Gold Nanoparticle: Recent Progress on Its Antibacterial Applications and Mechanisms
Abstract
Gold nanoparticles (AuNP) is a new type of metal nanomaterials used in the biomedical field. Compared with ordinary metal materials and other metal nanomaterials, AuNP can be very unique. AuNP has been proven to have good performance against a variety of pathogens, and research on its antibacterial activity and mechanism has also become a hot topic in recent years. This article summarizes the antibacterial properties and clinical applications of AuNP against different kinds of bacteria and analyzes and discusses its existing and potential antibacterial mechanisms. At the same time, we also put forward the current challenges and problems to be solved by AuNP and look forward to its future development prospects, in order to correctly and comprehensively introduce this new antibacterial method.
1. Introduction
Gold nanoparticles (AuNPs), containing gold whose diameter varies from 1 to 100 nanometers, have been considered a novel material in recent years, especially in the field of nanomedicine. Generally speaking, metal nanomaterials possess distinct and even unique properties compared with their nonnanoforms. At the same time, their properties and superiority vary when their size and shape change, leading to the exploration of the potential use of metal nanoparticles [1–6]. Gold nanoparticles are capable of being synthesized by a considerable number of simple techniques, covering physical methods like laser ablation, chemical routes like solvothermal methods, and green approaches which are conducted in a biological way [7]. In the case of the conventional chemical synthetic method, gold primarily presents as chloroauric acid (HAuCl4), and, with the help of aqueous citrate through redox reaction, AuNP is formed. Unconventional synthetic approaches utilize microorganisms and derivatives from plants as reaction agents. As environmentally friendly approaches, harmful intermediate products are avoided, and the morphology and size of AuNPs are easier to control by adopting different agents [8]. Meanwhile, the applications of AuNPs touch upon various areas. When it comes to the biomedical field, AuNPs’ fine biocompatibility constitutes the basis of in vivo application. AuNPs have already shown promising potential in anticancer treatment and nanoscale biosensing for their surface modification ability, their role as macromolecular carriers, and their physical features, especially the optic properties. Localized surface plasmon resonance (LSPR) is referred to as the key optic feature and one of the most fascinating characteristics of AuNPs from which the nanogold-based biomedical imaging detection and photothermal therapy benefit [9]. Furthermore, surface-enhanced Raman scattering (SERS), the consequence of plasmon resonance in AuNP, helps to reduce the limitations of molecular detection [10].
The superiority of AuNPs as antibacterial agents has become a research hotspot nowadays. Bacterial drug resistance is a severe challenge in antibacterial therapy because of the abuse of antibiotics and the formation of biofilms. Studies have verified their autologous bacteria-killing activity. Additionally, AuNPs are able to conjugate with aptamers, antibiotics, antimicrobial peptides, and bacterial specific antigens, which demonstrate their antibacterial activity in multiple ways: good drug delivery performance and immune response mediation. In research, interactions between various microorganisms and AuNPs have been studied, and it appears that AuNPs have promising results against several microorganisms and biofilms in terms of growth inhibition or cell damage. Recent research has also looked into the antimicrobial mechanisms.
The present review concentrates attention on the antibacterial applications of AuNPs, summarizing their individual antimicrobial activities upon different kinds of bacteria and analyzing the existing and potential mechanisms behind (Figure 1) them. We also focus on the special and fascinating properties which help shape them as antibacterial agents. Eventually, we will figure out that the challenges and problems AuNPs are presently confronted with and look forward to their future possibilities, aiming to develop a proper and comprehensive concept of this brand-new antibacterial approach.
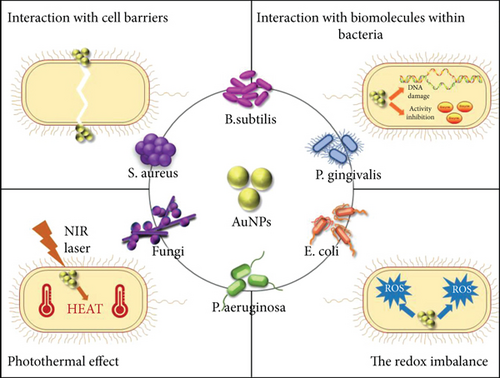
2. The Bactericidal Mechanism of AuNPs
2.1. Interaction with Cell Barriers
The bacterial cell barrier includes the cell wall and cell membrane and plays an important role in drug resistance; although, the compositions and properties of gram-positive bacteria and gram-negative bacteria are slightly different. It has been reported that AuNPs can destroy the bacterial cell membrane or cell wall, thereby causing damage to the bacteria [11].
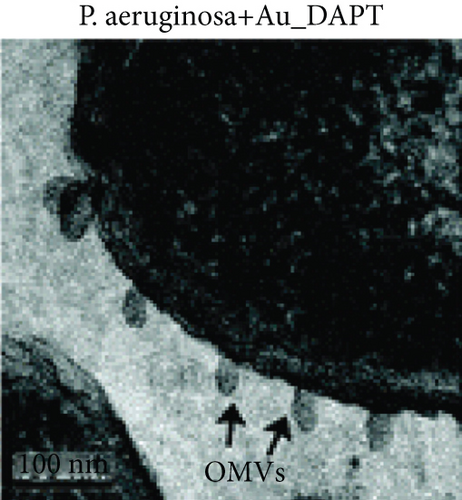
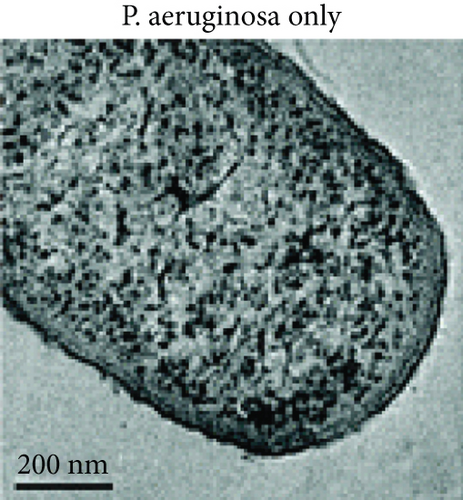
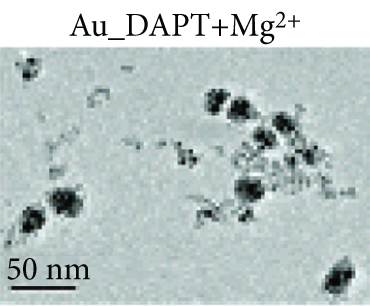
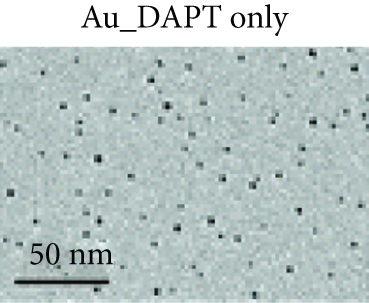
Zhao et al. synthesized 4,6-diamino-2-pyrimidinethiol (DAPT) capped gold NPs (Au_DAPT), in which DAPT itself has no antibacterial effect. 70% of Escherichia coli treated with Au_DAPT had enhanced cell membrane permeability, and some bacterial nucleic acids leaked from the cells, while only 5% of E. coli in the control group that had this change. Similarly, treatment with Au_DAPT increased the number of Pseudomonas aeruginosa with enhanced membrane permeability by 42%. In addition, Au_DAPT destroyed the membrane stability and induced the formation of bacterial outer membrane vesicles. Magnesium ions played an important role in maintaining outer membrane permeability, which could be chelated with Au_DAPT. Therefore, scholars speculated that Au_DAPT changed the concentration of magnesium ions by chelating with them, thereby increasing the permeability of the cell membrane [12] (Figure 2). Hayden et al. observed that AuNPs formed different aggregation patterns on the cell membrane surfaces of E. coli and Bacillus subtilis, causing significant lysis [13]. Adhikari et al. obtained similar results and pointed out that, as the dose of AuNPs increased, the bacterial membrane permeability gradually increased, and the bacterial viability gradually decreased [14].
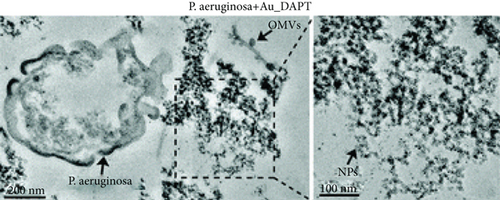
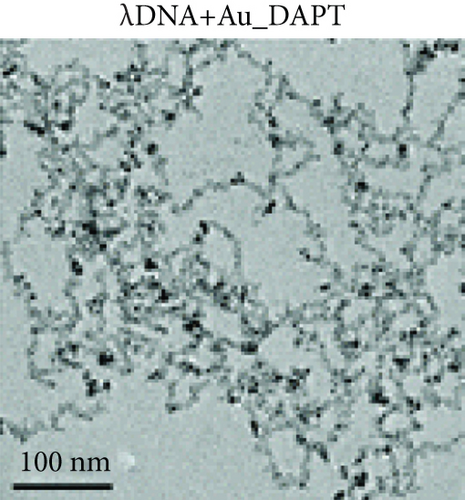
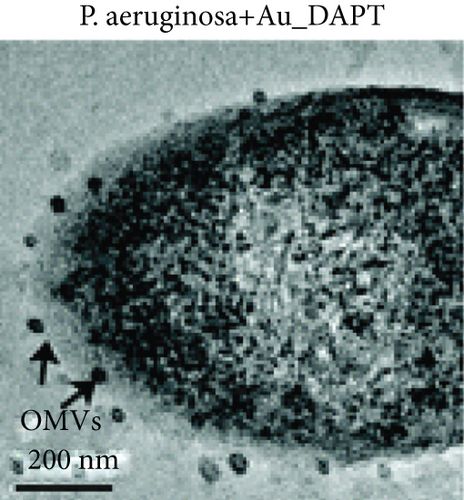

AuNPs increase the permeability of bacterial cell membranes, which cannot only produce antibacterial effects on their own but also enhance the antibacterial effects of antibiotics by combining with them. E. coli is resistant to vancomycin, because vancomycin cannot penetrate the gram-negative bacteria’s outer membrane, but the combination of vancomycin and AuNPs appeared to have an antibacterial effect on E. coli [15]. These AuNPs were densely packed on the outer membrane, and some of them penetrated the cells, suggesting that AuNPs can easily pass through the inner and outer membranes of E. coli, which may damage the stability of the lipopolysaccharide membrane and change the permeability of the cell membrane. In addition, for vancomycin-resistant Staphylococcus aureus (VRSA), vancomycin-coated AuNPs also showed excellent antibacterial effects. Fayaz et al. speculated that the mechanism is that AuNPs nonspecifically bound to the transpeptidase of the VRSA cell wall and effectively lysed the VRSA cell wall [16]. However, some scholars pointed out that the fluorescent dye did not enter the bacteria after treatment with AuNPs, indicating that AuNPs had no effect on the integrity of cell membranes [17].
2.2. Interaction with Biomolecules within Bacteria
Nanoparticles can kill bacteria by inhibiting enzyme activity, binding to DNA, or interfering with protein synthesis [18]. AuNPs neutralized the charge of the plasmid and prevented its movement in E. coli. When the weight ratio of AuNPs to plasmids was 15 : 1, the plasmids could be completely bound by AuNPs with the significantly reduced number of plasmids [12]. Lee et al. further proposed a hypothesis regarding the antibacterial mechanism of bacterial apoptosis-like cell death [17]. AuNPs led to the depolarization of the bacterial cell membrane and the continuous increase of calcium ion concentration in the cytoplasm. Compared with the control group, it was found that a greater degree of nuclear concentration appeared in the E. coli treated with AuNPs, and the degree of DNA fragmentation increased by 19.68%. Subsequently, cell elongation occurred in the process of repairing damaged DNA. All these processes are similar to cell apoptosis [17, 19]. In addition, compared with the control group, the caspase-like protein in E. coli treated with AuNPs was overexpressed and showed stronger protein bands in Western blot analysis. Scholars speculated that caspase-like protein further activated the ROS response and SOS response and induced bacterial apoptosis. These characteristics are similar to those of eukaryotic cell apoptosis; so, they are called bacterial apoptosis-like cell death [17].
2.3. Photothermal Effect
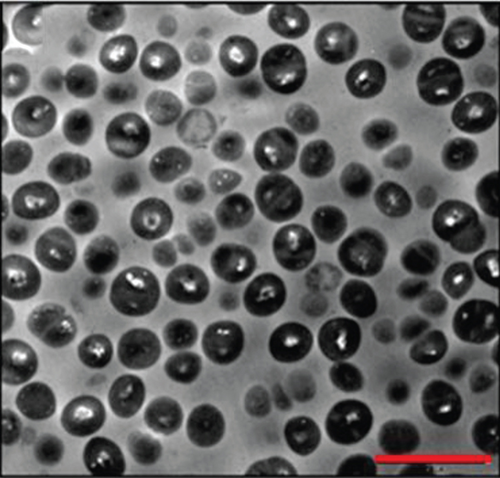
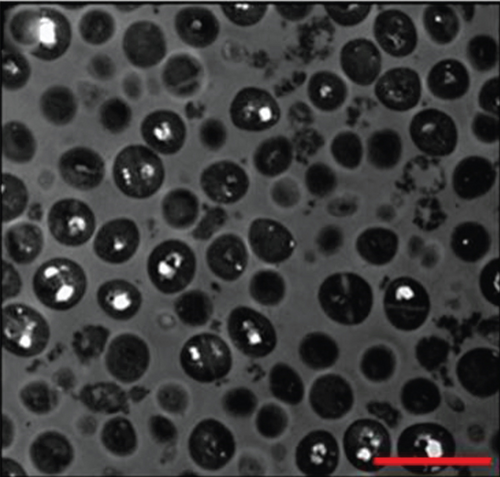
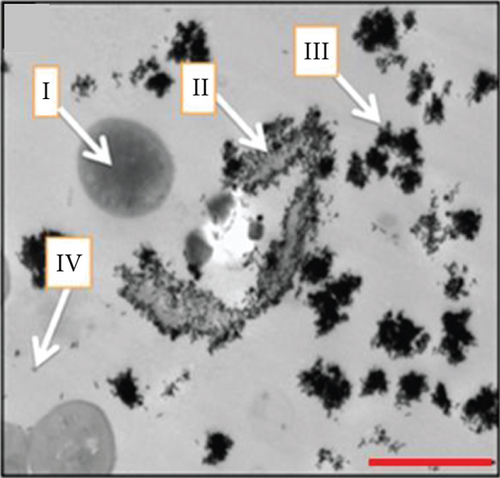
AuNPs have excellent light-to-heat conversion properties and kill bacteria by converting near-infrared light into local heat [20]. Among them, gold nanorods exhibit a high light-to-heat conversion rate [21]. Mahmoud et al. used hydrophilic and hydrophobic gold nanorods to pretreat S. aureus and Propionibacterium acnes (P. Acnes), and the subsequent near-infrared light irradiation significantly reduced the number of bacteria. When the concentration of hydrophilic gold nanorods was 0.25-0.06 nM, near-infrared light irradiation almost completely killed S. aureus and P. Acnes. Compared with the group not irradiated with near-infrared light, the laser irradiation increased the bacterial viable count of this group by at least 4 log10. Although the temperature rise caused by gold nanorods was dose dependent, enough heat can be generated to kill bacteria even at low concentrations. The results seen via transmission electron microscopy (TEM) showed that the local heat generated by laser irradiation caused lysis and the disintegration of bacteria, while the shape of bacteria without gold nanorod treatment was regular and complete, and there was no cell disintegration even after laser irradiation. These results prove that the local heat generated by the gold nanorods after laser irradiation leads to cell lysis and disintegration [22] (Figure 3). Specific mechanisms may include protein denaturation, cell fluid evaporation, and cell structure breakdown [23]. In addition, Zharov et al. observed that bubbles were generated around clustered AuNPs and caused damage to bacteria. During laser irradiation, due to the overheating of the surrounding liquid layer, bubbles were formed around the AuNPs and burst quickly. The lifetime of bubbles with a size between 1 and 8 μm was between 0.1 and 2 μs [24]. The formation of these bubbles damaged the bacterial cell wall and promoted the penetration of the cell wall by nanoparticles. Coupled with the effect of local heat, the bacteria were completely decomposed [25].
2.4. The Redox Imbalance
Excessive reactive oxygen species (ROS) in cells can cause oxidative stress, destroy biological macromolecules, and cause cell apoptosis and necrosis [26]. There is still controversy about whether AuNPs can induce oxidative damage in bacteria. Studies have pointed out that gold ions produced extracellular ROS and further induced the production of intracellular ROS, thereby causing oxidative damage, usually without visible membrane damage [27]. Perni et al. found that adding AuNPs enhanced the bactericidal effect of methylene blue on methicillin-resistant S. Aureus (MRSA) and E. coli, but had no effect on the concentration of singlet oxygen produced by methylene blue. The authors speculated that AuNPs enhanced the light-induced antibacterial activity of methylene blue by increasing the production of ROS other than singlet oxygen [28]. Lee et al. believe that AuNPs triggered redox imbalance by affecting glutathione (GSH) instead of ROS, ultimately leading to oxidative damage. AuNPs did not significantly increase the ROS concentration in E. coli, but reduced GSH concentration, which resulted in the breakdown of the balance between the oxidative system and the antioxidant system in E. coli. This imbalance induced apoptosis, similar to the ROS-independent apoptosis of mammalian cells [17].
2.5. Others
Because of their stable properties and easy modification, AuNPs widely serve as carriers for drugs and can concentrate drugs on the surface, which is called a multivalent effect [29]. The gold nanoclusters loaded with ampicillin and lysozyme demonstrated a long-lasting antibacterial effect on MRSA. This was because the gold nanoclusters increased the local antibiotic concentration by concentrating ampicillin on the surface and enhanced the binding of ampicillin to the bacterial cell wall, thereby improving the bactericidal effect [30]. Similarly, the vancomycin-coated AuNPs interacted multivalently with the bacterial surface receptors, thereby enhancing its antibacterial activity [31].
The formation of bacterial biofilm is crucial to bacterial toxin effects [32]. AuNPs have been reported to destroy the bacterial biofilm to kill bacteria. Ahmed et al. observed that S. aureus that were not treated with AuNPs adhered to each other. But after treatment with AuNPs, the bacteria were dispersed, their spacing increased significantly, and the biofilm was significantly destroyed [27]. In addition, Singh et al. also proposed that AuNPs’ polymers inhibited the biofilm formation by interfering with the quorum sensing system [33]. However, the components in the polymer were mixed, and the AuNPs have not been separated to study their effects alone; so, the mechanism remains to be further confirmed.
Apart from the above mechanisms of antibacterial activity, indepth studies in Candida demonstrated the mechanisms of AuNP antifungal capability as well. One hypothesis suggested that AuNPs are able to interact with the H + -ATPase-mediated proton pump of Candida, therefore disturbing the maintenance of the proton gradient, leading to disorders of nutrient transportation and further fungi death. In addition, gold may interact with sulphur and phosphorus, which compose fungal protein and DNA, directly having a harmful influence on their functions [34]. Another hypothesis indicated that endonuclear AuNPs are capable of inducing karyopyknosis and DNA fragmentation, while cytoplasmic AuNPs interact with mitochondria, leading to the alteration of mass, calcium ion concentration, and the disorder of mitochondrial membrane potential (MMP). The ROS level was found to have been substantially unchanged, suggesting that the fungi-killing effect is ROS independent [35]. Mechanisms of fungal biofilm disintegration can be attributed to the inhibition of adhesion between substrate surfaces and adhesin, which results in the collapse of fungal biofilms and the increase of planktonic fungi [36].
3. Antibacterial Activities against Various Microbial Species
3.1. Staphylococcus aureus
Staphylococcus aureus is a species subject to Firmicutes phylum, contributing to skin mucosa, respiratory tract infection, and other purulent infections [37, 38]. Exotoxins are major virulent factors that cause infection-related diseases [39]. The formation of biofilms enhances bacterial resistance to antimicrobial agents, in which S. aureus communicate with each other via quorum-sensing mechanisms. By secreting phenol-soluble modulins and surfactant peptides, the S. aureus communities are able to regulate and control their status, flexible during times of environmental changes [40]. Due to the abuse of antibiotics, S. aureus has been found to be multidrug-resistant. Among these superbugs, methicillin-resistant Staphylococcus aureus (MRSA) raises a wide range of concerns. First reported in the 1960s, MRSA can encode proteins inducing β-lactam antibiotic resistance. However, recent studies have found that AuNPs are embedded with activity against biofilms and muitidrug-resistant S. aureus, which presents an alternative for treatments against these ubiquitous gram-positive bacteria.
The autologous antimicrobial activity of AuNPs is still in doubt, but some research has indeed observed bacterial growth inhibition after a single AuNP utilization [41, 42]. The high water-soluble, cationic AuNPs containing a hydrophobic end, synthesized by Li et al. [43], show a promising inhibition effect against several multidrug-resistant bacteria. Minimal inhibitory concentrations (MIC) reach 32 nM and 64 nM for S. aureus and MRSA, respectively.
Apart from an autologous antibacterial effect, AuNPs also possess a great capacity to act as carriers of antibacterial agents. Some research reports that AuNPs individually enhance the effectiveness of other antibacterial materials against S. aureus [44, 45]. Stacy Jones et al. [46] generated a kind of cellular structure of graphene oxide membrane, on which chitosan-immobilized AuNPs conjugate. It has been observed that the synthetic products are equipped with significantly stronger antimicrobial capacities. The graphene oxide-based product works in capturing, separating, and killing bacteria, and most interestingly, it possesses excellent activity in the detection and eradication of MRSA.
Some nonantibiotic agents or drugs have been observed to be in conjugation with AuNPs, which exhibit effective bioactivity against S. aureus. In one study, a multivalent aminosaccharide-gold nanoparticle was described as a narrow-spectrum anti-S. aureus agent [47]. In parallel, broad-spectrum antibacterial properties were generated by Zhao et al. [48] through the binding of the antihyperglycemic drug dimethylbiguanide (DMB). Results showed that the MIC of S. aureus and MRSA were 8 and 16 μg/mL with MBC of both 64 μg/mL. Additionally, the antibiofilm activity was embraced by this product. These trials introduced new ideas for the treatment of clinical drug-resistant bacteria.
Due to their special optic properties, AuNPs are capable of inhibiting and killing bacteria by utilizing their optic features. Dyes like toluidine blue, toluidine blue O, methylene blue, curcumin, and triarylmethane are verified photosensitizers generating photodynamic effects for the inhibition of S. aureus or MRSA [28, 49–53]. On the other hand, antibiotics can also be conjugated with AuNPs to kill S. aureus via the photodynamical or photothermal way. It has been observed that amoxicillin-covered AuNPs (amoxi@AuNPs) are promising photosensitizers, which are capable of reducing S. aureus biofilms by 60% under white light [54]. MRSA are evidently inhibited in the same situation [55].
An antibody can specifically kill targeted bacteria; the attachment with AuNPs can also lead to the photothermal killing of S. aureus as well as its biofilms via the immunological way [56–58] (Figure 4).
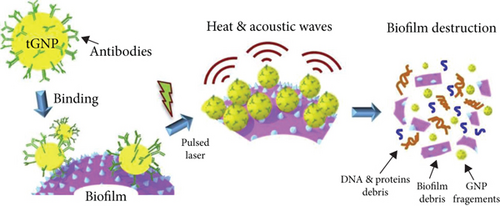
The promising anti-S. aureus activity can contribute to the treatment of skin wound healing. In a study conducted by Mona G. Arafa et al., spherical AuNPs were employed and unemployed in burn wound healing models. In AuNP-treated mouse, the mean wound diameter dropped to zero in ten days, with an average healing speed of 0.9 mm or so per day, while the wound diameter in the normal saline group diminished from 10 mm to around 8 mm after 10 days’ treatment [59]. As for patients suffering from diabetes, AuNPs also have potential in the clinical treatment of secondary superficial infections [60]. In another study, bioglass, the bioactive material related to bone regeneration, demonstrated anti-S.aureus properties produced with the mixture of AuNP. However, poor activity was shown for confronting E.coli [61]. Further studies can focus on approaches to boost the effectiveness of wound healing and find out more applications for eliminating S. aureus-based infections and inflammations.
3.2. Bacillus subtilis
Bacillus subtilis (B. subtilis) is a bacterial species ubiquitous in nature and a kind of probiotic bacteria in the human body. For its ability to produce antibiotics and bacteriocins, B. subtilis has much potential in the inhibition of malignant bacteria [62, 63]. However, in the event of hypoimmunity, such as debilitating diseases and drug abuse, infections caused by B. subtilis are possible [64].
Research has specifically aimed to explore if the anti-Bacilllus subtilis activities of AuNPs are deficient. Compared to common pathogenic bacteria such as S. aureus, for B. subtilis, the effectiveness is relatively reduced. The outcome of Shamaila et al. [65] was the synthesization of AuNPs by sodium borohydride- (NaBH4-) reduced method, which can be divided into two samples: G1 possessed a diameter of 7-34 nm, and G2 had a diameter of 20-40 nm. AuNP solutions were manually categorized into 3 different doses. In the high dose of G1 sample condition, the growth percentages of S. aureus and B. subtilis were 22.4% and 45.2%, respectively, and the growth percentages in the high dose G2 sample were 23.7% and 49.4%, respectively. Horizontal comparison suggests a smaller size with high antibacterial effects. MICs were also measured: MIC of G1 is 7.56 μg/mL, and MIC of G2 is 8.61 μg/mL, double the concentrations of that of S. aureus. Other studies utilized plant-synthesized and bacteria-synthesized AuNPs to test anti-B. subtilis activities. Although there were positive results, the effectiveness was much lower compared with S. aureus [66, 67].
Similarly, AuNPs can form conjugations with other compounds to enhance the ability to inhibit B. subtilis. Acridine derivatives, poly vinyl chloride, tetrazine, graphene oxide, or silk sericin are capped or combined with AuNPs, and corresponding indicators are observed (specific AuNP performances are on Table 1) [68–72]. In addition, a sort of hydrated microgel system with dispersed AuNPs achieves high antibacterial activity [73]. When AuNP/microgel reaches 20%, the inhibition ratio of B. subtilis exceeds 90%, a fairly high percentage and is comparable to the amoxicillin control of this experiment. Moreover, the microgel can play the role of the drug carrier as well.
| Gram-positive bacteria | AuNPs | Characterization of AuNPs or drugs | Antibacterial indicators | Antibacterial effects | References |
|---|---|---|---|---|---|
| Bacillus subtilis | Citrate stabilized AuNP-acridine derivatives: 9-aminoacridine hydrochloride hydrate (9AA-HCl), acridine yellow (AY), acridine orange (AO), and proflflavine (pro) |
|
Inhibition zone (mm) |
|
[68] |
|
AuNP-modified poly(vinyl chloride) (AuNP@PVC) for two types: A ring (PVC+[S- + AuNPs]) and B ring ([PVC+ S-] + AuNPs) | AuNP size: 4-18 nm | Bacterial density after 24 h (A) and 72 h(B): OD600 |
|
[69] |
|
Tetrazine-modified AuNPs (GNP-Tz) react with trans-cyclooctene derivative of vancomycin (Van-TCO) (photothermal therapy) |
|
Bacteria viability | More than 90% are dead after NIR irradiation for 5 min (808 nm, 2 W cm-2) | [70] |
|
|
|
Minimum inhibitory concentration (MIC) (μg/mL) |
|
[74] |
|
Multiresponsive microgel system functionalized AuNP | AuNP diameter: 10 ± 2 nm | Inhibition ratio | Under AuNP/microgel =0.2: Bacillus subtilis, Micrococcus luteus, and listeria monocytogenes reach 90% enterococcus feacalis stays only in 15% | [73] |
| Bacillus subtillis | Colloidal AuNP of two simples: G1 and G2 |
|
Minimum inhibitory concentration (MIC) (μg/mL) |
|
[65] |
|
Peptide (extracted from Vespa orientalis wasp venom) immobilized AuNP | AuNP diameter: 23.2 ± 2.7 nm |
|
|
[75] |
| Listeria monocytogenes | 1018 K6 peptide-stabilized AuNP | NA |
|
|
[76] |
| Streptococcus pneumoniae | Spherical AuNP | Various sizes |
|
AuNPs with the size of 20 ± 0.9 nm and concentration of with concentrations of 1024 mg/mL and 512 mg/mL have the best performance | [77] |
| Bacilllus subtilis MTCC 8364 | AuNP synthesized by the cell lysate supernatant of Bacillus licheniformis |
|
Inhibition zone (mm) | NA | [66] |
| Bacillus licheniformis | Polydisperse, spherical AuNP synthesized by flavonoid tricetin | AuNP diameter: 12 nm |
|
|
[78] |
| Bacillus subtilis | Quaternized carboxymethyl chitosan (QCMC) induced AuNP-graphene oxide conjugation: Au-QCMC-GO (photothermal therapy) | NA | Inhibition zone (mm) |
|
[71] |
| Bacillus subtilis | Silk sericin-capped dispersed spherical AuNPs (S-AuNPs): S-AuNP0.25, S-AuNP0.5, and S-AuNP1 (10 mL sericin with 0.25, 0.5, and 1% wt/vol) | AuNP diameter: <10 nm | MIC | With the addition of S-AuNP, the MIC of streptomycin can be lowered | [72] |
| Listeria monocytogenes |
|
AuNP diameter: 21.7 nm | MIC and MBC (μg/mL) |
|
[79] |
|
AuNP inclusion body | AuNP concentration: 512 μg/mL | NA | NA | [80] |
| Streptococcus pyogenes | Lincomycin capped, polyethylene glycol functionalized AuNP | AuNP diameter: 187.2-242.9 nm | Inhibition zone (mm) | 16 (in the temperature of 40°C and 60°C) | [81] |
| Bacillus subtilis ATCC 21332 | Spherical AuNP synthesized by thyme extract | AuNP diameter: 6-26 nm | MIC and MBC (μg/mL) |
|
[67] |
| S. epidetmitis | Photo-produced gold nanosphere and nanorod | AuNP diameter: 20 nm | Zone of inhibition (mm) | 31 | [82] |
| Group B Streptococcus | β galactosidase-capped spherical AuNP | AuNP diameter: 12-26 nm with average of 19.25 nm | MIC and MBC (μg/mL) |
|
[27] |
Compared with gram-positive bacteria, the structure of peptidoglycan cell walls, which gram-negative bacteria have, may cause differences in the antibacterial effect of AuNPs [83]. For example, it has been found that the ampicillin-loaded AuNPs have a significantly stronger killing effect on S. aureus than on P. aeruginosa and E. coli [84]. Below, we will introduce the antibacterial effect of AuNPs on Gram-negative bacteria in detail.
3.3. Escherichia coli
E. coli is an opportunistic pathogen that can cause various local tissues and organ infections, such as gastrointestinal or urinary tract infections, and even cause a wide range of hospital-acquired infections [85]. The emergence of multidrug resistance in E. coli poses a serious threat to global health. Fortunately, AuNPs have shown strong antibacterial activity against E. coli. Zhou et al. found that AuNPs with concentrations of 0.1 μg/mL and 1.5 μg/mL killed E. coli with an effectiveness as high as 90%, and the TEM images showed cell lysis [86]. Cui et al. pointed out that AuNPs played a role by inhibiting the production of ATP or inhibiting the binding of tRNA to the subunits of ribosomes [87].
In order to enhance the antibacterial activity of AuNPs, various antibacterial substances are loaded onto these particles to form a complex [88]. For example, Ahmady et al. combined AuNPs with lysozyme to form an AuNP-lysozyme complex, which had an inhibitory effect on the activity of E. coli as high as 98%-99% [89]. AuNPs coated with cinnamic acid (CA) have promising effects on neuropathic E. coli K1 [90]. In addition, the combination of antibiotics or antimicrobial peptides with AuNPs is also an effective method to improve the antimicrobial efficiency and bioavailability of drugs [91]. For example, compared with free polymyxin B sulfate, AuNPs-coupled polymyxin B sulfate (PMB-AuNPs) was found to increase the rate of killing E. coli by about 10% [92].
PTT and PDT also occupy a position in anti-E. coli therapy [93]. Luo et al. found that the complex of AuNPs and graphene oxide (GO) (AuNPs-GO) exhibited excellent inhibition properties against both gram-positive and gram-negative bacteria after light exposure [71]. At the same time, the light increased the temperature by 80°C, showing the enhanced photothermal synergy of AuNPs and GO. In another experiment, E. coli was exposed to AuNPs loaded with Coptis chinensis and given light [94]. Compared with AuNPs without illumination, the diameter of the inhibition zone of AuNPs after illumination was enlarged by 30%. The TEM images showed that the E. coli cells had severe morphological deformation after illumination, and the intracellular ROS concentration increased significantly.
3.4. Pseudomonas aeruginosa
Pseudomonas aeruginosa, one of the multidrug-resistant bacteria, is the main cause of infection and death in patients with impaired immune function [95]. P. aeruginosa will cause ventilator-associated pneumonia and other diseases, with an infection rate as high as 30% in the examination rooms of various endoscopes and catheters [96]. Scholars have discovered that AuNPs will produce antibacterial effects on P. aeruginosa through various methods, such as by carrying antibacterial substances including antibiotics, antibacterial peptides, and chitosan and by performing photothermal therapy or photodynamic therapy [97].
AuNPs are able to kill P. aeruginosa without the combination of antibacterial substances [11]. The number of P. aeruginosa after treatment with positively charged AuNPs decreased significantly within 3 hours, which was caused by cell membrane damage. Similarly, Hameed et al. found that after using 0.2 μg/mL and 0.4 μg/mL cubic-shaped AuNPs to treat P. aeruginosa, all bacteria were killed after 30 minutes [98]. The results from transmission electron microscopy (TEM) showed that cell integrity was destroyed, flagella was lost, and nucleic acid leaked outside the cell. In addition to mechanical cutting and destroying the structure of bacteria, AuNPs can also kill bacteria by generating heat or ROS. Norman et al. prepared AuNPs carrying P. aeruginosa-specific antigens [99]. The experiment found that the bacterial viability decreased by 75% with near-infrared light (NIR). Within 10 minutes (Figure 5), the temperature in the light area increased, and a large number of cell membrane ruptures were observed in this area. Zheng et al. found that, after treating P. aeruginosa with Au nanoclusters, more than 90% of the bacteria were killed, and the ROS production increased significantly by 2-3 times [100].
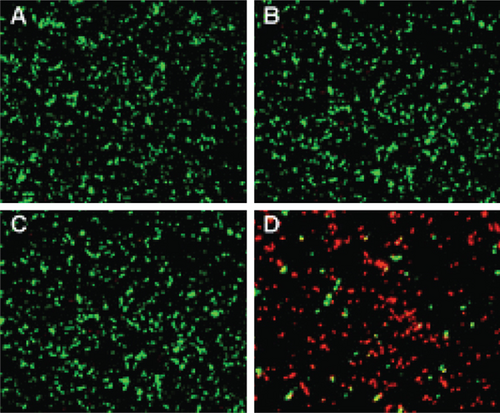
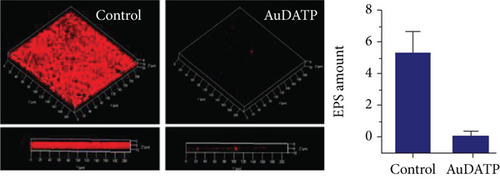
AuNPs can also be loaded with antibacterial substances to resist bacterial resistance, including antibacterial active substances extracted from plants, antibiotics, chitosan, antibacterial peptides, etc. Jobina et al. prepared a complex of phytocompound baicalein and AuNPs and observed that biofilm formation was reduced by 76.51 ± 4.27%, and the production of extracellular polysaccharide (EPS) was significantly reduced by 81.29 ± 2.96% [101]. In addition, the swimming and group movements of bacteria are also effectively prevented. After being combined with spherical AuNPs and rod-shaped AuNPs, the toxicity of imipenem to P. aeruginosa increased by 2.05 times and 7.54 times, respectively [99]. In addition, compared with chitosan alone, the chitosan-AuNP complex exhibited enhanced biofilm inhibition and clearance and significantly reduced the production of hemolytic and virulence factors related to P. aeruginosa [102]. The combined use of antimicrobial peptides and AuNPs also supports the above conclusions [103, 104].
3.5. Porphyromonas gingivalis
P. gingivalis is closely associated with the occurrence and development of periodontal diseases [105]. In addition, P. gingivalis has been found to be closely related to systemic diseases, including atherosclerosis, diabetes, and Alzheimer’s disease [106, 107]. Therefore, it is necessary to research methods for anti-P. gingivalis.
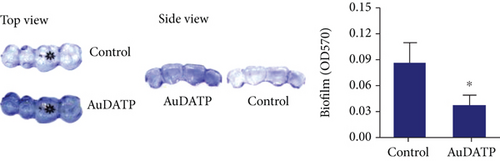
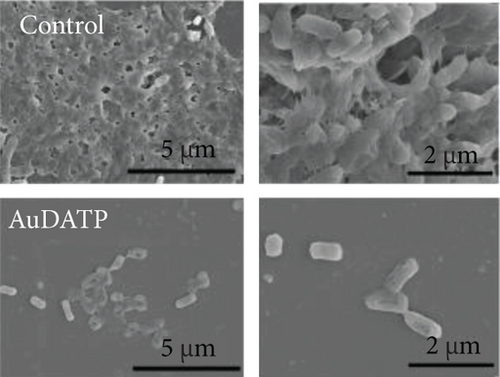
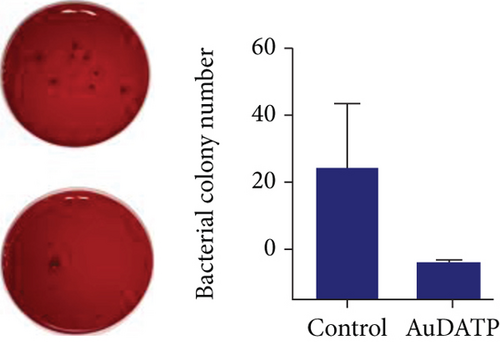
AuNPs are potential drugs for anti-P. gingivalis therapy. Miyata et al. found that AuNP-based nanocomposites had excellent antibacterial strong antibacterial effects on P. gingivalis [108]. They reported that after exposure to 50 and 500 μg/mL Au nanoclusters and blue LED light irradiation, the turbidity of the bacterial suspension decreased significantly, and the bacterial viability was inhibited. In this experiment, AuNPs also showed high antibacterial effects on Streptococcus mutans and Actinomyces. In addition, AuNPs have been used in dental instruments to reduce the incidence of periodontitis in orthodontic patients [109]. Zhang et al. coated the transparent appliance with AuNPs modified with 4,6-diamino-2-pyrimidinethiol (AuDAPT) and found that this transparent appliance had high antibacterial effects on P. gingivalis [110] (Figure 6). The number of suspended bacterial colonies in the biofilm was reduced from 27/μL to 0.6/μL. They believe that, in order to maintain the effective antibacterial concentration of AuDAPT, transparent appliances coated with AuDAPT can be replaced every 1-2 weeks in clinical practice, thereby effectively reducing the incidence of periodontal disease and related systemic diseases in orthodontic patients.
3.6. Fungi
Though small in number, pathogenic fungi can cause troublesome diseases. Among all these kinds, Candida albicans (C. albicans) can cause infectious diseases mostly in the urogenital canal and digestive tract. It leads to infections through various means: changes of host immunity, reconstitution of in situ microbiota, stress, etc. [111, 112]. Ways to deal with fungal infections are limited due to their complex eukaryotic structures, and the formation of biofilms makes it even harder to eradicate them by traditional therapies [113].
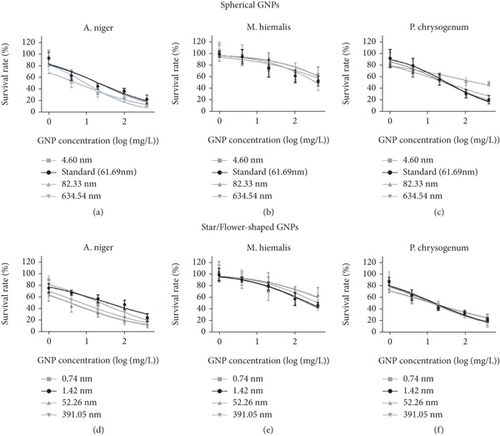
AuNPs are also synthesized and applied in the conflict against harmful fungi. In one study [113], the antifungal effect of AuNPs with diameters of around 40 nm against four species achieved similar outcomes: 2-4 mg/mL for fungous inhibition and 2-8 mg mL for fungus damage. For the shape-dependent antifungal activities, Liu et al. [114] utilized colloidal AuNPs with three shapes (spherical, star, and flower-shaped) to test their different antifungal capabilities. The dose–response curves from this study demonstrated that the AuNP antifungal activities are size dependent, and shapes of AuNPs have less influence (Figure 7), but the observed outcomes indicated poor regularity between size/shape and fungi-damage effect according to IC50, suggesting that the decisive effect may lie in fungi species.
Similarly, AuNPs are able to combine with antifungal drugs to expand their antifungal activity [115]. In terms of drug-resistant fungi (fluconazole-resistant C. albicans for instance), indolicidin-conjugated AuNPs, whose MIC is only a sixth of that of free indolicidin, display high anti-C. albicans effects [116], becoming a new method of t antifungal therapy in burn infections. The use of liposome-carried flucytosine-AuNP had promising effects in the treatment of fungal intraocular endophthalmitis [117]. In addition, photosensitizers like methylene blue (MB), Rose Bengal, and aluminum phthalocyanine are utilized to treat C. albicans (biofilm) infection by coating AuNPs [118–120]. Superficial fungal infections like onychomycosis have received promising results with the application of MB-coated AuNPs [121].
4. Advantages and Concerns in Clinical Use
AuNPs are nontoxic and easy to obtain by diverse synthesis methods, including physical synthesis, chemical synthesis, green synthesis, and other methods [122]. Whether AuNPs themselves have antibacterial activity that is still controversial, but it is undeniable that, because of their good surface modification and high photothermal conversion efficiency, AuNPs have shown great potential in actual antibacterial therapy [72]. As an effective carrier of antibacterial substances, AuNPs not only reduce the necessary doses of antibacterial drugs, thereby reducing the side effects of drugs, but also enhance the overall antibacterial effect [14]. Their antibacterial activity comes from a variety of mechanisms, including interaction with cell barriers or biomolecules within bacteria, which generate heat or induce oxidative damage after exposure to light. Although AuNPs have the above advantages, there are still some problems that need to be solved before AuNPs are used clinically.
First of all, although AuNPs themselves are not toxic, the toxicity of modified AuNPs to normal cells is a huge challenge in the clinical application of AuNPs. The cytotoxicity of AuNPs is affected by surface charge, size, shape, and surface modification [123]. Enea et al. found that rod-shaped AuNPs were more toxic than spherical AuNPs to human cerebral microvascular endothelial cells [124]. In another experiment, Engstrom et al. proved that the toxicity of AuNPs to embryonic zebrafish is size dependent [125]. At the same concentration, the cytotoxicity of smaller AuNPs is higher. In another interesting study, anionic AuNPs proved to be nontoxic to cells, while cations were moderately toxic to all cell lines [126]. In addition, chitosan-coated AuNPs had been shown to be noncytotoxic—even after a long period of exposure, more than 85% of the cells survived [127]. However, PEG-coated AuNPs caused immune responses and apoptosis in mice [128]. Therefore, the cytotoxicity of modified AuNPs is complex and different, and detailed verification is required before clinical use. Secondly, we mentioned earlier the combined use of AuNPs with NIR, for which the operation process was a little complex, which may limit the application of AuNPs [129]. In addition, bacteria may still develop resistance if we rely on existing drugs to modify AuNPs [12].
There is no doubt that the abov-mentioned problems limit the clinical application of AuNPs in reducing bacterial resistance. In addition, how to deliver AuNPs to the area that needs treatment without staying in other healthy areas also needs to be considered [130]. As far as we know, AuNPs have great therapeutic potential for epidermal infections; so, the chemical and physical methods of the surface modification of AuNPs are expected to solve the above problems, which can enhance their antibacterial activity and targeting ability.
5. Conclusion and Perspective
Recent years have witnessed the promising antibacterial activities of AuNPs. Controversial as they are, both individual AuNPs and conjugations containing AuNPs have been verified by a number of experiments for their ability to inhibit certain microbial growths, including some MDR bacteria. In the case of figuring out a drug carrier, the bright point of AuNP falls on its low drug resistance and expanded antibacterial range, paving a new way to confront multidrug-resistant bacteria and reduce the dose of antibiotics. Present studies mostly utilize S. aureus as a substitute for gram-positive bacteria, and E. coli for gram-negative bacteria. This is done, for one reason, to bear out the antimicrobial universality of AuNP, and it is worth considering typical strains for experiments; another reason is, since the antibacterial mechanisms are not completely clear, the actual effects on diverse pathogens differ, and more research on other bacterial species is a necessity.
AuNP is nontoxic to mammal tissues and cells, and in vitro experiments, most of which employed zones of inhibition on culture medium and MIC (MBC) as indicators on effectiveness, have observed positive results. However, in order to step into clinical use, animal models must be established for in vivo antibacterial activity tests. Internal experiments have more to consider: the measures of targeted delivery, clinical and lab indicators of infection inhibition degree, monitoring system for intravital existence forms and stability of AuNPs, and interactions between AuNPs and internal environment, etc. In vivo structural controllability of AuNPs is a valuable issue. In parallel, research on bacteriostatic mechanisms undoubtedly needs further development.
Much attention is paid on the diameter of AuNPs, and smaller one has higher antibacterial activity within limits. However, the morphology of AuNPs is still worth studying. Much research has synthesized spherical AuNPs in their experiments. The fraction of superficial area and volume is a significant factor in their capacity for drug adsorption, but the relation between AuNP forms and direct antimicrobial effect is unclear. Some nanogolds with uncommon appearance are not mentioned for antibacterial application, and their contribution in biomedical fields mainly lies in anticancer therapies. There is some similarity of these AuNPs between anticancer and antibacterial treatment, for instance, AuNP acts as drug carrier and AuNP-based photodynamic therapy; next, we can transfer anticancer application of differently shaped AuNPs to the antibacterial field. When it comes to photodynamic and photothermal therapies, combination of two strategies can be achieved for the treatment of superficial infections; furthermore, given that NIR has relatively low penetrability, methods to treat deep bacterial infections and approaches for precise delivery are urgent problems to be solved. Further research on antibacterial activity and mechanisms of AuNPs is expected to simplify the issue of MDR bacterial infections and pave the way of a new strategy against pathogens.
Conflicts of Interest
The authors declare no conflict of interest.
Authors’ Contributions
Er-Kang Tian, Yue Wang, and Ruiyang Ren contributed equally to this work.




