Preparation of Carbopol 934 Based Ketorolac Tromethamine Buccal Mucoadhesive Film: In Vitro, Ex Vivo, and In Vivo Assessments
Abstract
The goal of present investigation was to formulate and evaluate ketorolac tromethamine (KTM) mucoadhesive buccal films. The films were prepared by solvent evaporation method using PVP K30, HPMC K4M, HPMC K15M, carbopol 934, chitosan, and sodium alginate as polymers and propylene glycol as plasticizer. The films were evaluated for thickness, weight variation, folding endurance, surface pH, swelling index, in vitro residence time, in vitro diffusion, release kinetics, ex vivo permeation, in vitro-ex vivo correlation, and in vivo pharmacological activities such as anti-inflammatory and analgesic activity. Thickness, weight, drug content, and folding endurance were found to be uniform for the films. Surface pH was 6.85 ± 0.10, and swelling index was the highest (27.27 ± 0.37) for the best film containing carbopol 934 along with sodium alginate and PVP K 30 (formulation code F2). In vitro residence time was greater than 5 h, and in vitro % drug release was 98.71% for F2. It exhibited 55.49% of swelling inhibition at 5 h, and above 38.88% was maintained at even 8 h. The film F2 has shown maximum analgesic response of 17 sec at 5 h, and the response of 11 sec was maintained at even 8 h. The anti-inflammatory and analgesic effect of F2 was found be maximum while sustaining the effect for prolonged period when compared to free drug solution. Thus, KTM mucoadhesive buccal film containing carbopol 934, sodium alginate, and PVP K30 could be an effective alternative for conventional therapy with improved efficacy.
1. Introduction
One of the best alternatives to other routes of drug administration is buccal route [1]. Among the various buccal drug delivery systems, mucoadhesive delivery has received extensive consideration in treating local and systemic diseases due to their capability to stick to mucosa that prolongs the residence time at the site of administration by eliminating the chance of involuntary swallowing [1, 2]. Among the various mucoadhesive drug delivery systems such as tablets, gels, patches, and films [3], mucoadhesive buccal films (MBFs) are relatively new dosage forms. They are flexible, single, or multilayer films made up of bio adhesive polymers responsible for forming strong adhesive bond with the buccal mucosa [4]. MBFs also ensure an accurate dose of drug as other unit dosage forms when compared to liquids or gels [5]. The ease of scale up and manufacturing of the buccal films makes the cost of dosage form also affordable [6]. MBFs are highly flexible in nature but sufficiently tough enough to resist mouth movements therefore provides better patient compliance [5, 6]. Mucoadhesive polymers are the main components of these formulations; therefore, by sensibly optimizing the type and concentration of polymers along with other excipients such as permeation enhancers, plasticizers, flavouring agents, sweetening agents, and colorants, the best MBF with desired qualities can be prepared [7, 8].
Ketorolac tromethamine (KTM) is a nonsteroidal anti-inflammatory drug with a potent analgesic and anti-inflammatory activity due to prostaglandin-related inhibitory effect of drug. It is a nonselective cyclo-oxygenase inhibitor. The drug is currently used orally and intramuscularly in multiple divided doses, clinically for the management arthritis, cancer pain, postsurgical pain, and in the treatment of migraine pain [9–11]. The previous studies proved that KTM is more potent than indomethacin in the pain management of arthritis [12] and more potent than phenyl butazone, naproxen, and morphine in suppressing inflammation induced by carrageenan [13]. Since the drug is a nonselective cyclo-oxygenase inhibitor of arachidonic acid with no increase of lepoxygenase pathway, the adverse effects are very much severe, and the drug is implicated as a contributing cause of increased postoperative bleeding [14], renal failure, and gastritis [15]. The severity of these side effects is dose related [16, 17]. KTM has short biological half-life of 4 to 6 hours, which necessitates frequent dosing to retain the action [18]. The frequent occurrence of gastrointestinal bleeding, perforation, peptic ulceration, and renal failure leads to the development of KTM loaded mucoadhesive buccal film.
The objective of the present study was to develop KTM incorporated mucoadhesive buccal film using various mucoadhesive polymers like hydroxy propyl methyl cellulose (HPMC) K4M, HPMC K15M, carbopol P 934, chitosan, and polyvinyl pyrrolidone (PVP) K30 and evaluated in vitro. It was examined ex vivo whether and to what extent the transbuccal permeation occurs. In vivo pharmacological activities such as analgesic and anti-inflammatory activities were performed further to support the results.
2. Materials and Methods
2.1. Materials
KTM was obtained from Aizant Drug Research Solutions Pvt Ltd, Hyderabad, India. Carbopol P 934, HPMC K4M, chitosan, PVP K30, and sodium alginate were obtained from Sigma-Aldrich, Mumbai, India. The solvents and other chemicals used were generally regarded as safe (GRAS).
2.2. Preparation of KTM Buccal Film
MBFs were made by solvent evaporation method. Quantity of drug to be added was calculated as 10 mg/cm2 based upon the surface area of Petri plate since once the MBF forms it covers the entire surface area of the Petri plate. Weighed quantity of drug was dissolved in 1 mL of ethanol. The required amount of PVP K30 and 4 mL of water was added to the above solution (carrier solution). The remaining polymers were added as per composition to carrier solution (Table 1). 5% propylene glycol was further added to the above solution. The volume of the solution was made up to 15 mL with the remaining water. The whole mixture was vortexed for 1 h at 400 rpm using magnetic stirrer. The drug polymer mixture was poured in to Petri plate having inside diameter of 60 mm and dried at room temperature keeping on the Petri plate an inverted funnel to control air entry.
| Contents | Formulation code | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| KTM (mg/cm2) | 10 | 10 | 10 | 10 |
| PVP K30 (w/w) | 7% | 7% | 7% | 7% |
| Sodium alginate (w/w) | 1.5% | 1.5% | 1.5% | 1.5% |
| HPMC K15M (w/w) | 1% | — | — | — |
| Carbopol 934 (w/w) | — | 1% | — | — |
| HPMC K4 M (w/w) | — | — | 1% | — |
| Chitosan (w/w) | — | — | — | 1% |
| Propylene glycol (v/v) | 5% | 5% | 5% | 5% |
| Water (mL) | 15 | 15 | 15 | 15 |
The films were stored in desiccator after drying [19].
2.3. Thickness Uniformity
Micrometer was used for measuring the thickness of the films at six different spots of film, and the average was calculated [19].
2.4. Weight Uniformity of Films
The weight of each film was taken using balance, and the weight variation of films was calculated (n = 6) [20].
2.5. Percentage Moisture Loss
2.6. Folding Endurance
The folding endurance of the films was measured by constantly folding the film to 180° angle at the same place till it breaks or up to 300 times, which is well-thought-out satisfactory. Folding endurance is one of the norm for the mechanical strength of buccal films. The greater folding endurance ensures the larger mechanical strength of the film. Folding endurance is also an important element in patient compliance and convenience. The maximum number of times, the films possibly be folded at the same place without breaking, provides the measure of the folding endurance (n = 6) [21].
2.7. Surface pH
2% w/v agar in phosphate buffer solution pH 6.8 was used as the supporting gel media plate to measure surface pH of buccal films. Measurement was done by allowing the films to swell for 1 h on the surface of the plate and by bringing the pH meter to be in contact with the surface of the swollen patch. The mean of six results was noted [19].
2.8. Drug Content Uniformity of Films
MBFs were tested for the content uniformity. A film size of 1 × 1 cm2 was cut and placed in a beaker containing 10 mL of pH 6.8 phosphate buffer solution. The film was completely dissolved by constant stirring, and drug content was estimated against blank at 333 nm [22] spectrophotometrically using a standard calibration curve constructed for the calibration concentration range of KTM solutions from 2 to 12 μg/mL at λmax of 333 nm. The calibration curve was linear with the R2 value of 0.999.
2.9. Swelling Index
Six trials were conducted, and average was recorded.
2.10. In Vitro Residence Time
The in vitro residence time for buccal film was measured using a locally modified disintegration apparatus USP. The medium was composed of 500 mL of phosphate buffer pH 6.8 maintained at 37 ± 0.5°C. A part of goat buccal mucosa of 3 cm length was glued to a glass slab. After hydrating the film surface using phosphate buffer pH 6.8, the film was set into contact with the mucosal membrane. The glass slab was secured vertically such that there is complete immersion of the film into the buffer solution at the lowest point and was available out at the highest point. The time taken for the complete detachment of the film from the mucosal surface was recorded (n = 6) [24].
2.11. In Vitro Diffusion Studies
In vitro drug diffusion was studied in a modified diffusion cell using sigma membrane (12000 molecular weight cut off) kept between the donor and receptor compartment of the diffusion cell. This model consists of a beaker (250 mL) as receptor compartment and a glass tube of diameter 17.5 mm opened from both the ends as donor compartment. Sigma membrane (Sigma 12000 MW cutoff) was tied at one end of the tube, and the other end left free. After securing buccal film in sigma membrane, the assembly was dipped into the beaker containing 90 mL of diffusion medium (phosphate buffer pH 6.8). The temperature was maintained at 37 ± 0.5°C. The entire assembly was secured on a hot plate magnetic stirrer and stirred at 100 rpm at the temperature of 37 ± 0.5°C. 5 mL aliquot samples were withdrawn at regular intervals and analysed by UV spectroscopy at 333 nm [19, 25, 26].
2.12. Mucoadhesion Strength
Mucoadhesion strength of the film was measured on a modified physical balance employing the method as described by Gupta et al. [27] using goat buccal mucosa as model mucosal membrane. Fresh goat buccal mucosa was obtained from local slaughter house and was used within 2 h of slaughtering. The mucosal membrane was washed with distilled water and then with phosphate buffer pH 6.8. A double beam physical balance was taken, and to the left arm of balance, a thick thread of suitable length was hanged, and to the bottom side of thread, a glass stopper with uniform surface was tied. The buccal mucosa was tied tightly with mucosal side upward using thread over the base of inverted 50 mL glass beaker which was placed in a 500 mL beaker filled with phosphate buffer pH 6.8 kept at 37°C such that the buffer reaches the surface of mucosal membrane and keeps it moist. The buccal film was then stuck to glass stopper from one side of the membrane using an adhesive. The two sides of the balance were made equal before the study, by keeping a weight on the right hand pan. A weight of 5 g was removed from the right hand pan, which lowered the glass stopper along with the film over the mucosal membrane. The balance was kept in this position for 3 min. Then, the weights were increased on the right pan until the film just separated from mucosal membrane. The excess weight on the right pan, i.e., total weight minus 5 g, was taken as a measure of the mucoadhesive strength. The mean value of six trials was taken for each formulation. After each measurement, the tissue was gently washed with phosphate buffer pH 6.8 and left for 5 min before placing a new film to get appropriate results for the formulation.
2.13. Ex Vivo Permeation Studies
Ex vivo drug permeation was studied in a modified diffusion cell as discussed in Section 2.11 using the goat buccal mucosal membrane separating the donor and the receptor compartment of the diffusion cell. The film was positioned on the buccal mucosal membrane using aluminum foil as the backing layer. 90 mL of phosphate buffer pH 6.8 was used as the receptor compartment media. The entire assembly was secured on a hot plate magnetic stirrer and stirred at 100 rpm at the temperature of 37 ± 0.5°C. 5 mL aliquot samples were withdrawn at regular intervals and analysed by UV spectroscopy at 333 nm.
2.14. Release Kinetics
In order to predict out the order and mechanism of KTM release from buccal films, the in vitro drug release data was exposed to the following mathematical models such as zero-order kinetic model, first order kinetic model, Higuchi’s kinetic model, and the Korsmeyer-Peppas models [28].
2.15. In Vivo Studies
2.15.1. Animals
Adult male albino rats were obtained from animal housing facility, Department of Pharmacology, Malla Reddy College of Pharmacy, Secunderabad (an approved and registered facility under CPCSEA 2010).
The experimental protocol for the all in vivo studies was approved by Institutional Animal Ethical Committee, which is an approved body under CPCSEA (registration number CPCSEA/MRCP/1217). Animals were maintained under controlled conditions of temperature and humidity in polypropylene cages filed with sterile paddy husk. They fed balanced diet obtained from Mahaveera Enterprises (registration number 146/1999/CPCSEA) and water ad libitum.
2.15.2. Anti-Inflammatory Activity of KTM Buccal Film
Carrageenan-induced rat paw edema method was used to study the in vivo performance of the prepared film, and the study was approved by Institutional Animal Ethical Committee of Malla Reddy College of Pharmacy, Hyderabad (CPCSEA/MRCP/1217).
Adult male albino rats (Wistar strain) of each weighing 160 ± 5 g were divided into 3groups with each group comprising of 6 animals. All the animals were marked on the right hind paw just behind the tibia-tarsal junction to ensure constant paw volume up to the fixed mark whenever dipped into the plethysmograph. Initial paw volume of the rats was measured by dipping rat paw into the plethysmograph just before carrageenan administration. All the animals were administered with 0.1 mL of 1% w/v of carrageenan (in 0.9% normal saline) in the subplantar region of the right hind paw. Group I was control group/untreated group. Group II (reference group) animals were treated with KTM free drug solution (dose 5 mg/kg) after half an hour of administration of 0.1 mL of 1% w/v of carrageenan (in 0.9% normal saline). Group III is posttreated group where all the animals were treated with KTM loaded buccal film (dose of 5 mg/kg body weight) by administering suspension of film formula in water. Buccal mucosal absorption of the drug can be estimated by determining the extent of the drug dissolution and then by the rate of the drug penetration through the buccal mucosa. The presence of permeation enhancer in the formula can promote the penetration of the dissolved drug in the buccal mucosa. Addition of 5% propylene glycol in formula F2 might enhanced the buccal absorption of the drugs. In addition, the greater anti-inflammatory activity of F2 compared to free KTM solution recommends that the anti-inflammatory effect of F2 cannot be described wholly on the basis of systemic oral absorption, and KTM might enter the systemic circulation through the buccal mucosal membrane. This discussion might support the practical benefit of administering the suspension of film while using smaller animal models like rats and mice [29].
where V0 is the paw volume before carrageenan injection (mL), Vt is the paw volume after carrageenan injection (mL), Ec is the edema rate of the control group, and Et is the edema rate of the treated group [31].
2.15.3. Assessment of Analgesic Activity
(1) Tail Flick Test. Tail flick test was performed on mice of 3 groups, each group comprising of 5 animals. Group I was untreated/control group. Group II was the reference group treated with 5 mg/kg dose of free drug solution. Group III was administered with KTM buccal film suspension in water. The test was done by focusing radiant heat on the dorsal surface of tail. Latency or the time it took for the mice to withdraw tail from a noxious thermal stimulus was measured using a tail flick meter. To minimize tissue damage, a maximum latency of 30 sec was imposed. The nichrome wire was about 1/8 below the tail. Each mouse was thus tested at 0, 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, and 8 h.
(2) Hot Plate Test. Animals were divided into groups and treated as discussed in the above section. The hot plate test was performed by placing the mice on aluminum hot plate at a temperature of 62 ± 0.5°C for a maximum time of 30 sec [32]. The reaction time was noted, when rats were licking their fore and hind paws or jumped at 0, 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, and 8 h.
2.16. Statistical Analysis
Statistical analysis of the experimental results was done by using one-way ANOVA with 95% confidence interval. Unpaired t-test was carried out for conducting statistical analysis using GraphPad Prism (version 5, GraphPad Software, San Diego, California, USA). A P value of less than 0.05 was considered as the level of significance. Mean value and standard errors were calculated out of six trials.
3. Results and Discussion
3.1. Preparation of KTM Buccal Film
Mucoadhesion is one of the most widely examined strategies in delivering drugs for rapid/sustained onset of action and better bioavailability. The rich vasculature of buccal mucosa affords outstanding stage for drugs targeting into systemic circulation provided that there is minimum dosage as compared to the oral route of drug delivery.
Buccal films of KTM were prepared by solvent evaporation method using mucoadhesive polymers such as HPMC K15M, carbopol P934, HPMC K4M, and chitosan at constant proportion of PVP K30 and sodium alginate in combination (Table 1). All the films prepared appeared elegantly without any sign of cracks and with optimum flexibility.
3.2. Physico-Chemical Characterization
The films were assessed for various physicochemical characteristics like thickness, surface pH, weight uniformity, content uniformity, folding endurance, and swelling index.
The thickness of the films was in the range of 0.62 ± 0.02 to 0.87 ± 0.03 mm. The results of thickness measurement revealed that all the films were perfect and uniform (n = 6) without any significant differences at different points. Formulation F1 displayed the least thickness while F4 displayed the extreme. Weight in gram of the films was from 0.04 ± 0.001 to 0.08 ± 0.007. From the results shown in Table 2, it was detected that the weight of buccal films was uniform without significant differences as compared to the average value with less than 5% of the weight variation. The thickness and weight uniformity of the films is an indication of uniform distribution of drug, polymers, and plasticizer. The percentage moisture loss was observed to range between 1.886 ± 1.50% and 4.761 ± 2.50%. It was found to be less (1.886 ± 1.50) for both F1 and F2. It can be hypothesized that the initial moisture content is the deciding factor in moisture loss. F3 and F4 showed comparatively high % moisture loss; the reason could be that F3 and F4 might have had initially high moisture content. The folding endurance was measured by folding the film repeatedly at 180° angle of the plane at the same place until it breaks. The folding endurance was found to be the highest for formulation F2 (256 ± 2) and the lowest for formulation F4 (198 ± 6). Carbopol is a cross-linking polymer, and cross-linking between the carbopol chains might maximised the mechanical properties of the film F2 and folding endurance. Presence of correct quantity of plasticizer propylene glycol also takes part in enhancing the folding endurance. The small molecules of propylene glycol penetrate the polymer chains well, interact with them, and by reducing the glass transition temperature (Tg) of system, improve the mechanical properties including flexibility and therefore improve folding endurance. The folding endurance is the measure of the flexibility of the films that is compulsory for easy handling of the films. Almost the entire films were consistent enough without cracking while folding for more than 200 times at a place except formulation F4. The folding endurance of the films other than F4 was fine, and therefore, the films demonstrated excellent physical and mechanical properties. High folding endurance always preferred in mucoadhesive buccal delivery because the film would neither dislocate from the site of application easily nor break during handling and administration. In order to explore the possibility of any irritation effects in vivo, the surface pH of the buccal films was measured since extremely acidic or alkaline pH prone to irritate the buccal mucosal membrane. The film F2 showed the surface pH of 6.85 ± 0.10 correlating with salivary fluid pH. The value was very close to neutral pH as it is known that neutral pH of the film does not irritate the mucosal membrane. Swelling index was found to be the highest for F2, i.e., 27.27 ± 0.37% (Table 2 and Figure 1).
| Formulation code | Thickness in mm | Surface pH | Weight uniformity (g) | Content uniformity (%) | Folding endurance | Swelling index (%) | In-vitro residence time (h) | % moisture loss |
|---|---|---|---|---|---|---|---|---|
| F1 | 0.62 ± 0.02 | 7.23 ± 0.21 | 0.06 ± 0.003 | 97.52 ± 0.36 | 245 ± 7 | 14.51 ± 0.97 | 3 ± 0.29 | 1.886 ± 1.50 |
| F2 | 0.80 ± 0.04 | 6.85 ± 0.10 | 0.04 ± 0.001 | 99.57 ± 0.17 | 256 ± 2 | 27.27 ± 0.37 | >5 | 1.886 ± 1.10 |
| F3 | 0.79 ± 0.03 | 7.48 ± 0.34 | 0.07 ± 0.006 | 96.27 ± 0.32 | 248 ± 3 | 16 ± 0.52 | 3 ± 0.58 | 4.761 ± 2.50 |
| F4 | 0.87 ± 0.03 | 5.14 ± 0.26 | 0.08 ± 0.007 | 97.59 ± 0.25 | 198 ± 6 | 22.73 ± 0.77 | 4 ± 0.26 | 2.352 ± 2.30 |
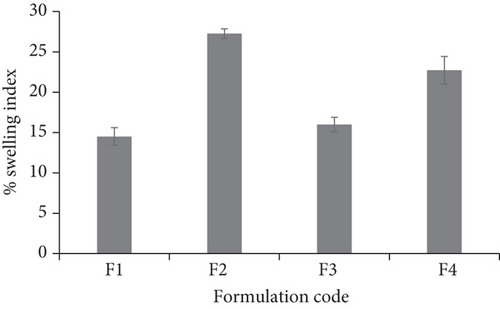
It was assumed that the high hydrophilic nature of polymer in F2 resulted in extreme swelling as compared to other formulations. There exists a direct relationship between the swelling index and presence of polymer carbopol. It is formed by polyacrylic acid chains’ cross linking. It is well-known statement that swelling reduces generally due to cross-linking of polymeric chains. But presence of carboxylic acid (COOH) groups in the polymeric chains of carbopol gets converted to carboxylate groups (COO-) after neutralization that leads to self-repulsion of polymeric chains themselves and forms hydrogen bonds with water. This particular characteristic of carbopol marks this polymer as one of the swellable polymers despite being cross-linked. The quicker the polymer swells, the quicker the bonds between mucus and the polymer form; hence, the adhesion to mucus occurs quicker. Drug content percentage was even for all the films ranging from 96.27 ± 0.32 (F3) to 99.57 ± 0.17 (F2) (Table 2). It was found as per the results of content uniformity test that there might be uniform distribution of the drug throughout the film irrespective of polymer concentration and composition.
3.3. In Vitro Residence Time
In vitro residence time test was carried out using modified disintegration apparatus. In vitro residence time results showed that formulation F2 having carbopol P934 as the mucoadhesive polymer showed higher mucoadhesion strength and hence greater in vitro residence time of more than 5 h. The film made using carbopol P934 as the mucoadhesive polymer showed the highest degree of swelling, and the reason for this could be optimum hydration and swelling of carbopol P934 [19]. The least % of swelling and in vitro residence time were found to be with film F1, and the reason might be over hydration of HPMC containing carboxylic groups, which form hydrogen bonds with the tissue. Some buccal films (F1 and F3) did not protect the integrity throughout the experimentation and were disintegrated within 3 h. Based upon the above results, F2 was chosen as the formulation having optimum characteristics.
3.4. In Vitro Diffusion Studies
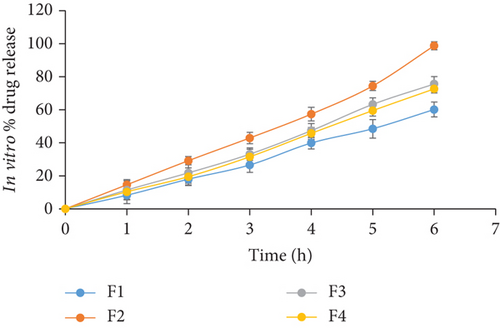
In vitro diffusion studies were conducted using modified diffusion assembly. The study revealed that the drug release was independent of the drug concentration. Among all the formulations, formulation F2 showed drug release of 98.71 ± 0.48% (Figure 2). This result might be due to optimum swelling of film F2 makes a thick gel barrier that slows down the drug release since diffusional path length for the drug to traverse increases [20].
3.5. Mucoadhesion Strength
The mucoadhesive strength was measured by a modified two-arm balance using goat buccal mucosa. All the films had shown good mucoadhesive strength with the range of 9-15 gm. The formulation F2 shown maximum strength (Table 3).
| S. no | Formulation | In vitro mucoadhesive strength (g) |
|---|---|---|
| 1. | F1 | 12.01 ± 0.75 |
| 2. | F2 | 15 ± 0.95 |
| 3. | F3 | 10.50 ± 0.45 |
| 4. | F4 | 9.50 ± 0.41 |
- ∗Each value was an average of six determinations (AM ± SD).
The attachment of a polymeric macromolecule to mucosal surface is known as mucoadhesion. It happens in four major phases: wetting, interpenetration, adsorption, and formation of secondary chemical bonds between mucus membrane and polymer. There are various characteristics such as molecular weight of the polymer, contact time with membrane, degree of swelling of the polymer, and the type of biological membranes used in the study that influences the mucoadhesive strength [19]. It was confirmed that increase in degree of hydration increases the adhesion but at the same time over hydration results sudden weakening in mucoadhesion, and it could be owing to the disentanglement at the polymer/tissue interface. The film made using carbopol P934 as the mucoadhesive polymer showed the highest mucoadhesive strength, and the reason for this could be optimum hydration and swelling of carbopol P934 [19]. Carbopol P934 is a high molecular weight polymer of cross-linked acrylic acid with polyalkenyl alcohols. Carbopol P934 hydrates and swells in aqueous phase due to hydrogen bonding and electrostatic repulsion after neutralization reaction (conversion of carboxylic acids in polymer chain to carboxylates). Thus, the hydration potential of carbopol P934 permits the film to rapidly establish contact with the mucus upon application. Hydrogen bonding and interchain penetration between carbopol and mucin components make the mucoadhesion consolidated.
3.6. Ex Vivo Diffusion Studies
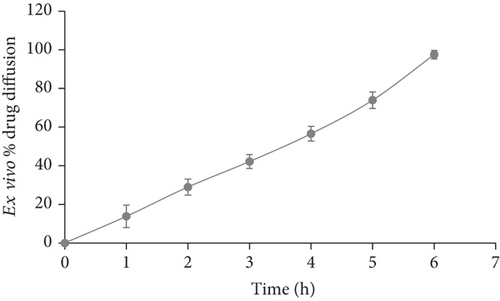
Ex vivo diffusion studies were carried out for the formulation F2 using goat buccal mucosal membrane separating donor and receptor compartment of the modified diffusion cell. The results are shown in Figure 3. Further the ex vivo % drug diffusion was well correlated with in vitro results for the formulation F2 (Figure 4).
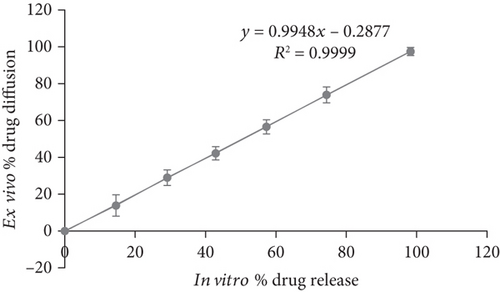
3.7. Release Kinetics
In vitro drug release data of formulation F2 was subjected to appropriate test to find out release kinetics, and the results revealed that the drug release followed zero order kinetics which was confirmed from regression coefficient of plot drawn between cumulative % drug releases vs. time. Mechanism of drug release was found to be diffusion mediated which was confirmed from regression coefficient of plot drawn between cumulative % drug release vs. square root time (Higuchi’s plot), and it is of non-Fickian type that was confirmed from slope of plot drawn between log cumulative % drug releases vs. log time (Table 4). Formulation F2 followed controlled release phenomena for the drug release and fitting the in vitro data in to Peppas model indicated with proved n and R2 values that the release of KTM was non-Fickian type of diffusion (Table 4) [33]. This results lead to an assumption that the drug followed both paracellular and transcellular pathways for its permeation [21].
| Formulation | Zero order | First order | Higuchi | Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| K0 | R2 | K1 | R2 | KH | R2 | N | R2 | |
| F2 | 15.81 | 0.9974 | 0.16 | 0.9586 | 55.51 | 0.9533 | 0.94 | 0.9960 |
3.8. Assessment of Anti-Inflammatory Activity
The anti-inflammatory activity of the film (F2) when compared to free drug solution was carried out in carrageenan-induced rat paw edema model against control. It was found that the buccal film F2 reduced the inflammation to the larger magnitude and also sustained the magnitude. Maximum inhibition of 55.49% was observed at 5 h, and above 38.88% was maintained at even 8 h. However, in case of free drug solution, maximum inhibitory effect was found to be at 5 h with the magnitude of 30.65%, and just after 5 h, it reduced drastically, and at 8 h, only 2% of effect was observed. Thus, buccal film resulted in larger as well as sustained inhibitory effect throughout the study period. This might be the result of effective and controlled drug release of buccal film and enhanced accumulation of drug in inflammatory area (Table 5).
| (Batch) | Edema rate (%) ± SD (inhibition rate (%)) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | 0.25 | 0.50 | 0.75 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Control | 42.50 ± 0.81 | 44.37 ± 0.52 | 48.71 ± 1.04 | 49.31 ± 1.14 | 54.41 ± 0.23 | 57.26 ± 1.17 | 59.11 ± 1.34 | 62.36 ± 0.96 | 55.91 ± 1.25 | 48.71 ± 0.92 | 43.03 ± 1.15 |
| KTM solution | 39.68 ± 0.89 (8.24) | 37.50 ± 0.78 (17.77) | 36.83 ± 1.09 (23.40) | 37.50 ± 1.16 (23.47) | 39.84 ± 1.25 (27.78) | 41.98 ± 1.11 (28.07) | 42.35 ± 1.26 (28.81) | 43.95 ± 1.91 (30.65) | 48.94 ± 0.48 (12.78) | 46.91 ± 0.92 (4.17) | 43.20 ± 0.66 (2.27) |
| F2 | 39.63 ± 0.53 (6.75) | 35.16 ± 0.46 (20.73) | 31.67 ± 0.86 (34.98) | 27.07 ± 0.49 (45.12) | 28.34 ± 1.17 (47.93) | 28.55 ± 1.03 (50.16) | 27.65 ± 1.82 (53.27) | 27.77 ± 0.95 (55.49) | 30.96 ± 1.29 (44.64) | 28.88 ± 0.49 (40.37) | 26.29 ± 0.41 (38.88) |
- Presented values are mean of 6 determinations ± SD.
Data was expressed as mean ± SD and statistically assessed by one-way analysis of variance (ANOVA). Values for edema rate percentage of the film were compared to the control, and the differences were determined statistically. P < 0.05 was considered significant.
3.9. Assessment of Analgesic Activity
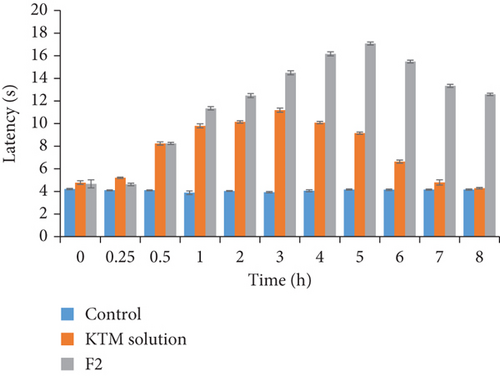
Administration of KTM buccal film increased tail flick and hot plate latency when compared to control. There was no change in latent period of control group animals in both hot plate and tail flick test. The buccal film has shown maximum analgesic response of 17 sec at 5 h, and the response of 11 sec was maintained at even 8 h. In drug solution, analgesic response peaked at 3 h, i.e., 11 sec; after that, it reduced drastically, and minimum response of 5 sec was observed at 8 h (Table 6 and Figure 5). Thus, analgesic activity was very much comparable in buccal film to free KTM solution. So not only the rapid onset of action but also the prolonged release (as indicated by in vitro release and ex vivo permeation data) can be achieved with the present buccal film of KTM.
| Group (sec) | 0.0 h | 0.25 h | 0.5 h | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h | 8 h |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 4.42 ± 0.16 | 4.10 ± 0.04 | 4.10 ± 0.04 | 3.89 ± 0.16 | 4.05 ± 0.04 | 3.92 ± 0.08 | 4.06 ± 0.09 | 4.168 ± 0.05 | 4.143 ± 0.07 | 4.16 ± 0.05 | 4.16 ± 0.06 |
| KTM solution | 4.65 ± 0.14 | 5.20 ± 0.08 | 8.22 ± 0.15 | 9.79 ± 0.18 | 10.14 ± 0.12 | 11.18 ± 0.18 | 10.09 ± 0.09 | 9.85 ± 0.10 | 9.64 ± 0.14 | 7.79 ± 0.23 | 5.23 ± 0.06 |
| F2 | 4.92 ± 0.05 | 5.62 ± 0.12 | 8.23 ± 0.08 | 10.34 ± 0.15 | 12.46 ± 0.19 | 13.47 ± 0.18 | 15.17 ± 0.18 | 17.08 ± 0.13 | 14.48 ± 0.12 | 10.35 ± 0.14 | 10.58 ± 0.11 |
4. Conclusion
In this study, KTM mucoadhesive buccal films using carbopol P934, HPMC K15M, HPMC K4M, chitosan blended with sodium alginate, and PVP K30 were developed successfully and characterized to circumvent all the adverse effects of KTM and to produce better therapeutic outcome. The optimized buccal film containing combination of carbopol P934, sodium alginate, and PVP K30 (F2) was appeared smooth, nongritty, and flexible without cracks having folding endurance of 256 ± 2 and nominal thickness and weight of 0.80 ± 0.04 mm and 04.96 ± 0.001 g, respectively, confirms the suitability of the formulation for buccal application. The surface pH of 6.85 ± 0.1 offers patient compliance since no irritation would be expected at this pH. The drug distribution in the optimized film was around 99.57 ± 0.17%. The film displayed mucoadhesive strength of 15 g ± 0.95 and swelling index of 27.27 ± 0.37% that are considered to be optimum for mucoadhesion. The film exhibited an in vitro release of 98.71% and ex vivo permeation of 97.47%. The prominent in vivo anti-inflammatory activity on carrageenan-induced rat paw edema model and analgesic activity further confirms the therapeutic efficacy of the film. These findings suggest that the buccal film could be a promising and alternative approach for the delivery of KTM.
Ethical Approval
The experimental protocol for the all in vivo studies was approved by Institutional Animal Ethical Committee, which is an approved body under CPCSEA (registration number CPCSEA/MRCP/1217).
Consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Contributions
We declare that this work was done by the authors named in this article, and all liabilities pertaining to claims relating to the content of this article will be borne by the authors.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research Group (Small) (Project number RGP.1/330/42). The authors also extend their thanks to Malla Reddy College of Pharmacy, Hyderabad, India, for providing animal house facilities to carry out the work.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.