Neonatal Hypoglycemia Related to Glycine Levels in Uncontrolled Gestational Diabetes Mellitus during Mid-Late Pregnancy: Multicenter, Prospective Case-Cohort Observational Study
Abstract
Aims. To explore the relationship between gestational diabetes mellitus (GDM) and neonatal cord blood amino acid and carnitine levels after GDM was diagnosed among pregnant women monitoring glycosylated haemoglobin levels of 5.5%-6.4% during mid-late gestation. Methods. In all, 7289 qualified participants were recruited and divided into two groups (GDM and control groups) between 1 July 2015 and 1 July 2020, and all maternal-neonatal data were collected and analyzed at three centers. Results. Interestingly, glycine in cord blood was not only significantly different between groups (15.52 vs. 6.67, P < 0.001) but also associated with neonatal hypoglycemia (r = 0.132, P < 0.001). Although glycine was an independent positive factor with neonatal hypoglycemia, it had lacked effective size to predict the risk of neonatal hypoglycemia (b = 0.002, P < 0.001). Conclusion. The study identifies some differences and relationships in maternal-neonatal data when the GDM group has fluctuating glycosylated haemoglobin levels of 5.5%-6.4% without hypoglycemic drug intervention, compared with the control group. Although umbilical cord blood of glycine levels has a lack of effective power to predict the risk of neonatal hypoglycemia, it is probably an independent factor involved in the maternal-neonatal glucolipid metabolism.
1. Introduction
Maternal disorder of glucose metabolism probably affected maternal-neonatal health and metabolism during pregnancy [1, 2]. Meanwhile, on the basis of the International Association of Diabetes and Pregnancy Study Groups (IADPSG), over 17% of pregnant women would be diagnosed with gestational diabetes mellitus [3]. Therefore, GDM could be a common disturbance of carbohydrate metabolism in pregnant women and caused adverse pregnancy outcomes, such as maternal preeclampsia and neonatal hypoglycemia according to the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study [4].
All pregnant women in GDM would accept the dietary therapy and physical exercise treatment after the strict diagnosis standard of OGTT according to IADPSG. Additionally, glycosylated haemoglobin (HbA1c) levels were widely used for diabetic control. Some pregnant women were poor with the glucose regulation control based on the IADPSG recommendation range of HbA1c levels below 5.5% during mid-late pregnancy. Meanwhile, those pregnant women in GDM were probably reluctant to accept drugs such as insulin even for serious uncontrolled hyperglycemia.
According to IADPSG and American Diabetes Association (ADA), HbA1c levels could be a useful and effective evaluation to blood glucose control, but it was still an argument as to what range was appropriate and what therapeutic schedule should be used on a critical range. It was considered that worse glucose control as HbA1c levels between 5.5% and 6.4% among pregnant women with GDM,and it was unnecessary for further treatment, immediately. In China and India, most pregnant women were recommended to follow IADPSG because of racial and economic factors [5].
Pregnancy complicated with diabetes, with out of control blood glucose levels as an obvious dysglycemia, it was associated with a highly increased risk of adverse pregnancy outcomes, such as neonatal hypoglycemia [6, 7]. If pregnant women in GDM appeared with HbA1clevels ≥ 6.5%, it also was diagnosed as pregestational diabetes mellitus (PGDM) following the standard of IADPSG. Insulin as a first-line treatment should be considered among those pregnant women. However, mid-late pregnancy maternal blood glucose monitoring by HbA1c fluctuated in 5.5%-6.4% which was an unclear and potentially risky range, especially for those pregnant women in GDM.
Some recent studies reported that maternal-neonatal metabolites were correlated with insulin resistance and diabetes [8, 9]. For example, fetal carbohydrates [10], lipid profiles [11], carnitines [12], and amino acids [13], particularly branched-chain amino acids [14] and aromatic amino acids [15], could be influenced by GDM. However, little articles reported the change and relationship of neonatal amino acids and carnitines when maternal consistent dysglycemia because of the failure of glucose control during pregnancy.
On the other hand, maternal glucose homeostasis tended to remain elevated because placental hormones and maternal insulin resistance occurred particularly in mid-late pregnancy [16, 17], but it was still unclear. Maternal dysglycemia had been proven to be a contribution for fetal macrosomia and neonatal hypoglycemia [18]. However, the exact effect of how maternal glycolipid dysfunction influenced neonatal metabolism in offspring remained unclear because it was rarely reported and early interfered by researchers. In the study, we carried out a prospective observational study to explore the associations between GDM in dysfunction status and neonatal amino acids and carnitines when GDM monitored by HbA1c average levels was between 5.5% and 6.4%.
Therefore, the first research shows a rigorous accurate study on the part of maternal-neonatal metabolism with GDM ranged HbA1c average levels between 5.5% and 6.4% because of the elimination of drug interference and the safety of pregnancy. There was also a further study on the argument of the failure of controlling glucose when HbA1c average levels were between 5.5% and 6.4% during mid-late pregnancy and maternal-neonatal metabolism and outcomes. It may provide effective evidence for the prevention of GDM and adverse maternal-neonatal outcomes and a new perspective for understanding the mechanism of maternal and neonatal metabolism.
2. Materials and Methods
2.1. Ethics Statement
The present study had been approved by the ethical committees of the Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai People’s Hospital, and Zhuhai Center for Maternal and Child Health Care, and all participants provided written informed consent.
All subjects were informed that they would be followed up and collected blood sample. It was none of interference and difference compared with daily clinical advice and treatment in the follow-up.
2.2. Sample Size
According to the multicenter prospective cohort study of 1813 pregnant women, the OR rate of neonatal hypoglycemia (OR 4.86 [95% CI 2.04, 11.53]) was used to determine the sample size [19]. We estimated that 1107 patients per group would be required to find the morbidity of hypoglycemia in neonates approximately being 3% in the control group using two tailed α = 0.05 and one-sided β = 0.10 and the power of test = 0.90. We increased the study size to 1200 per group to allow for possible dropouts or patient loss as a result of a clinical situation.
2.3. Study Design
All participants who delivered in our centers between 1 July 2015 and 1 July 2020 were willing to engage in the follow-up, test HbA1c levels each month at least three times and donate maternal-neonatal blood sample during mid-late pregnancy. Meanwhile, with more and more large-scale multicenter clinical research studies, the importance of quality management was needed. Those hospitals were the third grade hospitals which had the same clinical protocol for follow-up and collected all blood samples to the key national laboratory for storage and analyzation.
First, potential participants that meet the inclusion criteria would be invited by research assistants, and the objective, procedure, benefits, and risks of the study would be explained. Pregnant women would be invited to participate in this study if they were between 18 and 40 years of age and had had their OGTT in our centers before 24-28 weeks of gestation.
There was a clear recommendation to screen for GDM in high-risk pregnant women using the IADPSG and ADA criteria, and thus, pregnant women would be excluded from the study if they had any of the following risk factors or conditions: (1) history of GDM or preexisting diabetes mellitus (DM); (2) family history of DM (first-degree relative with diabetes or a sister with GDM); (3) body mass index (BMI) > 30 kg/m2 before pregnancy; (4) previous macrosomia (baby with birthweight > 4000g) or a history of stillbirth; (5) polycystic ovary syndrome; (6) medications: corticosteroids, antipsychotics, and drugs influencing glycolipids such as statins; (7) participant not willing to take OGTT at 24-28 gestational weeks and HbA1c levels each month until labor (less three times), not willing to have a series of prenatal care visits and delivery, or refusing blood sample collection during mid-late pregnancy.
2.4. Diagnosis Standards and Medical Care
The diagnosis of GDM was based on a 75 g oral glucose tolerance test (OGTT) performed between 24 and 28 gestational weeks, according to IADPSG criteria (fasting ≥ 5.1mmol/L, 1h ≥ 10.0mmol/L, and 2h ≥ 8.5mmol/L). Recruited subjects accepted the standard of treatment for GDM.
The diagnosis of neonatal hypoglycemia was based on a fingertip blood glucose test after labor within 5 minutes, according to the American Academy of Pediatrics criteria (fingertipbloodglucose < 2.22mmol/L) [20].
2.5. Blood Sample Collection
In brief, all pregnant women had blood tests from the prenatal examination or hospitalization examination before their delivery. All neonates included in our study had amino acids and carnitines measured in dried blood specimens collected from umbilical cord blood after the second stage within 10 minutes.
2.6. Study Clinical Test
All subjects took blood tests including OGTT, HbA1c, and lipid tests such as triglycerides (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C).
Blood samples in the anticoagulative tubes for measuring glycated haemoglobin, lipid profiles, and glucose in plasma were sent to the clinical laboratory within 1 hour of collection.
Maternal glucose levels were measured using a GOD-PAP kit (Human, Shanghai, China). Glycated haemoglobin was tested by an ADAMS™ A1c HA-8180 kit (Arkray USA Inc., Kyoto, Japan) and based on the standardization of the National Glycohemoglobin Standardization Program and Diabetes Control and Complications Trial. Triglyceride (TG) levels were measured using a glycerol phosphate oxidase test kit (Shanghai, China). Total cholesterol (TC) levels were measured using an oxidase test kit (Human, Shanghai, China). High-density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein-cholesterol (LDL-C) levels were determined by direct method kits (Human, Shanghai, China).
2.7. Laboratory Measurements
Neonatal blood was collected and analyzed for 17 types of amino acids and 30 types of carnitines after the delivery of the placenta.
A single 3.2 mm diameter dried newborn’s blood disc per sample was punched from the dried blood spot card and extracted with 90 μL of methanol (including internal standards from Cambridge Isotope Inc.) at 30°C for 30 minutes. After centrifugation at 2000 g for 5 minutes, 50 μL of supernatant was transferred and nitrogen-dried at room temperature, followed by addition of 50 μL of derivatization reagent (acetyl chloride : butanol (v : v) =1 : 9), incubation at 60°C for 30 minutes, nitrogen-drying, and reconstitution with 75 μL of 80% acetonitrile/H2O (v/v). After centrifugation at 4000 g for 15 minutes, 50 μL of supernatant was taken for liquid chromatographic-mass spectrometric (LC-MS) analysis.
An Acquity UPLC I-Class Xevo TQD mass spectrometer (Waters) was used to measure the concentration levels of multiple amino acids and carnitines in the dried blood spot with the application of direct flow injection and multiple reaction monitoring (MRM). The sample was introduced into the electrospray ionization interface by the mobile phase of 80% acetonitrile/H2O (v/v) with scheduled flow rates. The mass spectrometer was tuned as recommended by the manufacturer with resolution, capillary voltage, source temperature, and desolvation temperature set at 0.7 Da, 3.5 kV, 120°C, and 350°C, respectively. All the targeted analytes were quantitatively measured according to the ion pair transitions of both their own and corresponding internal standards. The MassLynx NT 4.1 Software Suite was used to control the instrument and for data processing and analysis.
2.8. Clinical Data
Clinical data, including age, gestational weeks, BMI, mode of delivery, fetal birth weight, and maternal-neonatal outcomes, were obtained from subjects and measured by researchers.
2.9. Statistical Analysis
Continuous variables and categorical variables are presented as the median (25th, 75th percentile) or mean (SD) and counts (percentage) as appropriate. Differences between two groups were analyzed with the Mann-Whitney U test, t test, or chi-square test. Correlations between maternal data or neonatal clinical data and fetal blood test indexes were analyzed by the Spearman rank correlation test. Significance was assumed at P < 0.05. Then, an ANCOVA analysis was performed to study the relationship between neonatal hypoglycemia and neonatal blood test by adjusting other confounders. Statistical analysis was performed with SPSS for Windows software (version 22, SPSS, IBM, New York, NY).
3. Results
3.1. Baseline Characteristics
Figure 1 showed 7289 pregnant women with fully completed blood tests in those diagnosed with GDMa and non-GDM (1116 cases and 6173 cases, respectively) in the study. The GDMa group had tested HbA1c at three times to evaluate diabetic dysfunction and the prevention of delaying treatment for pregnant women. Meanwhile, the control group had the HbA1c test for three times as well.
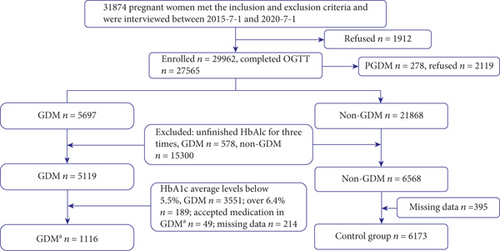
Compared with noncases, participants who developed GDM-monitoring HbA1c levels within 5.5%-6.4% were more likely to be older, have higher antepartum BMI, and have higher incidence of hypoglycemia in neonates as well as premature but less gestational weeks (Table 1).
| GDM | Control | χ2 | P | |
|---|---|---|---|---|
| N | 1116 | 6173 | ||
| Maternal age (years) | 29.6 ± 3.56 | 28.32 ± 3.00 | — | <0.001 |
| Gestational weeks (days) | 267.08 ± 10.82 | 274.87 ± 12.23 | — | <0.001 |
| Body mass index (kg/m2) | ||||
| Prepregnancy | 23.19 ± 3.33 | 23.21 ± 3.27 | — | 0.723 |
| Antepartum | 26.25 ± 3.46 | 27.14 ± 3.56 | — | <0.001 |
| Mode of delivery, no. (%) | 5.974 | 0.113 | ||
| Spontaneous vaginal | 722 (64.7) | 4074 (66.0) | ||
| Assisted vaginal | 93 (8.3) | 395 (6.4) | ||
| Elective cesarean | 172 (15.4) | 944 (15.3) | ||
| Emergency cesarean | 129 (11.6) | 760 (12.3) | ||
| Neonatal outcomes | ||||
| Gender, no. (%) | 0.008 | 0.931 | ||
| Male | 581 (52.1) | 3205 (51.9) | ||
| Female | 535 (47.9) | 2968 (48.1) | ||
| Birth weight (g) | 3242.49 ± 456.30 | 3198.92 ± 449.14 | — | 0.004 |
| Hypoglycemia, no. (%) | 145 (13.4) | 123 (2.0) | 322.926 | <0.001 |
| Premature, no. (%) | 166 (14.9) | 362 (5.7) | 114.199 | <0.001 |
| Meconium-stained amniotic fluid, no. (%) | 5.677 | 0.128 | ||
| Clear | 812 (72.8) | 4432 (71.8) | ||
| I | 218 (19.5) | 1346 (21.8) | ||
| II | 65 (5.8) | 315 (5.1) | ||
| III | 21 (1.9) | 80 (1.3) | ||
| Bad outcomes after delivery, no. (%) | ||||
| Apgar ≤ 7 at 1 min | 36 (3.2) | 278 (4.5) | 3.743 | 0.053 |
| Apgar ≤ 7 at 5 min | 7 (0.6) | 48 (0.8) | 0.285 | 0.593 |
| Apgar ≤ 7 at 10 min | 7 (0.6) | 40 (0.6) | 0.006 | 0.936 |
| Admission to intensive care unit | 9 (0.8) | 56 (0.9) | 0.108 | 0.742 |
- Data given as the mean ± SD or no. (%). GDM: gestational diabetes mellitus; BMI: body mass index.
3.2. Comparison of Maternal and Fetal Blood Tests between Groups
Considering the maternal blood test levels during mid-late pregnancy in Table 2, all maternal blood glucose test results in the GDM group were higher than those in the non-GDM group (all P < 0.001). Meanwhile, HbA1c levels were averaged by three or four times in two groups and TG levels were slightly higher in the GDM group (4.87 vs. 4.39, P < 0.001).
| All groups | GDM | Control | P | |
|---|---|---|---|---|
| OGTT (mmol/L) | ||||
| Fasting blood glucose | 4.45 ± 0.66 | 5.35 ± 0.91 | 4.29 ± 0.44 | <0.001 |
| 1-hour postprandial blood glucose | 7.55 ± 1.23 | 10.1 ± 0.93 | 7.09 ± 0.50 | <0.001 |
| 2-hour postprandial blood glucose | 6.37 ± 1.32 | 8.48 ± 1.56 | 5.99 ± 0.81 | <0.001 |
| HbA1c (average, %) | 4.95 ± 0.49 | 5.96 ± 0.20 | 4.76 ± 0.25 | <0.001 |
| Plasma lipid test (before delivery, mmol/L) | ||||
| TG | 4.44 (3.7, 5.19) | 4.87 (3.86, 5.88) | 4.39 (3.69, 5.1) | <0.001 |
| TC | 6.27 (5.34, 7.17) | 6.31 (5.34, 7.23) | 6.26 (5.34, 7.16) | 0.437 |
| HDL-C | 2.05 (1.59, 2.57) | 2.07 (1.57, 2.56) | 2.05 (1.6, 2.57) | 0.640 |
| LDL-C | 2.98 (2.06, 3.87) | 2.95 (2.05, 3.93) | 2.98 (2.06, 3.86) | 0.694 |
| Cord blood test (μM) | ||||
| Gly | 7.53 (1.71, 308.40) | 15.52 (2.37, 346.09) | 6.67 (1.63, 298.50) | <0.001 |
| Isovalerylcarnitine | 0.11 (0.08, 0.15) | 0.12 (0.09, 0.25) | 0.1 (0.08, 0.14) | <0.001 |
| Octenylcarnitine | 0.07 (0.05, 0.10) | 0.08 (0.05, 0.14) | 0.07 (0.05, 0.09) | <0.001 |
| Palmitoylcarnitine | 1.66 (1.05, 2.25) | 1.52 (0.92, 2.12) | 1.68 (1.08, 2.28) | <0.001 |
| Octadecylcarnitine | 0.64 (0.47, 0.82) | 0.68 (0.49, 0.96) | 0.63 (0.47, 0.80) | <0.001 |
| Octadecenoylcarnitine | 0.9 (0.68, 1.18) | 0.94 (0.68, 1.26) | 0.89 (0.68, 1.17) | 0.001 |
- Data given as the mean ± SD in maternal glucose profiles and as the median (25th, 75th) in others. GDM: gestational diabetes mellitus; HbA1c: glycosylated haemoglobin; OGTT: oral glucose tolerance test; TG: triglyceride; TC: total cholesterol; HDL-C: high-density lipoprotein-cholesterol; LDL-C: low-density lipoprotein-cholesterol; Gly: glycine.
Additionally, several long-chain acylcarnitines including isovalerylcarnitine, octenylcarnitine, palmitoylcarnitine, octadecylcarnitine, and octadecenoylcarnitine were also significantly different between two groups as well as glycine (all P < 0.05). The baseline concentrations of other carnitine species and amino acids in neonatal blood are presented in Table 3.
| All groups | GDM | Control | P | |
|---|---|---|---|---|
| HbA1c (24-28 weeks, %) | 4.97 ± 0.69 | 6.15 ± 0.17 | 4.76 ± 0.52 | <0.001 |
| HbA1c (28-32 weeks, %) | 5.08 ± 0.72 | 6.15 ± 0.17 | 4.69 ± 0.35 | <0.001 |
| HbA1c (32-36 weeks, %) | 4.94 ± 0.48 | 5.53 ± 0.78 | 4.8 ± 0.2 | <0.001 |
| HbA1c (average before delivery, %) | 4.93 ± 0.78 | 5.91 ± 1.17 | 4.75 ± 0.52 | <0.001 |
| Cord blood test (μM) | ||||
| Ala | 214.16 (160.26, 301.23) | 212.91 (160.3, 301.55) | 214.33 (160.23, 301.01) | 0.824 |
| Arg | 2.06 (1.34, 3.5) | 2 (1.38, 3.58) | 2.07 (1.33, 3.47) | 0.356 |
| Asp | 27.95 (19.38, 38.45) | 28.1 (19.51, 39.22) | 27.92 (19.36, 38.33) | 0.717 |
| Cit | 9.04 (7.35, 11.12) | 9.05 (7.3, 11.06) | 9.04 (7.35, 11.13) | 0.962 |
| Gln | 2.52 (1.39, 7.67) | 2.55 (1.41, 7.52) | 2.51 (1.38, 7.69) | 0.828 |
| Glu | 30.31 (2.29, 392.88) | 42.51 (2.25, 405.86) | 29.5 (2.3, 387.7) | 0.669 |
| His | 376.68 (218.92, 536.89) | 379.85 (218.63, 535.29) | 376.19 (218.92, 537.25) | 0.816 |
| Leu | 314.34 (125.43, 456.15) | 301.37 (112.69, 456.96) | 316.4 (128.63, 456.02) | 0.056 |
| Met | 28.43 (19.95, 39.82) | 27.87 (19.38, 39.51) | 28.5 (20.05, 39.9) | 0.463 |
| Orn | 87.64 (61.97, 111.26) | 85.71 (60.1, 110.02) | 87.92 (62.61, 111.36) | 0.184 |
| Phe | 27.07 (21.12, 40.02) | 27.66 (21.29, 43.02) | 26.99 (21.08, 39.43) | 0.142 |
| Ser | 61.43 (45.07, 87.31) | 63.63 (46.22, 89.01) | 61.03 (44.85, 86.95) | 0.054 |
| Thr | 49 (31.95, 67.83) | 48.9 (32.25, 66.37) | 49.01 (31.92, 68.14) | 0.926 |
| Trp | 85.6 (62.89, 109.61) | 84.87 (62.39, 110.19) | 85.66 (63.05, 109.47) | 0.560 |
| Tyr | 48.23 (34.32, 61.86) | 48.81 (34.03, 62.44) | 48.11 (34.38, 61.74) | 0.343 |
| Val | 56.06 (42.67, 71.32) | 56.79 (43.14, 71.62) | 55.79 (42.61, 71.23) | 0.157 |
| Free carnitine | 54.75 (38.34, 71.5) | 54.84 (38.07, 73.18) | 54.72 (38.42, 71.22) | 0.962 |
| Acetylcarnitine | 30.7 (20.81, 40.73) | 29.96 (19.94, 40.09) | 30.88 (20.99, 40.89) | 0.147 |
| Propionylcarnitine | 4.22 (1.82, 19.04) | 4.22 (1.9, 18.43) | 4.22 (1.81, 19.15) | 0.986 |
| Malonylcarnitine | 0.05 (0.04, 0.13) | 0.05 (0.04, 0.13) | 0.05 (0.04, 0.13) | 0.975 |
| Butylcarnitine | 0.13 (0.09, 0.18) | 0.13 (0.09, 0.18) | 0.13 (0.09, 0.18) | 0.661 |
| Succinylcarnitine | 0.19 (0.14, 0.26) | 0.19 (0.15, 0.26) | 0.19 (0.14, 0.26) | 0.847 |
| 3-Hydroxyisobutyryl-carnitine | 0.1 (0.07, 0.16) | 0.11 (0.07, 0.16) | 0.1 (0.07, 0.15) | 0.606 |
| Senecioylcarnitine | 0.03 (0.02, 0.03) | 0.03 (0.02, 0.03) | 0.03 (0.02, 0.03) | 0.448 |
| Glutarylcarnitine | 0.03 (0.02, 0.05) | 0.03 (0.02, 0.05) | 0.03 (0.02, 0.05) | 0.596 |
| 3-Hydroxyisovaleryl-carnitine | 0.14 (0.11, 0.17) | 0.13 (0.11, 0.17) | 0.14 (0.11, 0.17) | 0.849 |
| Caproylcarnitine | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.964 |
| Hexenoylcarnitine | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.059 |
| Adipoylcarnitine | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | 0.341 |
| Decoylcarnitine | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) | 0.363 |
| Octanedioylcarnitine | 0.02 (0.01, 0.02) | 0.02 (0.01, 0.02) | 0.02 (0.01, 0.02) | 0.421 |
| Decanoylcarnitine | 0.04 (0.02, 0.07) | 0.04 (0.02, 0.07) | 0.04 (0.02, 0.07) | 0.579 |
| Alkaloidcarnitine | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) | 0.102 |
| Lauroylcarnitine | 0.06 (0.04, 0.09) | 0.06 (0.04, 0.1) | 0.06 (0.04, 0.09) | 0.199 |
| Myrcenecarnitine | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.410 |
| Myristoylcarnitine | 0.14 (0.11, 0.19) | 0.15 (0.11, 0.19) | 0.14 (0.11, 0.19) | 0.591 |
| Alkenyl-myristoyl-carnitine | 0.05 (0.03, 0.08) | 0.05 (0.03, 0.08) | 0.05 (0.03, 0.08) | 0.226 |
| 3-Hydroxy-myristoyl-carnitine | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.02) | 0.282 |
| Palmitoyl-enoyl-carnitine | 0.09 (0.05, 0.13) | 0.09 (0.05, 0.13) | 0.09 (0.05, 0.13) | 0.877 |
| 3-Hydroxy-palmitoyl-carnitine | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.428 |
| 3-Hydroxy-octadecanoyl-carnitine | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.756 |
- Data given as the mean ± SD in maternal glucose profiles and as the median (25th, 75th) in others. GDM: gestational diabetes mellitus; HbA1c: glycosylated haemoglobin; Ala: alanine; Arg: arginine; Asp: aspartic acid; Cit: citrulline; Gln: glutamine; Glu: glutamic acid; His: histidine; Leu: leucine; Met: methionine; Orn: ornithine; Phe: phenylalanine; Ser: serine; Thr: threonine; Trp: tryptophan; Tyr: tyrosine; Val: valine.
3.3. Association between Maternal-Fetal Clinical Data and Blood Tests
Our data showed that neonatal hypoglycemia were positively correlated with GDM (r = 0.210, P < 0.001) and HbA1c average levels (r = 0.127, P < 0.001) but negatively correlated with gestational weeks (r = −0.101, P < 0.001). Meanwhile, compared with the premature correlation coefficient, neonatal hypoglycemia was stronger with maternal diabetic disorder (Table 4).
| Neonatal hypoglycemia | Premature | |||
|---|---|---|---|---|
| r | P | r | P | |
| GDM | 0.210 | <0.001 | 0.125 | <0.001 |
| Maternal age | 0.017 | 0.139 | 0.014 | 0.243 |
| Gestational weeks | -0.101 | <0.001 | -0.449 | <0.001 |
| Antepartum BMI | -0.019 | 0.107 | -0.025 | 0.032 |
| Maternal HbA1c (average) | 0.127 | <0.001 | 0.079 | <0.001 |
| TG | 0.020 | 0.092 | 0.014 | 0.233 |
- GDM: gestational diabetes mellitus; BMI: body mass index; HbA1c: glycosylated haemoglobin; TG: triglyceride.
Then, Table 5 summarizes the association between the maternal-fetal characteristics, and blood tests in those with significant differences were analyzed. Only GDM and HbA1c average levels were associated with those carnitine and glycine levels (r = 0.084, P < 0.001, and r = 0.064, P < 0.001), while palmitoylcarnitine, octadecylcarnitine, and octadecenoylcarnitine were negative with neonatal hypoglycemia. On the other hand, fetal blood glycine, isovalerylcarnitine, and octenylcarnitine levels were all positively correlated with maternal-neonatal glycolipid dysfunction including GDM, HbA1c average levels, TG, and neonatal hypoglycemia (all P < 0.05).
| Glycine | Isovalerylcarnitine | Octenylcarnitine | Palmitoylcarnitine | Octadecylcarnitine | Octadecenoylcarnitine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| GDM | 0.084 | <0.001 | 0.142 | <0.001 | 0.158 | <0.001 | -0.071 | <0.001 | 0.074 | <0.001 | 0.036 | 0.002 |
| Maternal age | -0.036 | 0.002 | 0.003 | 0.822 | 0.005 | 0.652 | 0.002 | 0.867 | 0.017 | 0.139 | 0.005 | 0.698 |
| Gestational weeks | -0.027 | 0.023 | -0.035 | 0.003 | -0.051 | <0.001 | 0.017 | 0.136 | -0.010 | 0.374 | -0.014 | 0.240 |
| Antepartum BMI | 0.009 | 0.465 | -0.006 | 0.593 | -0.022 | 0.060 | 0.002 | 0.895 | -0.026 | 0.028 | -0.015 | 0.191 |
| Maternal HbA1c (average) | 0.064 | <0.001 | 0.085 | <0.001 | 0.112 | <0.001 | -0.048 | <0.001 | 0.047 | <0.001 | 0.029 | 0.012 |
| TG | -0.025 | 0.031 | 0.072 | <0.001 | 0.063 | <0.001 | -0.044 | <0.001 | 0.025 | 0.034 | -0.001 | 0.901 |
| Neonatal hypoglycemia | 0.132 | <0.001 | 0.035 | 0.002 | 0.040 | 0.001 | -0.022 | 0.058 | 0.014 | 0.246 | 0.014 | 0.226 |
| Premature | 0.045 | <0.001 | 0.007 | 0.551 | 0.260 | 0.026 | -0.005 | 0.686 | 0.014 | 0.238 | 0.016 | 0.183 |
- GDM: gestational diabetes mellitus; BMI: body mass index; HbA1c: glycosylated haemoglobin; TG: triglyceride.
For further study, an ANCOVA analysis was performed to study the relationship between neonatal hypoglycemia and amino acid or carnitine levels in offspring, by adjusting related factors. Only glycine levels were significantly related with neonatal hypoglycemia and obviously elevated in umbilical plasma of neonates born to GDM mothers (15.52 vs. 6.67, b = 0.002, P < 0.001) (Table 6). Meanwhile, isovalerylcarnitine and octenylcarnitine were associated with maternal TG levels but only slightly higher than the control group.
| Glycine | Isovalerylcarnitine | Octenylcarnitine | ||||
|---|---|---|---|---|---|---|
| b | P | b | P | b | P | |
| GDM | 0.001 | 0.026 | 0.027 | <0.001 | 0.027 | <0.001 |
| Maternal age | 0.005 | <0.001 | — | — | — | — |
| Gestational weeks | — | 0.644 | — | 0.235 | — | 0.115 |
| Antepartum BMI | — | — | — | — | — | 0.258 |
| Maternal HbA1c (average) | — | 0.357 | — | 0.354 | — | 0.360 |
| TG | 0.005 | <0.001 | 0.041 | <0.001 | 0.040 | <0.001 |
| Neonatal hypoglycemia | 0.002 | <0.001 | — | 0.089 | — | 0.124 |
| Premature | 0.001 | 0.021 | — | — | — | 0.423 |
- GDM: gestational diabetes mellitus; BMI: body mass index; HbA1c: glycosylated haemoglobin; TG: triglyceride.
4. Discussion
Fetal metabolism, as indicated by carnitine levels and amino acids, may be related to the risk of preterm infants, maternal gestational age, and neonatal development [21, 22]. Dysglycemia was an important link between maternal serum amino acids and carnitines [23], maternal obesity/overweight [24], and maternal and fetal lipid profiles [25]. We explored the relationship between GDM and their neonatal carnitine levels and amino acids to figure out how to influence fetal outcomes.
Fetal development was influenced by neonatal and maternal metabolism [26, 27], and in the present study, we analyzed the effects of neonatal carnitine levels and amino acids on neonatal growth parameters. We found that cord blood glycine levels were different between two groups and correlated with maternal glucolipid when HbA1c levels were between 5.5% and 6.4%. Meanwhile, glycine levels in umbilical cord blood were also correlated with neonatal glucose dysfunction.
The association between maternal glycolipid metabolism and neonatal development had been reported in previous studies [28], and our results supported previous studies in a larger study population. There were 17 types of amino acids and 30 types of carnitines in cord blood to be analyzed, and it was the first original study to fully analyze those neonatal amino acid and carnitine metabolisms compared with previous studies [29]. However, the negative associations were between carnitine levels and neonatal hypoglycemia. The results in the present study not only found that glycine levels had an independently positive relationship with neonatal hypoglycemia but also firstly revealed the association between neonatal hypoglycemia and neonatal glycine levels with maternal glucose dysfunction in mid-late gestation in a new research population.
The result of the present study mainly showed that, although neonatal hypoglycemia was positively correlated with glycine levels in umbilical cord blood, it had a pretty low effect size to hypoglycemia in offspring. Unfortunately, glycine levels were probably not an effective biomarker for predicting the risk of neonatal hypoglycemia. Meanwhile, these findings also implicitly expressed that neonatal hypoglycemia was influenced by multi-maternal-neonatal factors and an extremely complicated multifactor, multivariable, and multilevel dynamic process and even multisystems, especially placenta.
Additionally, it was clearly proven that the incidence of adverse neonatal outcomes was higher when HbA1c levels ranged 5.5%~6.4% among pregnant women in GDM, and GDM can affect neonatal amino acid and carnitine levels. It was the first research to reveal how to influence fetal outcomes and specific amino acid and carnitine levels in offspring. It ineffectively predicted the risk of hypoglycemia for newborns, but it was confidently involved in the biological process on neonatal hypoglycemia.
There was no doubt that amino acid is the basic unit of protein and is involved in the entire glycolipid process during pregnancy and neonatal development in many studies from human beings or animals [30, 31]. Meanwhile, the amino acid amount can affect amount important signaling molecules and natural products during biological metabolism, then affect synthesis of most proteins involved in maternal-neonatal metabolism. However, it was too difficult to figure out which were key points to determine obvious adverse outcomes between mothers and neonates because of clinical interferences and lack of advanced animal experiments data, especially pregnancy researches.
Objectively, glycine levels were obviously higher than in the control group, but it was hard to explain the original biological resources and why it is higher because the umbilical cord blood was mixed with maternal and fetal blood. However, the first large prospective case-cohort observational study was a confident evidence to reflect real-time perspective on maternal-neonatal metabolic changes after the delivery. Meanwhile, glycine may be a potential biomarker to trace glucose metabolism for further study during prenatal development since the significant difference of glycine levels was higher in maternal glucose metabolism dysfunction and an independent factor to affect neonatal hypoglycemia as well.
Some studies showed that plasma glycine positively correlated with glucose disposal, and dietary glycine supplementation increases insulin [32]. Glycine levels rose higher in the GDM group than in the control group, and the elevation of insulin levels may be followed to contribute to neonatal hypoglycemia. However, it was unclear whether neonatal hypoglycemia was associated with self-insulin levels by the influence of glycine levels and whether glycine levels truly influenced glucose disposal during labor through the placenta. It needs more specific biological experiments and deserves further study to prevent adverse outcomes in offspring.
Recently, the crosstalk between GDM and fetal growth is well established [33]. During pregnancy, dysglycemia is typically accompanied by dyslipidemia [34], and together, they promoted an adverse metabolic intrauterine environment and led to preterm infants [35] or congenital malformations [36]. GDM is strongly correlated with fetal growth [37], but neonatal hypoglycemia was negative with different fetal carnitine levels in our study. Most studies reported that carnitine was associated with the development and progress of diabetes mellitus for multiperspectives such as microbiota [38] and lipid [39].
Besides, in our study, there were some significant differences between groups for the measured carnitine parameters in newborns and positively correlated with maternal glucose metabolism dysfunction. Meanwhile, isovalerylcarnitine and octenylcarnitine were positively correlated with GDM with glycosylated haemoglobin levels of 5.5%-6.4% but with low effective size the same as glycine. However, neonatal carnitine levels were not associated with their hypoglycemia in the present study.
There are several limitations in the present study. First, although the present study provided insight into the association between maternal glycolipid factors and neonatal metabolism and characteristic size, the observational design could be another limitation because of the lack of effective size for the prediction. The principle of “equilibrium” was mainly for aiming at maintaining high statistical efficiency in researches. However, it strongly ensured that the research object was close to the real world and reflected the real relationship in clinical phenomenon, especially observational studies and diagnostic accuracy tests [40].
Meanwhile, it was still unclear that how neonatal hypoglycemia was influenced by metabolism changes after delivery. The leading explanation was that maternal insulin levels were delivered to the baby or the neonatal liver produced higher insulin levels in GDM. However, it could not explain why the higher incidence of neonatal hypoglycemia was in preterm neonates [41].
Additionally, since insulin levels of cord blood in the present study were not checked in the two groups, the relationship between glycine and the function of insulin including C-peptide was unclear. Neonatal insulin and C-peptide levels were inaccurately analyzed by the normal clinical standard, as well as glycosylated haemoglobin for newborns [42].
Furthermore, variations in lipid concentration were considerable during gestation, but we only obtained lipid values in late gestation. Therefore, further prospective studies measuring maternal glycolipid concentrations across multiple gestational times in a wide-ranging population were needed to increase the validity of the results.
5. Conclusion
In summary, our results showed that glycine levels in cord blood were independently positive with neonatal hypoglycemia in maternal glucose dysfunction when pregnant women in GDM fluctuating HbA1c levels ranging from 5.5% to 6.4% during mid-late pregnancy, compared to the non-GDM group. Meanwhile, glycine levels have a positive relationship with maternal-neonatal diabetic outcomes, but lack of effective size to predict neonatal hypoglycemia. Glycine may be a potential biomarker as the key departure to trace the underlying mechanisms of neonatal hypoglycemia.
Ethical Approval
The study protocol was established, according to the ethical guidelines of the Helsinki Declaration, and was approved by the ethical committee of the Fifth Affiliated Hospital of Sun Yat-sen University.
Consent
Written informed consent was obtained from individual or guardian participants.
Disclosure
The funding organisation had no role in the study design, data collection, analysis, interpretation, or writing of the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests. No conflicts of interest that could be perceived as prejudicing the impartiality of the research are reported.
Authors’ Contributions
YL and ZS designed the experiments; YY, WL, and LC carried out the experiments; and YL, HZ, and WC analyzed the experimental results. YY, YL, LC, and WL analyzed the sequencing data and developed the analysis tools. FJ, TH, JW, YC, and JC made a series of inclusions and follow-ups of patients. DX randomly extracted samples to match. YL wrote the manuscript.
Acknowledgments
We appreciated Ran Xue for the analysis of the cord blood test in the manuscript. This project was funded by the Science and Technology Project of China, Zhuhai (to ZS).
Open Research
Data Availability
We can provide the data with permission from three official centers (The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai Center for Maternal and Child Health Care, and Zhuhai People’s Hospital).




