Study of the Antimalarial Activity of the Leaf Extracts and Fractions of Persea americana and Dacryodes edulis and Their HPLC Analysis
Abstract
In the present study, the antimalarial activity of the extracts and fractions of the leaves of Persea americana and Dacryodes edulis as well as their phytochemical compositions were examined. Each of the extracts of the plants was successively fractionated to obtain hexane, ethyl acetate, methanol, and water fractions. The extracts and fractions were tested against Plasmodium berghei in both curative and suppressive antimalarial mouse models. Their major phytochemical composition was studied by the standard chemical tests and HPLC analysis. The extracts and fractions of P. americana and D. edulis demonstrated significant (p < 0.05) maximal plasmodial inhibition as 52.16 ± 2.77% and 57.10 ± 1.98%, respectively, and chemosuppression of parasitemia as 64.01 ± 0.08% and 71.99 ± 0.06%, respectively. The major secondary metabolites identified in the plants include alkaloids, flavonoids, and saponins. It was concluded that P. americana and D. edulis possess promising antimalarial activity and they are potential sources of new lead compounds against malaria.
1. Introduction
Malaria is a major parasitic disease that is endemic in the tropics particularly in Africa [1, 2]. The disease has a great impact on the economy of the affected communities as there is associated loss of man-hours and consequent decline in the agricultural productivity of the people [3, 4]. In the year 2020, an estimated number of 229 million new cases of malaria and 409,000 deaths were reported by the WHO globally [5]. Nigeria is one of the countries in Africa where the disease causes a major healthcare problem [6, 7].
Not only are the conventional drugs often unaffordable or inaccessible but also the rate at which Plasmodium falciparum, the causative organism, has developed resistance to the conventional drugs warrants the quest for novel and more effective agents [8] particularly from natural resources. Medicinal plants have long been used for malaria treatment in many traditional settings [9, 10]. Two common ingredients in Nigeria ethnomedicine used for malaria treatment are the leaves of Persea americana and Dacryodes edulis which are used either singly or in combination.
Persea americana Mill. (Family: Lauraceae) is a plant that grows throughout the tropics and bears an edible fruit known as avocado. The seeds are used traditionally for the treatment of skin rashes, diarrhea, high blood pressure, dysentery, asthma, and rheumatism [11]. The plant is used in Nigerian [12, 13] and Guinean [14] ethnomedicine for the treatment of malaria. The antiprotozoal, antidiabetic, and anticonvulsant activities of the seed have been demonstrated [15, 16]. However, despite the reported use of the plant in ethnomedicines for malaria treatment, detailed investigations into the antimalarial potential of the plant have not been done [13].
Dacryodes edulis (G. Don) Lam (Burseraceae) is an evergreen tree that is traditionally used in several parts of Africa to treat various diseases including tonsillitis, sickle cell, skin diseases, and malaria [17, 18]. The analgesic, antiallergic, and antimicrobial activities of D. edulis have been investigated [17]. Moreover, the leaves of the plant have shown a significant in vitro antiplasmodial activity [19] while the stem bark extract and five isolated compounds were shown to have strong antiplasmodial activity in vitro [17].
Given the above, this study therefore aimed at assessing the antimalarial activity of P. americana and D. edulis extracts and fractions in mice as well as examining the major class of phytoconstituents of the plants.
2. Materials and Methods
2.1. Plant Materials
P. americana and D. edulis fresh leaves were collected within the University of Nigeria, Nsukka, and authenticated by Mr. Felix Nwafor of the Department of Pharmacognosy and Environmental Medicines, University of Nigeria, Nsukka, with the identification vouchers of PCG/UNN/0079 and PCG/UNN/0078, respectively. The plant materials were washed with distilled water, air-dried, powdered, and stored in the refrigerator prior to use.
2.2. Plant Extraction and Fractionation
Plant extraction and fractionation were done as previously reported with some modifications [20]. A certain quantity (500 g) of each of the powdered plants was weighed and macerated in 1.5 L of 95% methanol in water (BDH, England) at room temperature (28 ± 2°C) for 72 h with occasional agitation and daily washing. Each suspension was filtered and the filtrate concentrated using a rotary evaporator at 40°C to afford the crude extracts coded as PA (for P. americana) and DE (for D. edulis). Each of the crude extracts (DE and PA) was further subjected to fractionation by dissolving it successively with n-hexane, ethyl acetate, methanol, and water. This procedure afforded the respective fractions coded for P. americana and D. edulis respectively, as follows: PAH and DEH (for n-hexane), PAE and DEE (for ethyl acetate), PAM and DEM (for methanol), and PAW and DEW (for water) fractions.
2.3. Animals
Animals used for the study were albino mice of either sex and weight of 11–18 g. A total number of 126 mice were used for the experiments. The animals were obtained from the Faculty of Veterinary Medicine, University of Nigeria, Nsukka. The animals were acclimatized for 7 days before experimentation. They were given access to water and feed ad libitum. Animal handling was in accordance with the internationally accepted guidelines (EEC Directive of 1986; 86/609/EEC).
2.4. Acute Toxicity Studies
If no death was recorded in the second stage, the LD50 was taken as greater than the maximum dose administered.
2.5. Inoculation of Parasites
Blood was drawn from a donor mouse which has been infected with P. berghei. Blood was drawn by heart puncture and diluted to a final concentration of about 1 × 107 infected red blood cells (RBC) per 0.2 ml suspension, following the procedure earlier reported [22].
2.6. Curative Antimalarial Test (Rane’s Test)
On day 8, mice were sacrificed and blood samples were collected for hematological (Hb and PCV) analysis.
2.7. The Four-Day Suppressive Test
Each mouse was also observed daily for the determination of the survival time and the mean survival time (MST) calculated arithmetically from the date of infection over a period of 30 days (day 0–day 29).
2.8. Determination of the Hematological Parameters
The PCV was determined using standard Micro-Hematocrit Reader as previously described [22]. Hb was measured using a cyanmethemoglobin technique [25]. Determination of the hematological parameters was done using the extracts (PA and DE) only.
2.9. Preliminary Phytochemical Analysis
Preliminary phytochemical tests for the presence of the major class of phytoconstituents were conducted following standard procedures [20].
2.10. HPLC Analysis
HPLC (Dionex P580®) framework with a photodiode detector (UVD340S) was employed for the HPLC investigation. MeOH and 0.02% H3PO4 in H2O were utilized as mobile phase. Peak identification was ascertained by comparing their ultra-violet (UV) spectra with those on the local database of the Institute for Pharmaceutical Biology and Biotechnology, Heinrich Heine University, Düsseldorf, Germany. The analyzed samples were the crude extract of P. americana (PA) and its fractions (PAH, PAE, PAM, and PAW).
2.11. Statistical Analysis
Data analysis was done using IBM SPSS, version 21.0 package. The results were expressed as mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA) with Dunnett’s test for multiple comparisons was used to compare means across the groups. Mean values with p < 0.05 were considered statistically significant compared to the control group.
3. Results
3.1. Results of Acute Toxicity Test
The results of the acute toxicity study (Table 1) show that there was no mortality at the various doses tested for P. americana. This indicates that the LD50 is greater than 5000 mg/kg. There were no visible signs of overt toxicity such as tremors, loss of appetite, lacrimation, diarrhea, or salivation within 14 days of physical examination. However, mortality occurred at 5000 mg/kg of D. edulis extract. Hence, the LD50 for D. edulis was calculated to be 4243 mg/kg. These results formed the choice of the doses used for the extracts and fractions.
| Experiment | Number of animals | Extract | ||
|---|---|---|---|---|
| Dose (mg/kg) | PA | DE | ||
| Mortality rate | ||||
| Phase I | 3 | 10 | 0/3 | 0/3 |
| 3 | 100 | 0/3 | 0/3 | |
| 3 | 1000 | 0/3 | 0/3 | |
| Phase II | 1 | 1600 | 0/1 | 0/1 |
| 1 | 2900 | 0/1 | 0/1 | |
| 1 | 3600 | 0/1 | 0/1 | |
| 1 | 5000 | 0/1 | 1/1 | |
| LD50(mg/kg) | >5000 | 4243 | ||
3.2. Rane’s Curative Test Model
The results of the antimalarial test based on Rane’s curative model (Table 2) show that both extracts (PA and DE) produced dose-dependent inhibition (p < 0.05) of parasitemia. The inhibition produced by 400 mg/kg of D. edulis was 57.10 ± 1.90% while the same dose of P. americana produced a comparable but slightly lower inhibition of 52.16 ± 2.77%. Their effect was also comparable, a little lower than that of the standard drug, ACT (69.04 ± 3.02% inhibition).
| Group | Treatment | % parasitemia | % inhibition of parasitemia | |
|---|---|---|---|---|
| Day 3 | Day 8 | |||
| 1 | 100 mg/kg PA | 70.80 ± 3.71 | 46.80 ± 3.44 ∗∗∗ | 33.90 ± 2.13 ∗∗∗ |
| 2 | 200 mg/kg PA | 69.80 ± 3.54 | 41.80 ± 4.85 ∗∗∗ | 40.11 ± 5.10 ∗∗∗ |
| 3 | 400 mg/kg PA | 69.40 ± 1.44 | 33.20 ± 4.91 ∗∗∗ | 52.16 ± 2.77 ∗∗∗ |
| 4 | 100 mg/kg DE | 73.40 ± 1.50 | 66.60 ± 2.46 ∗∗∗ | 9.26 ± 0.08 ∗ |
| 5 | 200 mg/kg DE | 67.00 ± 3.51 | 34.20 ± 3.18 ∗∗∗ | 48.96 ± 2.11 ∗∗∗ |
| 6 | 400 mg/kg DE | 60.60 ± 2.94 | 26.00 ± 2.05 ∗∗∗ | 57.10 ± 1.98 ∗∗∗ |
| 7 | ACT | 78.80 ± 3.58 | 24.40 ± 5.02 ∗∗∗ | 69.04 ± 3.02 ∗∗∗ |
| 8 | Control | 71.20 ± 2.47 | 79.60 ± 5.4 | −11.80 ± 1.07 |
- Data are mean ± standard error of mean (SEM) (n = 5); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as compared with control group; % inhibition of parasitemia = (% parasitemia on day 3 − % parasitemia on day 8/% parasitemia on day 3) × 100; DE = D. edulis leaf extract; PA = P. americana leaf extract.
3.3. Hematological Parameters in Rane’s Curative Model
The results of Hb and PCV are shown in Table 3. Results show that there was a reduction of the hematological parameters as a result of the infection with the parasite from day 0 to day 3. The plant extracts improved (p < 0.05) these parameters after the 5-day treatment period. The results of Hb show that the two extracts produced a dose-dependent significant (p < 0.05) increase in the Hb from day 3 (the day of infection) to day 8 (a day after treatment) in the curative test model. The increment for P. americana ranges from 5.84 ± 0.08% to 33.94 ± 0.12% while that of D. edulis ranges from 22.54 ± 0.14% to 39.23 ± 0.25%. The increment values are however less than those of ACT (43.11 ± 0.17%). Similarly, the results of the PCV shows that both extracts also produced significant increment in the PCV of the parasitized mice though that of P. americana was in a non-dose-dependent manner (maximum increment was 26.06 ± 0.07%). D. edulis produced dose-dependent increment ranging from 4.69 ± 0.80% to 13. 04 ± 0.33%.
| Group | Treatment | Hb (mg/dL) | PCV (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 8 | % change in Hb# | Day 0 | Day 3 | Day 8 | % change in PCV# | ||
| 1 | 100 mg/kg PA | 11.00 ± 0.25 | 9.24 ± 0.11 ∗ | 9.78 ± 0.15 ∗∗∗ | 5.84 ± 0.08 | 40.80 ± 0.58 | 30.80 ± 1.85 | 35.80 ± 0.92 ∗∗∗ | 16.23 ± 0.06 |
| 2 | 200 mg/kg PA | 11.26 ± 0.23 | 8.42 ± 0.61 ∗ | 10.28 ± 0.21 ∗∗∗ | 22.09 ± 0.23 | 41.20 ± 0.80 | 28.40 ± 1.21 | 35.80 ± 0.66 ∗∗∗ | 26.06 ± 0.07 |
| 3 | 400 mg/kg PA | 11.40 ± 0.27 | 7.72 ± 0.30 ∗ | 10.34 ± 0.18 ∗∗∗ | 33.94 ± 0.12 | 41.80 ± 1.36 | 31.60 ± 0.93 | 36.80 ± 1.16 ∗∗∗ | 16.46 ± 0.40 |
| 4 | 100 mg/kg DE | 11.08 ± 0.13 ∗∗ | 7.72 ± 0.29 ∗ | 9.46 ± 0.24 ∗ | 22.54 ± 0.14 | 42.25 ± 1.10 | 32.00 ± 1.08 | 33.50 ± 0.95 ∗∗∗ | 4.69 ± 0.80 |
| 5 | 200 mg/kg DE | 11.20 ± 0.12 ∗ | 8.46 ± 0.63 ∗ | 10.50 ± 0.12 ∗∗∗ | 24.11 ± 0.23 | 42.20 ± 0.80 | 32.80 ± 1.66 | 35.80 ± 0.73 ∗∗∗ | 9.15 ± 0.90 |
| 6 | 400 mg/kg DE | 11.00 ± 0.09 ∗ | 7.80 ± 0.36 ∗ | 10.86 ± 0.19 ∗∗∗ | 39.23 ± 0.25 | 40.40 ± 0.68 | 32.20 ± 1.56 | 36.40 ± 0.68 ∗∗∗ | 13.04 ± 0.33 |
| 7 | Standard drug (ACT) | 11.28 ± 0.71 | 7.84 ± 0.29 | 11.22 ± 0.13 ∗∗∗ | 43.11 ± 0.17 | 41.40 ± 0.74 | 32.60 ± 1.60 | 39.80 ± 0.66 ∗∗∗ | 22.09 ± 0.87 |
| 8 | Control (infected but untreated) | 10.98 ± 0.13 | 7.06 ± 0.28 | 6.90 ± 0.31 | −2.27 ± 0.05 | 41.80 ± 0.58 | 28.60 ± 1.32 | 24.00 ± 1.44 | −16.08 ± 0.05 |
- Data are mean ± standard error of mean (SEM) (n = 5); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as compared with control group; #% change in Hb or PCV is that of day 3 and day 8.
3.4. Suppressive Antimalarial Test Model
The results of the suppressive antimalarial test model, mean survival time, body weight, and PCV are shown in Table 4. The P. americana extract at 400 mg/kg produced significant (p < 0.05) chemosuppression (55.00 ± 0.06%) in parasitemia. Similarly, the hexane (PAH), ethyl acetate (PAE), and water (PAW) fractions produced significant (p < 0.05) suppression by 56.03 ± 0.07%, 40.00 ± 0.05%, and 64.01 ± 0.08%, respectively. The rank order of chemosuppression of the fractions was water (64.01 ± 0.08%) > hexane (56.03 ± 0.07%) > ethyl acetate (40.00 ± 0.05%) > methanol (22.02 ± 0.09%). Likewise, the extract and fractions of D. edulis produced a significant (p < 0.05) chemosuppression of parasitemia. The extract of D. edulis at the dose of 400 mg/kg produced (suppression of 62.03 ± 0.01%) slightly better effect than P. americana extract (55.00 ± 0.06%) at the same dose. Also, the fractions of D. edulis showed chemosuppression ranging from 53.51 ± 0.09% (for the hexane fraction) to 71.99 ± 0.06% (for methanol fraction). The effect of the latter is slightly better than that of ACT (70.00 ± 0.06%).
| Group | Treatment dose (mg/kg) | Parasitemia level | Mean survival time (days) | Body weight (g) | PCV (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % parasitemia | % suppression | Day 0 | Day 4 | % change | Day 0 | Day 4 | % change | |||
| 1 | 400 mg/kg PA | 7.50 ± 0.65 ∗∗∗ | 55.00 ± 0.06 | 6.60 ± 1.08 | 12.44 ± 1.44 | 10.64 ± 0.52 | −14.47 ± 0.06 | 41.00 ± 1.00 | 37.25 ± 0.75 | −9.15 ± 0.03 |
| 2 | 200 mg/kg PAH | 7.33 ± 0.88 ∗∗∗ | 56.03 ± 0.07 | 6.00 ± 1.52 | 11.84 ± 0.91 | 11.10 ± 0.80 | −6.25 ± 0.04 | 39.00 ± 0.71 | 37.01 ± 0.58 | −5.10 ± 0.02 |
| 3 | 200 mg/kg PAE | 10.00 ± 0.58 ∗ | 40.00 ± 0.05 | 5.80 ± 1.69 | 15.48 ± 2.89 | 12.75 ± 2.08 | −17.64 ± 0.01 | 38.20 ± 0.66 | 39.67 ± 1.45 | 3.85 ± 0.03 |
| 4 | 200 mg/kg PAM | 13.00 ± 1.41 | 22.02 ± 0.09 | 10.6 ± 2.73 | 14.10 ± 2.27 | 12.36 ± 1.62 | −12.34 ± 0.03 | 38.80 ± 0.86 | 39.25 ± 0.63 | 1.16 ± 0.04 |
| 5 | 200 mg/kg PAW | 6.01 ± 0.91 ∗∗∗ | 64.01 ± 0.08 | 17.6 ± 3.67 ∗∗∗ | 13.82 ± 1.32 | 12.16 ± 1.10 | −12.01 ± 0.01 | 39.40 ± 0.60 | 37.75 ± 0.63 | −4.19 ± 0.02 |
| 6 | 400 mg/kg DE | 6.33 ± 0.33 ∗∗∗ | 62.03 ± 0.01 | 10.20 ± 2.97 | 19.76 ± 1.47 | 20.80 ± 0.48 ∗∗ | 5.26 ± 0.03 | 40.00 ± 0.32 | 36.33 ± 2.19 | −9.18 ± 0.02 |
| 7 | 200 mg/kg DEH | 7.75 ± 1.25 ∗∗∗ | 53.51 ± 0.09 | 10.40 ± 4.15 | 16.88 ± 0.69 | 21.70 ± 2.71 ∗∗ | 28.55 ± 0.07 | 39.40 ± 1.08 | 36.50 ± 1.70 | −7.36 ± 0.12 |
| 8 | 200 mg/kg DEE | 7.00 ± 0.58 ∗∗∗ | 58.01 ± 0.06 | 8.0 ± 4.29 | 19.36 ± 0.60 | 21.50 ± 0.15 ∗∗ | 11.05 ± 0.03 | 38.20 ± 0.66 | 34.00 ± 0.58 | −10.99 ± 0.05 |
| 9 | 200 mg/kg DEM | 4.67 ± 0.88 ∗∗∗ | 71.99 ± 0.06 | 11.60 ± 5.07 | 17.32 ± 1.02 | 19.78 ± 0.94 ∗∗ | 12.44 ± 0.02 | 40.60 ± 0.51 | 34.00 ± 0.58 | −16.26 ± 0.04 |
| 10 | ACT | 5.01 ± 0.58 ∗∗∗ | 70.00 ± 0.06 | 16 ± 1.14 ∗∗∗ | 17.52 ± 0.47 | 20.05 ± 0.52 | 14.44 ± 0.02 | 39.40 ± 0.51 | 39.67 ± 0.33 | 0.69 ± 0.02 |
| 11 | Control | 16.67 ± 1.45 | — | 4.8 ± 0.66 | 18.22 ± 1.69 | 14.90 ± 1.22 | −18.22 ± 0.07 | 40.00 ± 0.71 | 36.67 ± 1.76 | −8.33 ± 0.08 |
- PA = P. americana leaf crude extract, PAH = P. americana hexane fraction; PAE = P. americana ethyl acetate fraction; PAM : P. americana methanol fraction; PAW = P. americana water fraction; DE = D. edulis leaf extract; DEH = D. edulis hexane fraction; DEE = D. edulis ethyl acetate fraction; DEM = D. edulis methanol fraction. DEW (D. edulis water fraction) was not tested due to its limited quantity.
3.4.1. Survival Time in the Suppressive Antimalarial Test Model
While the untreated group (control group) caused a mean survival time of only 4.8 ± 0.66 days, the extract and fractions of P. americana prolonged the survival times in the range of 5.80 ± 1.69 to 17.6 ± 3.67 days with only the water fraction producing a significant (p < 0.001) effect (Table 4). In addition, D. edulis extract and fractions prolonged the survival time of the test animals in the range of 8.0 ± 4.29 to 11.60 ± 5.07 days with the methanol fraction producing the longest surviving time.
3.5. Body Weight and PCV in the Suppressive Antimalarial Test Model
Though there was a general decline in body weight in all the groups that received P. americana extract and fractions, there was a reduction of 18.22 ± 0.07% in the body weight in the control group while, for the hexane fraction, there was a moderate reduction of 6.25 ± 0.04%; ACT has an increment of 14.44 ± 0.02% (Table 4). None of the fractions and the extract produced a significant (p > 0.05) change in the PCV of the animals in the suppressive model within the 5-day observation period (day 0 to day 4) though the ethyl acetate and methanol fractions produced moderate increment in the parameter. For the D. edulis, there was a significant (p < 0.05) increase in body weight due to the effect of the extract and fractions in the range of 11.05 ± 0.03% to 28.55 ± 0.07%. Similar to P. americana, the extract and fractions of D. edulis did not produce significant (p > 0.05) change in the PCV of the mice in the suppressive model.
3.6. Results of Phytochemical Analysis
Results of phytochemical analysis (Table 5) revealed the presence of all the tested phytoconstituents like alkaloids, saponins, tannins, flavonoids, steroids, and glycosides in both crude leaf extracts of P. americana and D. edulis. All the plant samples contain saponins. As shown in Table 5, alkaloids are also present in the moderately polar fractions of both plants.
| Phytochemicals | PA | PAH | PAE | PAM | PAW | DE | DEH | DEE | DEM |
|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | + | − | + | + | − | + | + | + | + |
| Saponins | + | + | + | + | + | + | + | + | + |
| Tannins | + | − | + | + | + | + | − | + | + |
| Flavonoids | + | − | − | + | − | + | − | − | + |
| Steroids | + | + | − | − | − | + | + | + | − |
| Glycosides | + | − | − | + | − | + | − | + | + |
- PA = P. americana leaf crude extract, PAH = P. americana hexane fraction; PAE = P. americana ethyl acetate fraction; PAM : P. americana methanol fraction; PAW = P. americana water fraction; DE = D. edulis leaf crude extract; DEH = D. edulis hexane fraction; DEE = D. edulis ethyl acetate fraction; DEM = D. edulis methanol fraction; + = present; − = absent.
3.7. Results of HPLC Analysis
The results of HPLC analysis (Figure 1 and Table 6) suggested the presence of flavonoids, alkaloids, fatty acids, steroids, or terpenes as the major phytoconstituents in the extract and fractions of P. americana leaf. The major constituents of the hexane fraction (PAH) are steroids/terpenes and fatty acids while the moderately polar fraction (ethyl acetate) contains more flavonoids. The polar fractions (methanol and water fractions) contain mainly flavonoids and alkaloids. Quercetin and its glycosides are the most abundant flavonoids identified in the plant.
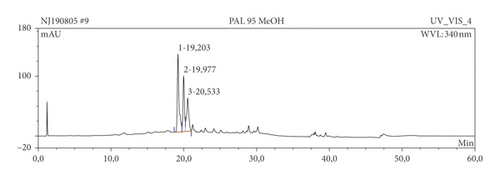

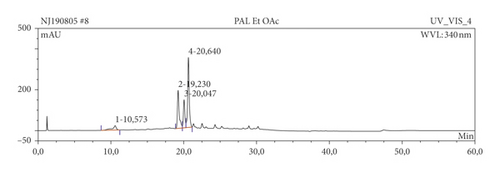
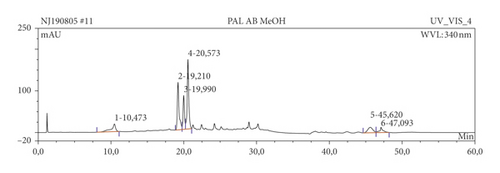
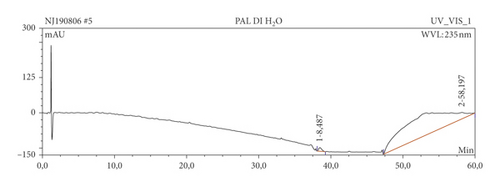
| Sample | Ret. time (min) | % peak area | UV maxima (rel. int.) | Suggested name of compound | class of phytochemical |
|---|---|---|---|---|---|
| PA | 12.65 | 17.39 | 239.6 (50), 321.1 (5), 357.7 (5) | Cytosporin | Flavonoids |
| 19.203 | 36.00 | 202.9 (50), 257.0 (30), 356.5 (45) | Hyperoside (quercetin-3-galactoside) | Flavonoids | |
| 19.977 | 19.08 | 203.2 (50), 256.7 (30), 356.5 (45) | Quercetin-3-O-galactoside | Flavonoids | |
| 20.533 | 21.47 | 201.8 (50), 255.9 (30), 349.7 (45) | Quercetin-3-O-rhamnoside | Flavonoids | |
| 47.08 | 6.06 | 201.8 (50), 280.4 (10), 371.7 (10) | Cyclopenol | Alkaloids | |
| PAH | 12.62 | 9.80 | 237.9 (50), 350.4 (10) | Cyteo-α-pyrone | Pyrones |
| 20.58 | 4.28 | 204.0 (50), 255.0 (30), 350.5 (20) | Quercetin-3-O-rhamnoside | Flavonoids | |
| 31.88 | 10.99 | 233.9 (50), 277.8 (5) | Fatty acid | Fatty acid | |
| 32.03 | 16.42 | 234.2 (50), 274.3 (5) | Fatty acid | Fatty acid | |
| 33.05 | 12.14 | 232.9 (50), 278.5 (5) | Cerebroside | Steroids/terpenes | |
| 40.89 | 4.38 | 251.8 (50), 405.9 (10) | β-Sitosterol-3-O-α-pyranoside | Steroids | |
| 47.07 | 19.56 | 202.4 (50), 280.3 (10), 371.6 (10) | Pretrichodermamide | Alkaloids | |
| PAE | 19.23 | 6.73 | 203.7 (50), 257.0 (30), 356.6 (30) | Hyperin | Flavonoids |
| 20.05 | 4.12 | 203.6 (50), 256.8 (30), 356.6 (30) | Quercetin-3-O-galactoside | Flavonoids | |
| 20.64 | 12.78 | 204.0 (50), 257.1 (30), 350.1 (30) | Quercetin | Flavonoids | |
| PAM | 19.21 | 21.40 | 203.2 (50), 257.1 (30), 356.7 (45) | Hyperoside (quercetin-3-galactoside) | Flavonoids |
| 19.99 | 11.90 | 203.2 (50), 256.9 (30), 356.7 (45) | Hyperoside (quercetin-3-galactoside) | Flavonoids | |
| 20.57 | 27.75 | 204.0 (50), 257.1 (30), 350.1 (30) | Quercetin | Flavonoids | |
| PAW | 38.49 | 1.05 | 201.2 (50), 226.1 (30), 276.2 (30) | Naamine | Alkaloids |
- PA = P. americana leaf crude extract; PAH = P. americana hexane fraction; PAE = P. americana ethyl acetate fraction; PAM : P. americana methanol fraction; PAW = P. americana water fraction.
4. Discussion
Toxicological investigation of the extracts was done to ascertain their safety for use in the animals with possible extension to humans. LD50 is used as an indication of acute toxicity [20, 21]. High values of the LD50 (>5000 mg/kg for P. americana and 4243 mg/kg for D. edulis) obtained from the present work indicate the safety of the leaf extracts of the plants and hence formed the bases for the doses chosen for the antimalarial studies. Further support for the safety of these plants is the fact that their fruits are edible and are used for both nutritional and therapeutic purposes [26]. However, some toxic compounds have been identified in the seed of D. edulis seeds; otherwise, the plant is considered to be safe [27]. In addition, in line with the present work, Kamagate et al. [28] reported the LD50 of P. americana leaf as greater than 5000 mg/kg.
The present study employed in vivo antiplasmodial model in rodents since the model takes into account the possible involvement of the immune system and possible prodrug effect in the activity of the plants against the parasites [29]. Moreover, several antimalarial drugs such as artemisinin derivatives and chloroquine have been identified using in vivo model [30]. Both curative and suppressive models were employed in order to establish the curative capability on established infection as well as the schizonticidal activity of the plants. In both models, the mean parasitemia level was lower than 90% which indicates that both plants were active as antimalarial agents [31]. Antimalarial agents are expected to decreased parasitemia and its symptoms. This can be achieved through various means like reducing parasite nutrient intake and interfering with the pathways such as the heme pathway [32] or they could negatively affect the growth and reproduction of parasites [33]. The extracts from both P. americana and D. edulis reduced parasitemia in both the curative and suppressive models and made some of the mice survive for a longer time. Comparatively, in both antimalarial models, D. edulis extract produced a slightly better effect (57.10 ± 1.98% inhibition in the curative model and 62.03 ± 0.01% chemosuppression) than P. americana extract (52.16 ± 2.77% inhibition in the curative model and 55.00 ± 0.06% chemosuppression) but their effects are somewhat lower than that of the ACT (69.04 ± 3.02% inhibition in the curative model and 70.00 ± 0.06% chemosuppression).
Likewise, the effects of the fractions further indicate the antimalarial activity of both plants as all the tested fractions (besides the methanol fraction of P. americana) from both plants produced (p < 0.05) chemosuppression. The antimalarial activity of D. edulis appears to concentrate more on the polar fraction (methanol fraction produced 71.99 ± 0.06% chemosuppression) than the nonpolar fractions. Similarly, the polar (water) fraction of P. americana produced a better effect (64.01 ± 0.08% chemosuppression) than others. Thus, the methanol fraction of D. edulis produced the largest parasitemia suppression (71.99 ± 0.06%) among all the tested extracts and fractions of both plants. The results of the present study validate the use of both plants, either singly or in combination in ethnomedicinal practice for the treatment of malaria [12–14, 17].
The decrease in PCV and Hb of the malarial-infected mice is in conformity with previous reports in malarial subjects [34]. In the present study, the leaf extracts of both plants in Rane’s curative model caused a significant increase in Hb and PCV as a result of increased production of RBC. Body weight increase, particularly by D. edulis extract, suggests that the extracts and fractions have an ameliorating effect on the malaria infection. Besides, the prolongation of the surviving times of the infected mice by the extract and fractions of both plants is a further proof of the strong antimalarial potential of the plants.
The observed antimalarial activity of both plants could be attributed to their phytochemical constituents including alkaloids, flavonoids, saponins, tannins, steroids, or glycosides either acting singly or synergistically as observed from the phytochemical and HPLC analysis. Some authors have previously isolated and identified some compounds having antimalarial activity in some parts of the plants. In a previous report, some compounds were isolated from D. edulis stem bark and shown to have a promising effect against P. falciparum with the most active compound being methyl 3, 4, 5-trihydroxybenzoate [17]. Compounds which were reportedly present in P. americana include peptone, b-galactoside, alkaloids, glycosylated abscisic acid, saponins, polyphenols, and fatty alcohols [29, 35, 36].
Our data indicate the presence of the antimalarial constituent of the leaf extract of P. americana in the water, hexane, and ethyl acetate fractions since these fractions produced significant (p < 0.05) suppression of parasitemia. The phytoconstituents of these fractions were varied and include saponins and tannins (for water fraction); saponins and steroids (for hexane fraction); and alkaloids, saponins, and tannins (for ethyl acetate fraction). HPLC analysis revealed the presence of main flavonoids in P. americana particularly quercetin and its glycosides. These results, therefore, suggest that the antimalarial constituents of P. americana are varied in nature. Likewise, methanol fraction, which was the most active fraction of D. edulis, contained alkaloids, saponins, tannins, flavonoids, and glycosides. One or more of these compounds could be responsible for the antimalarial activity of the plant [32, 37]. Thus, a higher concentration of these phytoconstituents in the active fractions could also be responsible for their high antimalarial property.
Various studies have supported the antimalarial activity of these plant constituents. Alkaloids, flavonoids, and sesquiterpenes have been reported to possess a broad spectrum of bioactivities including antimalarial activity [38, 39]. Many alkaloids, in particular, possess antimalarial activity and this is exemplified by quinine which is one of the most important and oldest antimalarial drugs [40, 41]. Other phytoconstituents including saponins, steroids, flavonoids [41], and terpenoids [42] have been reported to exhibit potent antimalarial activity. These previous reports are further support for the antimalarial activity of P. americana and D. edulis since these phytochemicals were identified in these studied plants.
Several mechanisms of action have been postulated for various natural antiplasmodial agents. Quercetin, a flavonoid, was shown to exhibit its antiparasitic effect through the destruction of mitochondrial function and the inhibition of different important enzymes and molecules, including heat-shock protein (HSP), acetylcholinesterase, DNA topoisomerase, and kinase [43]. Also, prenylated chalcones isolated from Humulus lupulus were reported to interfere with ham degradation in P. falciparum, suggesting a possible mechanism of action [44]. Flavonolignans and catechins also inhibit β-haematin formation in the parasite similar to chloroquine and H2O2-mediated heme degradation [45]. Some bromopyrrole alkaloids inhibited Plasmodium type II fatty acid synthase (FAS II) enzyme, suggesting that this might be part of the mechanism of action [46]. Also, some bromophycolides inhibited haemozoin formation by targeting heme crystallization [47]. Tajuddeen and Van Heerden [48] reviewed several other antiplasmodial mechanisms of action for natural compounds, including production of reactive oxygen species (ROS) and lipid oxidation products, inhibition of food vacuole falcipains, and Plasmodium falciparum glyoxalase I (PfGLOI), among others. These authors [48] also identified several natural compounds and medicinal plants that have been reported to exhibit a transmission-blocking effect by gametocidal activity. Though the actual mode of action of the antiplasmodial action of the plants was not studied in this present report, it is possible that the plants could exhibit their activity by some of the reported mechanisms of action.
The results of the present study provide justification for the anecdotal use of the two plants in the traditional medicines for the treatment of malaria. Further works are also underway to isolate and characterize the antimalarial constituents of the plants.
5. Conclusions
Herein, the activity of the leaf extracts and fractions of P. americana and D. edulis against malaria parasite in both curative and suppressive mouse models has been demonstrated. In addition, the extracts and fractions of the plants prolonged the survival time of the infected mice while also improving their hematological parameters. The most active antimalarial fractions of the two plants are the more polar fractions. The study provides further scientific justification for the use of the two plants in Nigerian and other ethnomedicines for the treatment of malaria. Additionally, based on our results, the leaf extracts may be a source for potential novel compounds against malaria parasite. Further studies geared towards the identification of the active compounds responsible for the antimalarial activity observed with the plants are envisaged.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Contributions
PFU designed the work; participated in phytochemical and animal studies and statistical analysis; and also wrote the first draft of the manuscript. CKO, APO, SAN, and OHU participated in the animal and phytochemical studies as well as the statistical analysis. NJN carried out the HPLC analysis. All the authors approved the final draft of the manuscript for submission.
Acknowledgments
The Institute for Pharmaceutical Biology and Biotechnology, Heinrich Heine University, Düsseldorf, Germany, is acknowledged for granting access to the HPLC facility. The authors are grateful to Ebube Uzor for typesetting the manuscript.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




