Evaluation of the Adsorption Efficiency of Glycine-, Iminodiacetic Acid -, and Amino Propyl-Functionalized Silica Nanoparticles for the Removal of Potentially Toxic Elements from Contaminated Water Solution
Abstract
In present work, mesoporous silica nanoparticles (MSNs) were prepared with a surface area of 1048 m2g-1 and a large pore size of ca. 6 nm, using Stöber process in the presence of expanding reagent (n-hexane). The surface of MSNs was modified with three different functional groups (amine, iminodiacetic acid, and glycine) and characterized by a variety of physicochemical techniques. The adsorption studies were carried out at different pH values in two extraction systems. In batch method, the maximum adsorption efficiency of heavy metals was measured to be 95% for all fabricated MSNs at pH 9. At pH 3, the adsorption efficiency of Pb and Cu was observed to be affected by the carboxylic moiety involved in the functional group. As the number of carboxylic moieties increase, the removal efficiency of Pb and Cu ions increased by two folds. The results demonstrated the selectivity of IDA-MSNs for the removal of Pb and Cu ions, even though the multielements are present in an aqueous solution. On the other hand, the incorporation of MSNs into the polymeric membrane showed high water permeability (9.96 ± 3 L/m2.h.bar), and 98% rejection was achieved at pH 7 for Cu+2 and Pb+2 ions.
1. Introduction
The 2018 edition of the UN World Water Development Report stated that more than 5 billion people could experience severe water scarcity by 2050. This is due to the increased demand for water, limited water resources, and increasing pollution of water, which is caused by dramatic population and economic growth [1]. Emerging contaminants such as heavy metals (e.g., Hg, As, Pb, and Cd) in the treated wastewater is of concern for the environment and human health. Even the presence of trace levels of heavy metals in the treated wastewater may have long-term health impacts. As a consequence, considerable attention has been paid on the development of new nanosystems for the fast, selective, and efficient removal of hazardous heavy metal ions from water [2–4].
Mesoporous silica nanoparticles (MSNs) have emerged as one of the most promising technologies for water remediation which can be used in the form of adsorbents [5, 6], hosts [7, 8], and sensors [9]. Indeed, MSNs become apparent as a promising water treatment technique due to their favourable chemical properties, thermal stability, and biocompatibility [10–12]. The surface modification of silica nanoparticle with a suitable functional group could enhance the efficiency, sensitivity, and selectivity of the material towards hazardous heavy metal ions [13–15]. For example, thiol terminated silica surfaces showed an improvement in adsorption of Ag, Hg, Cu, Zn, and Ni ions from aqueous solution [16], whereas amine-terminated surfaces improved sorption properties for Cr, Pb, and Cd ions [17, 18]. Theoretical modelling of binding heavy metals (i.e., Cd, Pb, Cu, and Zn) on surface modified with thiol, amino, or carboxyl functionalities demonstrated that the key features which determine the improved performance of the functionalized materials were as follows: (a) high metal loading capacities due to the ligands and (b) strong binding affinities for the selected metal ions due to the nature of the functional groups [19]. Gibson and coworkers reported the synthesis of silica nanoparticles with a broad pore-size distribution [20]. The researchers studied the effect of adsorbent pore size distribution on the rate of Cr (VI) ions uptake. The results showed that the adsorption behaviour of Cr (VI) ions was affected by the size of the particles [21]. Gupta et al. reported the synthesis of guanine functionalized MSNs for removal of toxic metal ions from aqueous medium [22]. The fabricated MSNs demonstrated proficient removal capacities of toxic metal ions (Hg2+, Cd2+, and Pb2+) from aqueous solution. Carbon quantum dots (CQDs) embedded in MSNs were prepared via hydrothermal approach [23]. It was found that MSNs/CQDs was high selectivity of Hg2+. Li et al. reported the synthesis of amino-functional mesoporous silica spheres by a one-step method, with large particle size (>1 mm) [24]. The material exhibited an excellent Pb2+ adsorption in both dynamic and static experiments. Sodium dodecyl sulfate- (SDS-) functionalized MSNs were prepared as adsorbent for removing toxic metal from aqueous solution [25]. The adsorption capacity was observed to be dependent on the metal ion and the pH of solution. Mesoporous silica was functionalized with triethylenetetramine (TETA) to be used for removal Cu2+ and Zn2+ metal ions from aqueous solutions [26]. Magnetic mesoporous nanoparticles were modified with EDTA and used to remove Cr(III) from water with high salinity [27]. The nanomaterials illustrate high adsorption capacity for Cr(III), with maximum adsorption amount of 30.59 mg.L−1 in acidic media.
The incorporation of nanoparticles into the polymeric thin-film composite membranes could enhance the physicochemical properties of the membranes: including mechanical stability, thermal resistance, and hydrophilicity as well as their permselectivity [28–31]. Importantly, the modification of the MSNs with hydrophilic groups could be beneficial for improving fouling resistance and permeability of the membranes [28, 32]. Shah et al. prepared amino-functionalized-multiwalled carbon nanotube/polysulfone composite membranes, such membranes were evaluated for the removal of heavy metals, showing maximum adsorption of 78.2% and 94.2% for cadmium and chromium, respectively [33]. Zhu et al. synthesised hollow fiber membranes by grafting polyamidoamine (PAMAM) on the interfacially polymerized layer of polyethersulfone (PES) membranes for removal of Pb(II), Cd(II), and Cu(II) [34]. The membrane showed an ion rejection of more than 95% and a water permeability flux of 3.6 L m-2 h-1 bar-1. Zhang et al. reported the removal of Pb(II), Zn(II), and Ni(II) using graphene oxide (GO) framework layer deposited on a modified Torlon hollow fibre [35]. The GO/Torlons composite membrane has rejections higher than 95% towards the target heavy metals with water permeability flux of 4.7 L m-2 h-1 bar-1. However, the high rejection percentage of ions comes with a cost of having low permeability flux. High throughput and significant rejection could be achieved by the incorporation of functionalized mesoporous silica materials into polymeric membranes.
The implementation of these objectives has largely been attempted in this study through the use of nanocomposite functionalized membranes utilizing surface-modified MSNs for the removal of heavy metal ions from aqueous solutions. As far as we know, very little works have been reported on the surface modification of silica nanoparticle with two functional groups (amine and carboxylic acid groups). The aim of this work is the fabrication of high surface area silica nanoparticles functionalized with amine and carboxylic acid groups and the use of such materials for hazardous heavy metal ions adsorptions. Herein, MSNs functionalized with amine, iminodiacetic acid, and glycine were prepared and characterized by a variety of physicochemical techniques, including FT-IR, elemental analysis, TGA, SEM, TEM, and BET analysis. Furthermore, the adsorption studies were carried out in batch mode at different pH values with nanoparticles being in the colloidal form, as well as the nanoparticles incorporated into the polymeric membrane. The membrane was then characterized in terms of permeability and the effect of solution pH on the removal of heavy metals.
2. Materials and Methods
2.1. Materials
Deionized water was obtained using an Elga Pure Nanopore 18.2 MΩ system. 3-Aminopropyltriethoxysilane (APTES, >98%), ethanol (99.8%, HPLC grade), N-cetyltrimethylammonium bromide (CTAB, 98%), toluene (analytical grade) tetraethylorthosilicate (TEOS, 98%), iminodiacetic acid (IDA, 95%), glycine (98.5%), n-hexane (HPLC grade), methanol (99.8% HPLC grade), and ammonium hydroxide (28 wt %) were purchased from Sigma-Aldrich. Hydrochloric acid (HCl) and dimethyl sulfoxide (DMSO) were obtained from Fisher Scientific. All the chemicals were used as received.
2.2. Mesoporous Silica Preparations and Functionalization
2.2.1. Synthesis of Mesoporous Silica Nanoparticles (MSNs)
Typically, 1 g of CTAB was dissolved in a solution of 160 mL of deionized water and 1 mL of concentrated ammonia water (28 wt %) under stirring. A mixture solution of n-hexane (20 mL) and TEOS (5 mL) was added into the solution within 30 min under continuous stirring at 35°C. After stirring for 12 h, the product was collected by centrifugation and washed with deionized water and ethanol. The collected solid sample was dried in an oven at 100°C for 2 h [24].
Then, the sample was submitted to solvent extraction treatment to remove CTAB templates by redispersing 1.5 g of the sample in methanol (160 mL), to which a concentrated aqueous solution of HCl (12 M, 9 mL) was added, and the mixture was heated under reflux for 24 h. The solvent extraction was repeated 2 times, and the sample was collected by centrifugation, followed with washing with ethanol 6 times, and finally vacuum dried overnight.
2.2.2. Synthesis of 3-Aminopropyl-Functionalized MSNs (AP-MSNs)
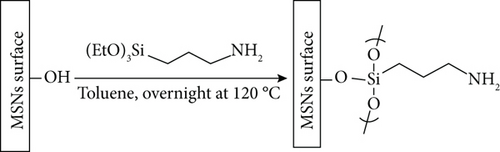
Amino functionalized silica surface was prepared by suspending the obtained nanoparticles (1.5 g) in a solution of APTES (2.5 mmol) in dry toluene (50.0 mL), and the resulting mixture was heated under reflux (130°C) for 24 h (Scheme 1(a)). The nanoparticles were collected by centrifugation, washed twice with toluene and five times with ethanol, and dried under vacuum.

2.2.3. Synthesis of (3-Glycidyloxypropyl)-Functionalized MSNs (Epo-MSNs)
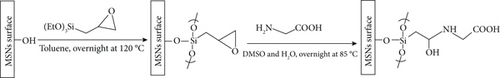
(3-Glycidyloxypropyl)-coated mesoporous silica surface was performed by suspending the obtained nanoparticles (1.5 g) in a solution of (3-glycidyloxypropyl) trimethoxysilane (2.5 mmol) in dry toluene (50.0 mL), and the resulting mixture was heated under reflux for 24 h. The nanoparticles were collected by centrifugation, washed twice with toluene and five times with ethanol, and dried under vacuum.
2.2.4. Preparation of Glycine-Modified MSNs (Gly-MSNs)
(3-Glycidyloxypropyl)-coated MSNs (1 g) were added to a flask containing 20 mL DMSO and kept at 60°C for 1 h with stirring. An aqueous solution (10 mL) containing glycine (3 g) was added to the mixture stirred for 48 h at 85°C (Scheme 1(b)). Gly-MSNs were collected by centrifugation and washed thoroughly with deionized water and ethanol, then dried in a vacuum.
2.2.5. Preparation of IDA-Modified MSNs (IDA-MSNs)
Before the reaction, iminodiacetic acid (IDA) is neutralized with KOH solution to keep carboxylic acid from reacting with epoxy ring of MSNs surface. Dipotassium salt of IDA solution (1 M, 100 mL) was added slowly to a water suspension (10.0 mL) of (3-glycidyloxypropyl)-coated MSNs (2.0 g). The mixture was kept at 65-70°C overnight under powerful stirring (Scheme 1(c)). The resulting material was centrifuged, washed extensively with water and ethanol, and dried under vacuum.
2.2.6. Preparation of Membranes
Three dispersions in this study were prepared using chitosan solution (0.5% w/v), which contained nanopartials (amine, glycine, and IDA) with a concentration of 0.1% (w/v). In a typical experiment, 15 mg of these nanopartials were dispersed in 15 mL of surfactant (Milli-Q® water and chitosan) using ultrasonics with a probe diameter of 10 mm for 10 min, which required time for complete dispersion of each nanopartials (amine, glycine, and IDA) through solutions. The vial was located inside an ice/water bath to keep a constant temperature during sonication process. The resulting solutions were then applied to a flat membrane (12 cm X 12 cm) of polysulfone (support layer) and dried at 21°C for 24 h.
2.3. Adsorption Experiments
The general procedure for the extraction of the selected elements from solution can be summarised as follows. Approximately 25 mg samples of AP-MSNs, IDA-MSNs, or Gly-MSNs were suspended in 25 mL solutions containing 10 ppm of the selected elements (i.e., Cu, Cd, Co, Cr, Pb, and Zn) at pH between 3 and 9. Solutions were stirred for approximately 2 h at room temperature and then filtered. The supernatants were analyzed using ICP/OES.
2.3.1. Permeability Studies
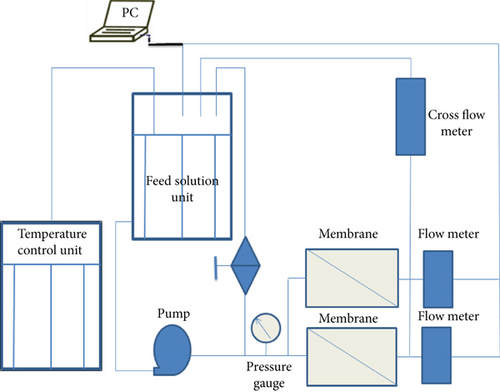
The permeability of three coating membranes (Gly-MSN, IDA-MSN, and AP-MSN) towards water was investigated using the cross-flow filtration system outlined in Scheme 2. The coating membrane was first located on a porous stainless steel that was used as support to the membrane and the active filtration area of the filtration cell was 40 cm2. Different pressures were applied to force water through the coating membrane in order to obtain a flux across the coating membrane. For the flux of water, the volume (mL) of water passing the coating membrane at each applied pressure was measured for 6 min by the cross-flow meter linked to a computer.
2.3.2. Heavy Metals Rejection Study
where Cp and Cf are the concentration of metal ions for the permeate and feed solution, respectively.
2.4. Measurement and Characterization
Surface area analysis: the surface area of silica nanoparticulate materials used in this study was measured using nitrogen physisorption isotherms on a Micromeritics Gemini 2375 volumetric analyzer. Each sample was degassed prior to analysis for 6 h at 150°C. The Brunauer–Emmett–Teller (BET) surface areas were calculated using experimental points at a relative pressure (P/P°) of 0.05–0.25. The total pore volume was calculated from the N2 amount adsorbed at the P/P° of 0.99 for each sample, and the average pore size distribution of the materials was calculated using the Barrett–Joyner–Halenda (BJH) model. FTIR spectroscopy: infrared spectra of all samples were obtained in KBr pellets in the 4000–400 cm-1 region with a resolution of 4 cm-1, using a Thermo Scientific Nicolet iS10. Elemental analysis (EA): elemental analysis was carried out using a Perkin Elmer Series II-2400 analyzer. Scanning electron microscopy (SEM): SEM images were collected using JEOL JSM-6380 LA scanning electron microscope. The dried samples were directly used for the observation without any treatment. Transmission electron microscopy (TEM): a drop of dilute sample suspension in ethanol was placed on a copper grid with a thin polymer coating and dried at room temperature prior to the measurement. A JEOL JEM-1230 transmission electron microscope was used for TEM imaging. Thermogravimetric analysis (TGA): TGA analyses were carried out on a SII TGA 6300 instrument with a heating rate of 10°C/min under N2. ICP analysis: the elemental compositions of the supernatants were determined by inductively coupled plasma/optical emission spectroscopy, ICP/OES (Varian 720-ES). For the supernatant samples, 0.4 mL of highly concentrated nitric acid was added, and the total volume was adjusted to 10 mL with DI water prior to ICP/OES analysis.
3. Results and Discussion
3.1. Characterization of Materials
The morphology, means diameter, and size distribution of the silica nanoparticles were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM characterization indicates that the obtained MSNs sample consists of nanospheres. The particle size distribution was estimated using the Image J software by analysing the SEM image. The majority of MSNs ranged between 150 and 280 nm. The average particle size was calculated to be 200 nm for all prepared nanoparticles, as shown in Figure 1(a). TEM observation (Figure 1(b)) reveals that the obtained mesoporous silica nanospheres are highly dispersed without aggregation and have regular morphology with a diameter ranging from 150 to 300 nm, agreeing well with the SEM results. The highly ordered arrays of uniform spherical mesopores can be clearly seen in the TEM images. The mesopores size was estimated from the TEM image to be ca. 6 nm, which is larger than those of typical mesoporous silica nanoparticles, due to the pore expanding effect of nonpolar n-hexane [15, 36].


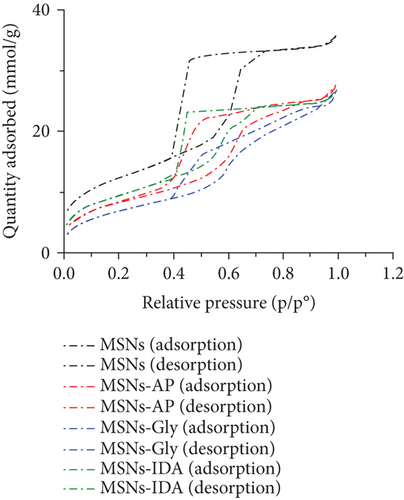
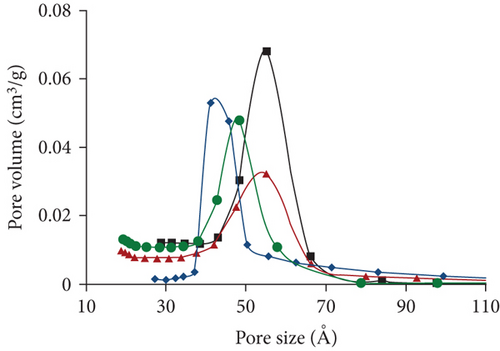
All samples of bare MSNs, AP-MSNs, Gly-MSNs, and IDA-MSNs were characterized using BET. The physicochemical properties of the samples were summarized in Table 1. As expected, reductions in surface area and pore volume were observed after the modification of the surfaces due to introducing the functional groups into the surface and the pores of MSNs. In Figure 2, the N2 sorption isotherms for all samples were found to be Type IV, confirming their mesoporous nature. A slightly different capillary condensation steps were noted at higher relative pressures for AP-MSNs, Gly-MSNs, and IDA-MSNs compared with nonmodified surfaces. The hysteresis loop was broader for MSNs, compared to the modified MSNs suggesting that the pore shapes and sizes of modified materials had been changed. All samples have a pore size of about 4-6 nm with a relatively narrow pore size distribution (Figure 3). The pore size of MSNs was found ca. 6 nm, as estimated from the TEM image. AP-MSNs sample had well-defined narrow pore size ranges and similar average pore size to MSNs, whereas a reduction in the average pore size of Gly-MSNs and IDA-MSNs.
| Material | BET surface area (m2.g-1) | Pore volume (cm3.g-1) |
|---|---|---|
| Bare MSNs | 1048 | 1.51 |
| AP-MSNs | 681 | 1.00 |
| Gly-MSNs | 570 | 0.95 |
| IDA-MSNs | 783 | 0.98 |
FTIR characterization shows that wide bands at 1240–1030 cm-1 were attributed to the asymmetric stretching of siloxane groups (Si–O–Si) bands of the condensed silica network. Peak at ~1630 cm-1 was ascribed to the bending vibration of water. Peak at ~806 cm-1 was assigned to stretching vibration of Si–O. These features were found in all samples. Peaks at ~1489 cm-1 and ~2928 cm-1 were attributed to C-H as -CH2- in template (CTAB). After extraction treatment, the peaks at ~1489 cm-1 and~2928 cm-1 were disappeared, indicating that the CTAB template was completely removed. When comparing MSNs spectrum with AP-MSNs spectrum, new peaks at ca. 694 cm-1, 1489 cm-1, 1630 cm-1, and 1565 cm-1 appeared after APTES modification. The absorption peaks for AP-MSNs at ~694 cm−1 were ascribed to N-H as bending vibration in –NH2. Peaks at ~1630 cm-1 and ~1565 cm-1 were ascribed to N-H as stretch vibration in -NH2. Peaks at ~1489 cm-1 were ascribed to C-H as -CH2- in APTES. Peaks around 2928 cm-1 ascribed to C-H as stretch vibration in -CH2- appeared after modification. Peak appeared at ~1489 cm-1, corresponding to C-H as -CH2- in the epoxy-functionalized MSNs. There is also a peak at ~2928 cm-1, as a C-H as stretch vibration in -CH2- was appeared after modification. After the reaction with glycine, and iminodiacetic acid (IDA), peaks appeared at ~1470 cm-1 assigned to N–H stretch and a weak band at 693 cm-1. Peaks at ~1720 cm-1 appeared, assigned to carbonyl groups, providing an evidence of successful reaction between the epoxy group and amino groups in glycine and IDA, as these peaks were absent in the epoxy-MSNs spectrum. Peaks around 1489 cm-1 and 2928 cm-1 ascribed to C-H was enhanced after modification, further indicating the successful reactions with epoxy surface.
The Lo values were calculated and demonstrated in Table 2.
| Material | %C | %H | %N | Lo (mmol/g)a |
|---|---|---|---|---|
| AP-MSNs | 6.38 | 1.87 | 1.62 | 1.16 |
| Epo-MSNs | 8.41 | 2.14 | — | — |
| Gly-MSNs | 8.29 | 2.33 | 1.18 | 0.84 |
| IDA-MSNs | 9.46 | 2.51 | 1.37 | 0.98 |
- aFunctionalization degree (in millimoles of ligand per gram of functionalized silica).
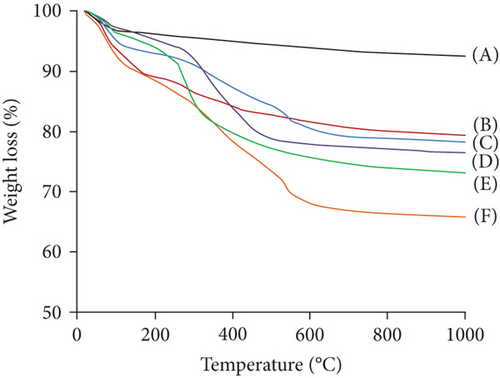
The successful surface modifications of the materials were evaluated by thermogravimetric analysis (TGA), when heating in a N2 atmosphere to 1000°C (Figure 4). After surface modification with APTES and (3-glycidyloxypropyl) trimethoxysilane, ca. 15 wt% of weight loss was observed. The TGA result revealed that the content of –NH2 and epoxy groups in the surface of MSNs were ca. 0.68 mmol/g. Also, the TGA result showed that the content of glycine in the Gly-MSNs was 0.53 mmol/g, whereas the content of IDA in the IDA-MSNs was 0.046 mmol/g. These results suggest that ca. 80% of epoxy groups on the surface was reacted glycine, and ca. 70% of epoxy groups on the surface was reacted IDA.
3.2. Adsorption Studies
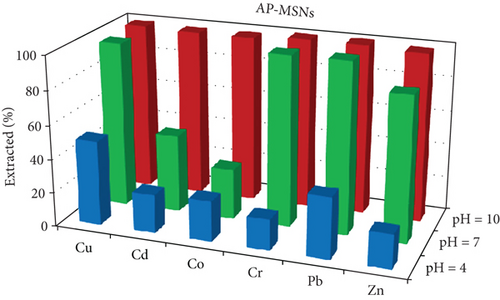
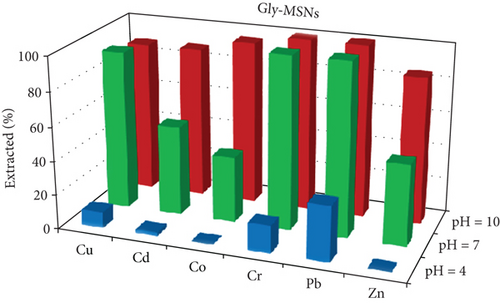
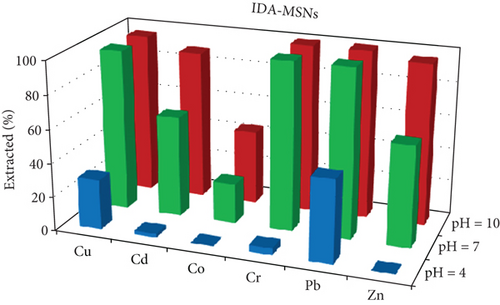
One of the most important factors in the adsorption process that influence the silica adsorbent-adsorbate interactions is the pH of the solution, due to its ability to change the ionic state of the analytes as well as the surface charge of adsorbent [37]. The effects of the pH on the extraction of Cu, Cd, Co, Cr, Pb, and Zn ions as an adsorbate on Gly-, IDA-, and AP-functionalized MSNs were investigated in the pH range of 3–9. The results are given in Figure 5. The results illustrate that at pH 9, most of the studied metal ions were removed with removal efficiency of more than 85% and 95% when Gly-MSN and AP-MSN were used, respectively. On the other hand, the behaviour of IDA-MSN was completely different in terms of the removal of cobalt ion as there was a decrease in the extraction efficiency to 40%, and that might be attributed to the formation of the hydroxyl complexes of cobalt Co(OH)2 at higher pH medium which can be hindered by the presence of dicarboxylic acid of IDA-MSN [24, 25]. In general, the removal efficiency was low at pH 3 across all samples towards the studied ions. However, there was a slight variation in the adsorption over the studied elements when AP-MSN is used. Interestingly, a completely different behaviour was observed when the surfaces were modified with Glycine and IDA; as some of the elements (Cd, Co, and Zn) were meanly hindered to interact with the surface of the materials. The removal efficiency of Pb and Cu ions increased from 20 to 45% and from 10 to 30%, respectively, when the number of carboxylic acid moieties increased in the functional group. This result was significant as it demonstrated the selectivity of IDA-MSN for the removal of Pb and Cu even when it was present in aqueous solution containing multielements.
3.2.1. Polymeric Membranes
| Material | Water permeability (L/m2.h.bar) |
|---|---|
| AP-MSNs | 9.96 ± 3 |
| Gly-MSNs | 9.15 ± 4 |
| IDA-MSNs | 4.24 ± 3 |
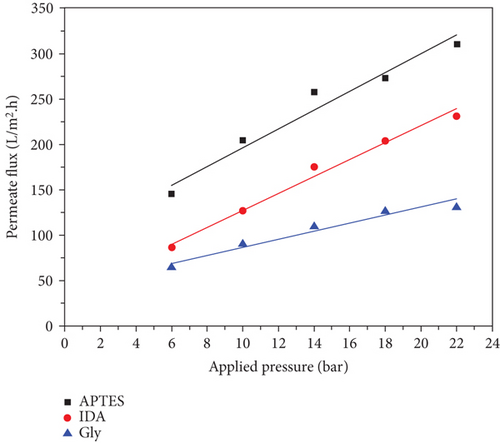
where J is the permeability of water, while Vp is the permeate volume, A is the efficiency membrane area, and t is the time (per hour). Figure 6 shows a comparison of the water permeability of the three types of membranes and is summarized in Table 3. Figure 6 shows that the permeate flux of prepared membranes was increased linearly with increasing the applied pressure as expected. It is also demonstrated that the prepared membranes have high water permeability and appropriate mechanical properties to withstand the high pressure applied to them. Table 3 shows that the AP-MSN/chitosan coating membrane has a significantly higher water permeability (9.96 ± 3 L/m2.h.bar) than other membranes, which are Gly-MSNs/chitosan and IDA-MSNs/chitosan (9.15 ± 4 and 4.24 ± 3 L/m2.h.bar, respectably). Additionally, it is noted that the water permeability values of coating membranes (AP-MSNs/chitosan, Gly-MSNs/chitosan, and IDA-MSNs/chitosan) investigated here were higher than those seen previously with other nanoparticles (CNTs) [40, 41]. Consequently, results suggest that the high water permeability of prepared membranes is a result from functionalized silica nanoparticles (Gly-MSN, IDA-MSN, and AP-MSN).
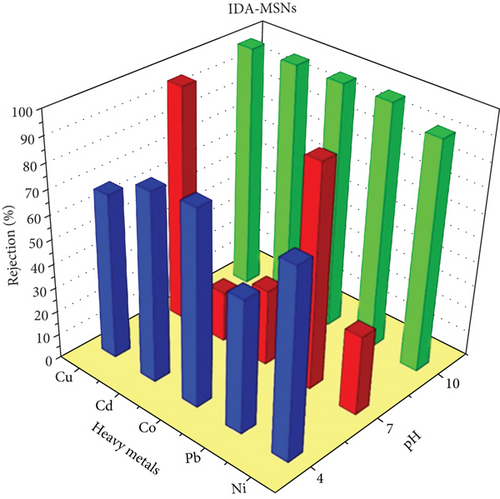
3.2.2. Heavy Metals-Rejection Capability
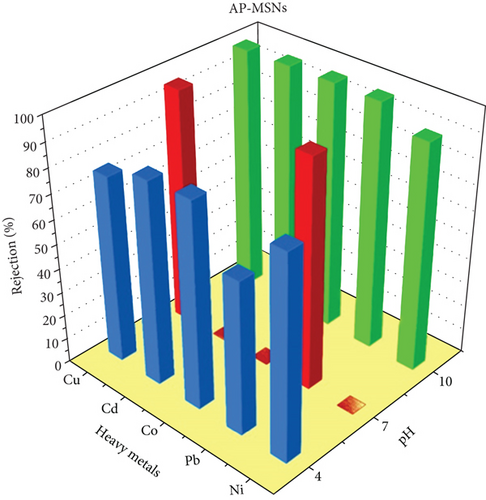
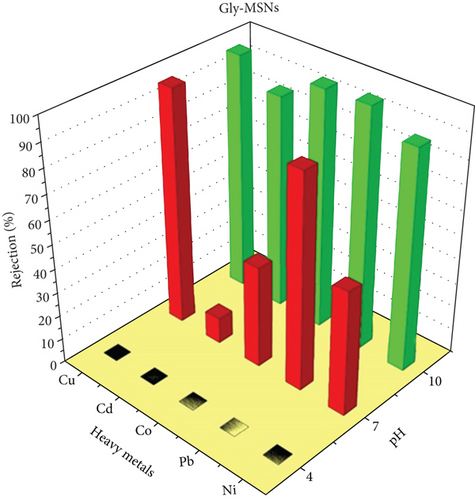
Three coating membranes AP-MSNs/chitosan, Gly-MSNs/chitosan, and IDA-MSNs/chitosan were evaluated for the rejection of heavy metals such as Cu+2, Pb+2, Co+2, Cd+2, and Ni+2 using a cross-flow system. The prepared membrane performances are shown in Figure 7. The pH of the solution affects the surface charge of the coating membranes and the ionization degree of heavy metals in the aqueous solution [42]. Consequently, the membrane ability to remove heavy metals from an aqueous solution can be affected by the pH of the solution as reported by Huang et al. [43]. The coating membranes AP-MSNs/chitosan, Gly-MSNs/chitosan, and IDA-MSNs/chitosan (Figure 7) show approximately similar pattern in heavy metals rejection at pH 10 and pressure at 6 bar. The prepared membranes show high rejection range between 80% and 98% for all heavy metals. While Cu+2 and Pb+2 were great rejected by membranes with 80-95% rejection at pH 7 with pressure at 6 bar, and Cd+2, Co+2, and Ni+2 were rejected with 10-40% range at the same pH and pressure. Both the AP-MSN/chitosan and the IDA-MSNs/chitosan coating membranes showed slight variation in the heavy metal rejection, ranging between 60% and 80% at pH 4 and 6 bar. While the Gly-MSNs/chitosan coating membrane was totally different in terms of the rejection of heavy metals as there was a decrease in the removal efficiency due to the creation of the hydroxyl complexes with the metals at lower pH medium.
4. Conclusions
We have studied a method for synthesizing mesoporous silica materials which were subjected to surface modification with three different functional groups (i.e., amine, iminodiacetic acid, and glycine) to be used as an adsorbent for heavy metals. The physiochemical characterization of the materials shows a mesoporous structure as confirmed by the BET isotherms with a surface area of 1048 m2g-1 and a large pore size of ca. 6 nm. Batch experiments were performed to study the effect of the pH on the presence of various metal ions (e.g., Cu, Cd, Co, Cr, Pb, and Zn ions). The obtained results showed a maximum adsorption efficiency of 95% could be achieved using the three functional groups at pH 9 for the studied heavy metals. Whereas, at pH 3, the adsorption efficiency of Pb and Cu was affected by the number of carboxylic molecules present in the attached functional groups, as increasing the number of carboxylic groups had led to increasing the removal efficiency of Pb and Cu ions by two folds. Further modifications of the MSNs by using it as a support for polymeric membrane have resulted in a significantly high water permeability (9.96 ± 3 L/m2.h.bar) compare to other coating membranes with a maximum ion rejection of 98% at pH 7 for Cu+2 and Pb+2 ions. Finally, the developed polymeric membrane modified with silica nanoparticles has the potential to become a promising polymeric nanocomposite membrane; it could be used to produce freshwater from a contaminated water sample.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Abdullah M Alswieleh and Khalid M Alotaibi contributed equally to this work.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no.RG-1441-304.
Open Research
Data Availability
(1) SEM and TEM images of materials are used to support the findings that the prepared are mesoporous silica nanoparticles. (2) Table is used to support the findings that the surface area of the materials reduced die to the functionalization. (3) Nitrogen adsorption isotherms are Type 4, which are used to support the findings that the materials are mesoporous. (4) FT-IR and TGA are used to support the findings that the functional groups are successfully functionalized on the surface of the materials. (5) Demonstration of the amount of toxic metal adsorbed on the nanomaterials at different pH to support the findings that the adsorption capacity is affected by different functional groups.




