developed TB incidence.
1. Introduction
The human immune deficiency virus (HIV) is the strongest risk factor for latent or new infection of tuberculosis (TB) through reduction of CD4 T-lymphocytes and cellular immune function [1].
The coinfections of TB/HIV are bidirectional, and the diseases reinforce each other, in that HIV sustains the progression of latent tuberculosis bacilli into active TB, while tuberculosis accelerates the progression of HIV disease to its advanced clinical stage [1, 2]. In 2019, there were an estimated 10.1 million new cases of TB and 1.7 million new deaths, making TB the leading cause of death from a single infectious agent (ranking above HIV/AIDS) [3]. In fact TB is responsible for one–third of TB/HIV-associated deaths for children living with HIV [4, 5]. The burden of this disease is higher in sub-Saharan Africa, where HIV remains a significant problem with inadequate coverage with antiretroviral drugs [6]. Globally, about 36.7 million patients were living with HIV/AIDS, and 2.1 million people became newly infected in 2015. The sub-Saharan Africa countries account for the largest proportion, with 25.6 million people living with HIV [1, 3]. Likewise, in 2018, there were about 251,000 deaths from TB among PLWHIV, which accounts for 33% of total deaths associated with HIV, which is much higher than the case fatality rate expected by WHO, which is ≤5% [1, 7]. Despite different interventions, TB incidence rate in children on ART is high in different settings at different times. Notably, 0.28 per 100 person-years reported in Latin America [8], 1.9 per 100 person-years reported in Zambia [9], 4.0 and 21.1 per 100 person years in South Africa [10], and 17.4 in East Africa [11]. Often in resource-limited settings, estimates of TB in children have been based on extrapolation from adult data [4], but children with TB differ significantly from adult TB patients in their immunological and pathophysiological responses [7].
The Ethiopian Federal HIV and AIDS Prevention and Control Office estimated that the single national HIV/AIDS is among the top ten high burden counties with an incidence rate of 341/100,000 of which 31% of TB patients are living with HIV [5, 7]. Still now, TB/HIV coinfection is the leading cause of death in people living with HIV/AIDS [12]. Although the incidence of TB among adult HIV patients has been exhaustively studied, it is overlooked for HIV-positive children receiving antiretroviral therapy. So, this study was intended to determine the incidence rate and predictors for the time to developed TB among HIV-positive children receiving antiretroviral therapy in North West, Ethiopia 2021.
2. Methods
2.1. Study Design, Area, and Populations
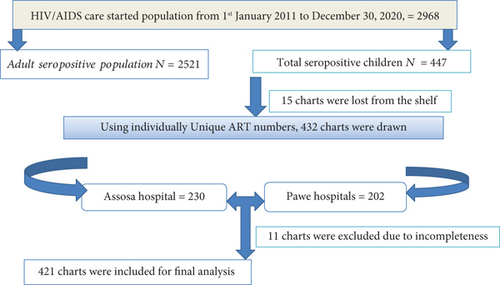
Facility-based retrospective cohort study was employed among 421 seropositive children from 1st January 2011 to December 31/2020 in two (Assosa and Pawe) general hospitals. Both are located in the Benishangul Gumuz region, Northwest of Ethiopia [13]. Apart from other services, these hospitals have been providing ART follow-up care services since 2007. In the hospital, the recorded number of HIV-positive people starting ART care was 2968, of who were 447 HIV-positive children files were there since January 1, 2011 and December 31, 2020 (Figure 1).
2.2. Sample Size Determination and Sampling Techniques
Exclusion criteria: patients taking anti-TB treatment at the time of HIV/AIDS enrolment were excluded from the study.
2.3. Outcome Ascertainment
The new incidence of TB considered as an event of interest, which is defined as the occurrence of TB in HIV-positive children during successive follow-up at any time after enrollment in the pediatrics HIV care clinic. Children who were lost, died, transferred out, or did not develop the events until the last visit were considered censored, whereas variables including sociodemographic such as age, sex, residence, family size, parental history of TB contact, plus clinical factors like WHO clinical stage, baseline cluster of differentiation (CD4 count), Hgb, functional status, and nutritional status like (stunting, wasting, underweight, MUAC) were considered as independent variables.
2.4. Operational Words
Smear-positive pulmonary tuberculosis: at least one sputum smear examination is positive for acid fast bacilli (AFB) by direct microscopy. Smear-negative pulmonary tuberculosis: sputum specimens negative for AFB and radiographical abnormalities were consistent with active TB and the decision by a clinician to treat with a full course of antituberculosis chemotherapy. Extra Pulmonary TB: defined as tuberculosis outside the lung usually results from hematogenous dissemination. Sometimes infection directly extends from an adjacent organ. Symptoms vary by site but generally include fever, malaise, and weight loss. Diagnosis is most often by sputum smear and culture and, increasingly, by rapid molecular-based diagnostic test. Serpositive children: children who had human immune deficiency virus (HIV) in their blood and catgories under age less than 15 years.
2.5. Data Collection Instruments and Quality Assurance
A structured English version checklist was developed and used for data extraction from the patients’ medical records sheet and Federal Ministry of Health Pediatrics antiretroviral therapy (ART) follow-up form [17]. Four diploma nurses and two degree nurses were recruited for data collection and supervision. One day of training was given for data collection and supervision procedures. A pretest was conducted on 5% of the final sample size at Felege Selam health center to check the reliability of the checklist. After the pretest, necessary modification of the data collection tool was made. Strict follow-up and supervision were carried out during data collection by the principal investigators, and feedback was given daily.
2.6. Data Processing and Analysis
EPI-DATA version 3.2 and STATA/14 software were used for data entry and analysis, respectively. Proportional hazard assumption was checked for each variable, and no variable was found with Schoenfeld residual test < 0.05. Categorical variables at bivariable Cox regression were assessed for candidates transferred at P value <0.25 for final multivariable Cox regression models, and variables associated with TB incidence in 95% CI at P < 0.005 were claimed as the predictor.
2.7. Ethical Approval and Consent of Participants
Ethical clearance and ethical approval were obtained from the research institute review board (IRB) of Debre Markose University with reference (Refill no: DMU IRB-984/118/13). A formal letter was submitted to all three hospitals for permission to be done. Debra Markose University waived consent from caregivers in addition to the national research, and ethical review guide waived consent for secondary files.
3. Results
3.1. Baseline Sociodemographic and Clinical Characteristics
After excluding 11 individuals’ files due to incompleteness, we reviewed a total of 421 charts registered from 1st January 2011 to December 31, 2020 as depicted (Figure 1). The mean (± SD) age of study participant was 10.6 ± 3.3 years. More than three-fifths of 261 (62%) participant children were aged ≥11 years, and the majority 219 (52%) of them were from the rural area. About 57.5% of participant had both parents (Table 1).
| Variables | Categories | Numbers (total = 421) | Percent |
|---|---|---|---|
| Sex | Male | 204 | 49 |
| Female | 217 | 51 | |
| Age | ≤5 years | 46 | 11 |
| Between 6 and 10 | 114 | 27 | |
| ≥11 years | 261 | 62 | |
| Residence | Urban | 205 | 48 |
| Rural | 216 | 51 | |
| Family size | ≤2 | 133 | 31 |
| 3-4 | 219 | 52 | |
| 5-6 | 50 | 11 | |
| ≥7 | 19 | 5 | |
| Hemoglobin | >10 mg/dl | 263 | 63 |
| ≤10 mg/dl | 158 | 38 | |
| WHO | Stage I | 128 | 30 |
| Stage II | 147 | 35 | |
| Stage III | 89 | 21 | |
| Stage IV | 57 | 13 | |
| CD4 count | <100 | 30 | 7 |
| 101-200 | 74 | 7 | |
| ≥201 | 317 | 75 | |
| Functional status | Working | 307 | 72 |
| Ambulatory | 72 | 17 | |
| Bedridden | 42 | 10 | |
| Adherence | Good | 224 | 59 |
| Fair | 121 | 29 | |
| Poor | 56 | 13 | |
| Isoniazid | Took | 258 | 61 |
| Missed | 163 | 38 | |
| Cocotrimoxazole | Took | 321 | 76 |
| Missed | 100 | 23 | |
| Opportunistic infections | Present | 126 | 29 |
| Absent | 295 | 70 | |
| Vaccination | Completed | 276 | 66 |
| Not updated | 76 | 18 | |
| Defaulted | 69 | 16 | |
| TB contact history | Yes | 135 | 32 |
| No | 286 | 68 | |
| Change of AR regiment | Present | 85 | 20 |
| Absent | 336 | 79 | |
| Parental status | Both parent alive | 242 | 57 |
| Paternal orphan | 115 | 27 | |
| Maternal orphan | 38 | 9 | |
| Both parent orphan | 26 | 6 | |
3.2. Baseline Clinical, Hematological, and Laboratory Characteristics
Of the total, 128 (30%) and 89 (21%) of participant children were found to be in clinical world health organization stages (WHO) I and III, respectively. Nearly two-thirds, 263 (62%), of the children had hemoglobin ≥ 10 mg/dl, whereas the majority, 317 (75%), of participants had CD4 cell count ≥ 201 cell/mm3. Moreover, 316 (75%) of children took cotrioxzol prophylaxis (CPT). Nearly two in five, 168 (39%), participant children missed isoniazid preventive therapy. Nearly one in five, 81 (19%), study participants shifted baseline ART regimens. The frequent reason was due to toxicity and TB incidence (24/81) and (22/81), respectively. Thirty nine percent of the children had an opportunistic infection. Bacterial pneumonia 53 (35.9%) and PCP 27 (21.6%) were the commonest. Of the total, 147 (35%) had hemoglobin ≤ 10 mg/dl, and more than two-thirds 276 (66%) children vaccinations. Majority, 338 (80%), of cases were in the appropriate developmental stage, while 56 (13.4%) children had poor ART adherence (Table 1).
3.3. Baseline Nutritional Status of HIV-Infected Children
Of the total, 33 (8%) and 72 (17%) cases had severe stunting and moderate wasting, respectively. However, 303 (72.3%) participants were on normal percentiles of weight for age > −2 Z score (Table 2).
| Nutritional parameter | Frequency | Percent | chi2(2) | P < 0.05 |
|---|---|---|---|---|
| Weight for height (WFH) normal (>-2 Z score) | 304 | 72 | 7.5724 | 0.023 |
| Moderate wasting ≤ −2 − 3 Z score | 71 | 17 | ||
| Severe wasting ≤ −3 Z score | 46 | 11 | ||
| Height for age (HFA) normal (>-2 Z score) | 284 | 68 | 5.9262 | 0.052 |
| Moderate stunting ≤ −2 − 3 Z score | 104 | 5 | ||
| Severe stunting ≤ −3 Z score | 33 | 8 | ||
| Weight for age(WFA) normal (>-2 Z score | 277 | 66 | 9.1780 | 0.010 |
| Moderate underweight ≤ −2 − 3 Z score | 115 | 27 | ||
| Severe underweight ≤ −3 Z score | 29 | 7 |
3.4. Time to Developed Tuberculosis
In this study, a total of 421 study participants were followed for a different period, contributing a cohort of 1043.1 PY of observation with minimum and maximum of observation 4 to 98 months.
During the follow-up period, 64 (15.2%) individuals were devloped new tuberculosis. this makes the overall incidence density rate(IDR) particicpant children was found 5.9 (95% CI: 4.7; 7.7) per 100 person-years of risk observation. Majority, 44 (70%), of new cases occurred after ART that started 3 years later. About 44 (69%) cases were EPTB, and the remaining 19 (30%) were PTB (Table 3).
| Years | No. of children at started | Withdrawn during year | At risk children | No. of TB case (63) | TB incidence | Cumulative probability | Survival rate | 95% CI |
|---|---|---|---|---|---|---|---|---|
| >1 year | 421 (100) | 13 (20.6) | 408(96.9%) | 13 (20.6) | 13 (3.1%) | 13 (20.6%) | 96.7% | 94.5-98.1 |
| 2 years | 408 (96.9%) | 18 (28.5%) | 390(92.6%) | 18 (28.5%) | 18 (4.3%) | 31 (49.2%) | 90.5% | 86.5-93.2 |
| 3 years | 390 (92.6%) | 8 (12.6%) | 382(90.7%) | 8 (12.6%) | 8 (1.99%) | 39 (61.8%) | 86.3% | 81.5-89.9 |
| 4 years | 382 (90.7%) | 5 (7.93%) | 377(89.5%) | 5 (7.93%) | 5 (1.15%) | 44 (69.7%) | 82.5% | 76.5-87.1 |
| 5 years | 377 (89.5%) | 12 (19.0%) | 365(86.6%) | 12 (19.0%) | 12 (2.8%) | 56 (88.7%) | 61.9% | 49.5-72.8 |
| 6 years | 365 (86.6%) | 4 (6.34%) | 361(85.7%) | 4 (6.34%) | 4 (0.98%) | 60 (95.1%) | 38.9% | 29.1-57.5 |
| 7 years | 361 (85.7%) | 2 (3.17%) | 359(85.2%) | 2 (3.17%) | 2 (0.047%) | 62 (98.2%) | 29.9% | 26.5-46.4 |
| End of follow | 358 (84.8%) | 1 (1.72%) | 358(84.8%) | 1 (1.72%) | 1 (0.023%) | 63 (100) | 28.5% | 22.3-36.9 |
| Total | 63 (100) | — | 63 (100) | — | 100 | — | — |
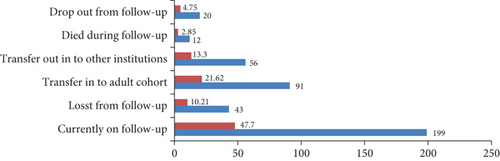
At the end of follow-up, 357 (85%) participant children were censored (excluded), among these 199 (47%) were on follow-up, 91 (21.6%) transferred into adult cohort, and 12 (4.7%) cases died (Figure 2).
3.5. Kaplan Meier TB Free Probability
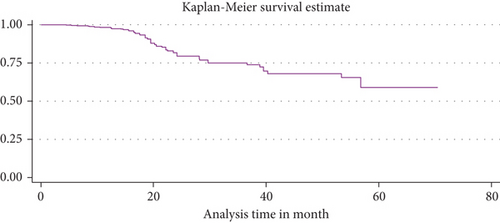
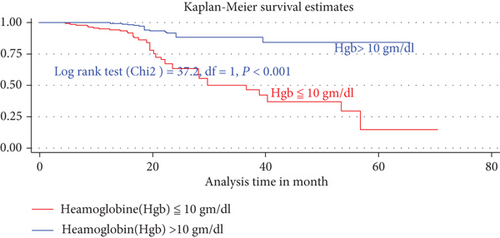
The median duration of TB-free probability time was found 35 (IQR = ±29) months. The mean TB-free survival time of the entire follow up was 74.5 months (95% CI: 73.8, 84.3 months). Tuberculosis-free survival probability by the end of the follow-up was determined as 28.5% (95% CI; 22.3-36.9%) (Figures 3–5).
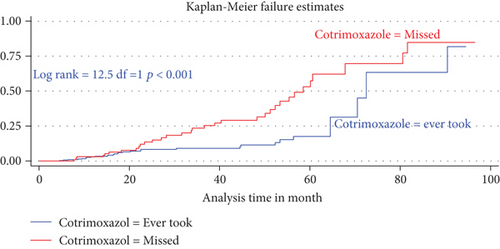
3.6. Predictors for Tuberculosis Incidence
In the final multiVariabel Cox regression model, only three variables found significantly associated with time to developed TB. Of this, the risks of developing TB for children who were not taking cotrimoxazole preventive therapy (CPT) were nearly three times higher as compared participant children who took CPT (AHR = 2.5: 95% CI,1.84-4.74, P < 0.021). Likewise, the risks of acquiring TB infection for seropositive children being nutritionally curve (height for age (HFA) ≤ −3 Z score) or being severely stunted were three times (AHR = 2.9: 95% CI,1.2-7.8, P < 0.03) higher than as compared with participant children having normal percentiles (HFA, >−2 Z score). Hemoglobin levels had a high predictive value for incident TB; indeed, baseline hemoglobin ≤ 10 mg/dl was four times increase the hazard of developing TB as compared to children having ≥10 mg/dl (AHR = 4.02: 95% CI, 2.1-8.1, P < 0.001) (Table 4).
| Variables | Categories | COR 95% CI | AOR=95% CI | P< 0.05 |
|---|---|---|---|---|
| Age of children | < =5 years | 1 | 1 | |
| 6-10 years | 3.4 ( 1.09 - 5.5) | 0.92 (0.28 ; 2.94) | 0.82 | |
| >=11 years | 3.6.(1.7 - 5.6) | 1.2 (0.32; 2.8) | 0.12 | |
| IPT | Given | 1 | 1 | |
| Missed | 2 .6(2.1 - 8.3) | 1.3(0.79 - 2.2) | 0.08 | |
| CPT | Given | 1 | 1 | |
| Missed | 3.7(2.8- 7.8) | 2.5(1.28; 5.3) | 0.021 | |
| TB history of contact | Yes | 1.2(1.08 - 3.4) | 1.09 (0.8 - 2.2) | 0.11 |
| No | 1 | 1 | ||
| Vaccination | Complete | 1 | 1 | |
| Not updated | 2.1 (0.9 - 2.7) | 1.2(0.72 ; 1.5) | 0.40 | |
| Defaulted | 1.7(1.2- 2.8) | 1.3(0.8; 1.9), | 0.12 | |
| Adherence | Good | 1 | ||
| Fair | 2.1(1.7 -- 4.1) | 1.8(0.87 ; 2.9), | 0.11 | |
| Poor | 3.1(2.05 -6.3) | 1.2 (0.93- 2.7) | 0.06 | |
| HFA>-2 Z score | No stunting | 1 | 1 | |
| Moderate stunting | 2.1(1.5 -- 2.8) | 1.2(0.8 ; 2.7) | 0.17 | |
| Sever stunting | 3.9(3.8 --5.1) | 2.9(1.2; 7.8) | 0.03 | |
| WHO clinical | stage I&II | 1 | 1 | |
| Stage III&IV | 9.1(4.9 16.4) | 1.3 (0.73-2.5) | 0.12 | |
| Hemoglobin | > 10 mg/dl | 1 | 1 | |
| ≤10 mg/dl | 9.62(5.13 18.0) | 4.02(2.1- 8.1) | 0.001 |
4. Discussion
This study is aimed at assessing TB incidence rate and its predictors in children on ART. Accordingly, at the end of the study period, 63 (15%) participants developed new TB incidence, making the overall incidence density rate 5.9 cases per 100 person-years (95% CI: 4.68; 7.68). This is higher than reported from Southern Ethiopia 2.6/100 PYs [18], Debre Markos 2.63/100 PYs [19], Northern Ethiopia 4.2/100 PYs [16], and Gonder 4.9/100 PYOs [20] but lower than findings in Adama hospitals 6.03/100 PYs [21] and South Africa 21.1/100 PYO [10]. This difference might be due to the higher burden of tuberculosis in resource-limited settings [1]. HIV was the immune system and acceleration viral replication responsible depletion of CD4 count [7] and associated with precipitation of new episode for opportunistic infections. This can be reduced early by addressing CPT and IPT, which are inexpensive and highly effective for reducing loads of endogenous reactivation of latent TB [22]. However, being seropositive children who missed CPT were significantly associated with risks of developed TB incidence. This finding is comparable with those reported in Gonder hospital [16] and Adam hospital [20]. The finding of this research also indicated that HIV-infected children having severe stunting were independently associated with the incidence of TB as compared with HIV-infected children who have no stunting. This is in line with the finding in Adama [21], Tanzania [23], Uganda, and Zimbabwe [9]. This might be due to HIV infection increasing nutrient malabsorption due to metabolic alterations that culminate in weight loss and stunting with time leading to early exposure for opportunistic infections [24]. Similarly, the existence of rapid viral replication consumed body energy and creates an arena for the incidence of TB [25, 26]. This finding also showed that children having hemoglobin ≤ 10 mg/dl were independently associated with TB incidence as compared with HIV-infected children having hemoglobin levels > 10 mg/dl. This is in line with the study finding in Adama hospital [21], Gonder hospital [20], Northern Ethiopia [16], Dar es Salaam, Tanzania [23], and England [27]. In fact, this is due to hemoglobin levels having a high predictive value for incident TB and death. TB incidence is directly associated with severe anemia [28]. Regardless of ART, moderate or severe anemia during ART follow-up can be an independent predictor for TB [28, 29].
4.1. Limitation of the Study
The retrospective nature of this study is one of the limitations of this study. Due to this, some of the clinically important predictor variables that have been independently associated with the incidence of TB occurrence in other studies, like the educational status of children and economic status of the family, were not included in this study.
5. Conclusion
Tuberculosis incidence was high among seropositive children attending HIV/AIDS care, especially in the first three years after ART initiated as compared with that of subsequent years, since more than half of 44 (69%) new cases of TB occurred. Levels of hemoglobin, missed cotrimoxazole preventive therapy, and nutritionallysevere stunting (HFA = ≤−3 Z score) were significantly associated with the incidence of tuberculosis. Besides, intensified screening for provisions of isoniazid preventive therapy to children living with HIV/AIDS is highly recommended.
Abbreviations
-
- HIV:
-
- Human immune deficiency syndrome
-
- TB:
-
- Tuberculosis
-
- AHR:
-
- Adjusted hazard ratio
-
- PLWHIV:
-
- People living with HIV
-
- WFH:
-
- Weight for height
-
- WFA:
-
- Weight for age
-
- HFA:
-
- Height for age
-
- PYOs:
-
- Person years of observations
-
- LLH:
-
- Log likely hood ratio.
Consent
There is no consent for this study.
Disclosure
In the role the funders took in the study, funders had no role in this study as I am a ph.D. candidate in the abovementioned university, and funders have no role except giving stay for their students on the time of research.
Conflicts of Interest
All authors declare that they have no competing interest in this research.
Authors’ Contributions
FK, TG, and TK were equally contributing to the work reported, conception, study design, execution, analysis, and interpretation and writing the manuscript, and finally, all authors approved for submission.
Acknowledgments
Our final heartfelt thanks goes to Tamirate Shewan (Assistance Professor of Epidemiology), for his unreserved cleaning, editing, and rewriting of this manuscript for final submission. This study material and financial support are from Debre Markose University’s Post-Graduate School as a Ph.D. student research support fund.
Open Research
Data Availability
The data of this original research are available from the corresponding author upon reasonable request.




