Chemical Constituents and Anti-Inflammatory Effect of Incense Smoke from Agarwood Determined by GC-MS
Abstract
Agarwood is generally used to make incense sticks in China and Southeast Asia. It emits smoke with a pleasant odor when burned. There are few reports on the chemical components of smoke generated by burning or heating agarwood. The agarwoods were produced by the whole-tree agarwood-inducing technique (AWIT), agarwood induced by axe wounds (AAW), burning-chisel-drilling agarwood (BCDA), wood of Aquilaria sinensis trees (AS), respectively. Herein, we used GC-MS to analyze the chemical constituents of incense smoke generated from AWIT, AAW, BCDA, AS, and the extracts of sticks from agarwood produced by the whole-tree agarwood-inducing technique (EAWIT), and 484 compounds were identified. A total of 61 chemical constituents were shared among AWIT, AAW, and BCDA. The experimental data showed that aromatic compounds were the main chemical constituents in agarwood smoke and that some chromone derivatives could be cracked into low-molecular-weight aromatic compounds (LACs) at high temperature. Furthermore, agarwood incense smoke showed anti-inflammatory activities by inhibiting lipopolysaccharide- (LPS-) induced TNF-α and IL-1α release in RAW264.7 cells.
1. Introduction
Agarwood, called chen-xiang in China, is a valuable resinous wood from Aquilaria spp. or Gyrinops spp. trees [1–3]. It has been applied in medicine and shown obvious medicinal effects, such as sedative, carminative, and antiemetic effects [4, 5]. Agarwood does not form until a tree has been affected by factors such as lightning strike, animal grazing, insect attack, and fungi [6, 7]. Moreover, it takes a long time (years or even decades) to form in the wild. Natural agarwood is considered to be the finest source of incense and has been applied in cultural, religious, and medicinal uses for centuries. The market demand for agarwood is increasing daily. As a result, the supply of wild agarwood is not enough to meet the market demand. Many Aquilaria plantations have been established in some Southeast Asian countries, such as Indonesia, Cambodia, Laos, Thailand, Vietnam, and Malaysia. Aquilaria trees have been planted in South China, for example, in Hainan, Guangdong, and Yunnan provinces [8]. Some artificial technologies designed to rapidly induce agarwood formation have been demonstrated to make A. sinensis (AS) trees produce agarwood [7, 9–11]. In 2009, Blanchette and Heuveling developed cultivated agarwood kits (CA-Kits) [12]. In 2013, Liu et al. developed a whole-tree agarwood-inducing technique (Agar-Wit) [11]. Recently, Peng et al. also developed a similar technology to induce agarwood formation [13]. The above methods induce agarwood formation simply and effectively.
Presently, agarwood and its volatile components are seen as important and efficient natural substances that can be used to produce valuable products such as perfumes and incense because of their fragrance characteristics. Many teams have researched the chemical constituents of agarwood [1, 14–16]. The chemical constituents of agarwood essential oil or solvent extracts have been studied by column chromatography, spectroscopic techniques, gas chromatography (GC), and multidimensional GC analysis. Many studies have reported the use of GC-MS to analyze the volatile components in agarwood smoke obtained by heating. For example, in 1993, Ishihara et al. analyzed the volatile constituents in agarwood smoke and identified 53 chemical compounds from Vietnamese agarwood [17]. Nurlaila et al. identified 8 significant compounds from agarwood smoke by Z-score analysis [18]. Recently, Zhou et al. used glass fiber pads to absorb volatile constituents of agarwood smoke from different kinds of agarwood from different countries and extracted the samples with dichloromethane (CH2Cl2) for GC-MS analysis [19, 20]. Kao et al. analyzed agarwood smoke from Kynam agarwood by headspace (HS) preheating with gas chromatography-mass spectrometry (HS GC-MS) and identified 40 compounds [21]. However, there are no reports on agarwood smoke produced by Agar-Wit. Herein, we analyzed the chemical constituents of agarwood smoke produced by Agar-Wit and identified 484 compounds.
2. Experimental Setup
2.1. Chemicals and Reagents
All chemicals were purchased from J&K Scientific (Beijing, China), unless otherwise indicated.
The agarwood samples were identified by Prof. Jianhe Wei (Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, China). Incense sticks of AWIT, AAW, BCDA, and AS were made by Bao Gong (Hainan Branch Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, China).
2.2. Sample Preparation
Each stick was placed in a gas washing bottle (250 mL) fitted with an air inlet/outlet tube. The smoke components were collected by bubbling through a 30 mL amount dichloromethane during the 10 min burn time. In addition, sticks made from AWIT (3.0 g) were pulverized and extracted with CH2Cl2 (30 mL).
2.3. Sample Analysis
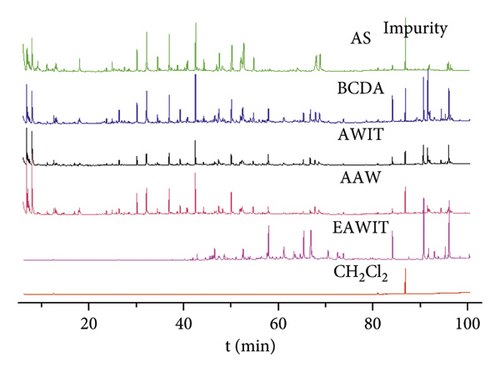
Chromatographic separation of the resulting mixture (1.0 μL) was undertaken on an Agilent 7890 A GC coupled to a 5975C quadrupole mass spectrometer and an automated 7683B sample injector system (Agilent Technologies, Santa Clara, California, USA). Chromatography was performed on a HP-5MS capillary column (30 m × 250 μm ID, 0.25 μm film thickness, 5% diphenyl methyl siloxane (Agilent Technologies, USA). Helium was used as carrier gas at a constant flow rate of 1.0 mL/min. The injections (1.0 μL) were performed in splitless injection mode (10 : 1) at 240°C. The operating parameters included the following temperature program: 40°C for 3 min, increase from 40°C to 140°C at a rate of 2.5°C/min, hold at 140°C for 5 min, increase from 140°C to 170°C at a rate of 1.5°C/min, hold at 170°C for 5 min, and increase to 280°C at a rate of 4°C/min. The total run time was 100.5 min as shown in Figure 1. The mass selective detector was operated with electron energy of 70 eV in electron ionization mode. The ion source and quadrupole temperatures were 230°C and 150°C, respectively. The scan range was 40–500 amu in full scan mode. Peak identification was completed by comparing mass spectra with those stored in the NIST 11 database and MSD ChemStation, or by comparing fragmentation patterns with those published by the Dai group [22]. Table 1 shows the representative data.
| No. | Name | RT (min) | Relative content (%) | ||||
|---|---|---|---|---|---|---|---|
| AAW | BCDA | AWIT | AS | EAWIT | |||
| 1 | Ethylbenzene | 6.774 | 6.215 (B) | 3.296 (B) | 6.384 (B) | 1.313 (B) | — |
| 2 | Furfuryl alcohol | 6.872 | — | — | — | 2.212 | — |
| 3 | 1,4-Xylene | 7.080 | 3.709 (B) | — | — | 1.765 (B) | — |
| 4 | 1,3-Xylene | 7.086 | — | — | 2.354 (B) | — | — |
| 5 | 1,2-Xylene | 7.098 | — | 1.326 (B) | — | 0.778 (B) | — |
| 6 | Phenylacetylene | 7.392 | 1.387 (B) | 0.698 (B) | 1.612 (B) | 1.013 (B) | — |
| 7 | Phenyl carbamate | 7.646 | — | — | — | 0.308 (B) | — |
| 8 | Phenylethylene | 7.901 | 8.596 (B) | 3.413 (B) | 7.973 (B) | 5.132 (B) | — |
| 9 | 2-Methyl-2-cyclopenten-1-one | 8.680 | — | — | — | 0.400 | — |
| 10 | 4,4-Dimethyl-2-cyclopenten-1-one | 8.952 | — | — | — | 0.302 | — |
| 11 | 2,5-Dimethyl-2,4-hexadiene | 9.056 | — | — | — | 0.408 | — |
| 12 | Anisole | 9.096 | 0.239 (B) | — | 0.21 (B) | — | — |
| 13 | 2(5H)-Furanone | 9.200 | 0.202 | 0.132 | 0.299 | 0.981 | — |
| 14 | 3,4-Dihydro-H-pyran | 9.356 | 0.202 | 0.202 | — | 0.381 | — |
| 15 | Methyl-2-oxo-1-pyrrolidineacetate | 9.605 | — | 0.222 | — | — | — |
| 16 | Tetrahydro-22-desoxy-tomatillidine | 9.610 | — | — | — | 0.211 | — |
| 17 | 1-Methylene-2-vinylcyclopentane | 10.962 | — | — | — | 0.113 | — |
| 18 | Benzaldehyde | 11.118 | 0.671 (B) | 0.28 (B) | 1.008 (B) | 1.146 (B) | — |
| 19 | 5-Methyl-furfural | 11.407 | — | — | — | 0.508 | — |
| 20 | 2,3-Dihydroxystearic acid | 11.875 | — | — | — | 0.209 | — |
| 21 | 2-Chloro-2,2-difluoro-acetonitrile | 11.950 | — | — | — | 0.157 | — |
| 22 | 2-Methyl-2-pentenal | 12.192 | — | — | — | 0.104 | — |
| 23 | Benzonitrile | 12.308 | — | — | — | 0.359 (B) | — |
| 24 | 2,2,4,6,6-Pentamethylheptane | 12.510 | 0.902 | 0.753 | 1.152 | — | 0.126 |
| 25 | Benzofuran | 12.793 | — | — | — | 0.541 (B) | — |
| 26 | Phenol | 12.839 | 1.225 (B) | 0.558 (B) | 0.592 (B) | 0.852 (B) | — |
| 27 | 2,2-Diethyl-3-methyl-oxazolidine | 13.105 | 0.751 | 0.785 | 0.808 | — | — |
| 28 | (2S, 3S)-2,3-Dimethoxy-N1,N1,N1,N4-tetramethyl-1,4-butanediamine | 13.111 | — | — | — | 0.846 | — |
| 29 | 5-Norbornene-2-carboxaldehyde | 13.654 | 0.169 | — | 0.189 | — | — |
| 30 | 4-Methylanisole | 14.185 | 0.305 (B) | 0.102 (B) | 0.135 (B) | 0.159 (B) | — |
| 31 | 2-Azido-2,4,4,6,6-pentamethylheptane | 14.445 | 0.255 | 0.187 | 0.220 | — | — |
| 32 | 2,3-Dioxabicyclo[2.2.2]oct-5-ene | 14.671 | 0.424 | 0.189 | 0.175 | — | — |
| 33 | 3-Methyl-1,2-cyclopentanedione | 14.717 | — | — | — | 0.369 | — |
| 34 | 2-Methyl-3-furanthiol | 15.918 | 0.106 | 0.201 | — | — | — |
| 35 | 3-Methyl-phenol | 16.635 | 0.219 (B) | 0.176 (B) | 0.12 (B) | — | — |
| 36 | 2-Methyl-phenol | 16.669 | — | — | — | 0.226 (B) | — |
| 37 | 3-Methyl-bicyclo[3.3.0]oct-2-en-8-one | 16.918 | — | — | — | 0.129 | — |
| 38 | 2,2,2-Bicyclo-2-octene | 16.935 | 0.496 | 0.183 (B) | 0.453 (B) | — | — |
| 39 | p-Cresol | 17.801 | 0.73 (B) | 0.473 (B) | 0.321 (B) | 0.205 (B) | — |
| 40 | Guaiacol | 17.998 | 0.877 (B) | 0.51 (B) | 0.608 (B) | 1.362 (B) | — |
| 41 | 2-t-Butylamino-acrylonitrile | 18.165 | 0.421 | 0.224 | 0.240 | — | — |
| 42 | 3,5-Dimethyl-4H-pyran-4-one | 18.182 | — | — | — | 0.107 | — |
| 43 | 3-Hydroxy-2-methyl-4-pyrone | 19.338 | — | 0.169 | — | 0.147 | — |
| 44 | 2,5-Dimethylphenol | 21.677 | 0.223 (B) | 0.129 (B) | — | 0.165 (B) | — |
| 45 | 2,3-Dihydroxybenzaldehyde | 22.064 | — | — | — | 0.148 (B) | — |
| 46 | 5-Ethyl-3-(3-methyl-5-phenylpyrazol-1-yl)-1,2,4-triazol-4-amine | 22.215 | 0.152 (B) | — | — | — | — |
| 47 | Ethyl disulfide | 22.220 | — | — | 0.094 | — | — |
| 48 | 1-Methylene-1H-indene, | 22.740 | 0.166 (B) | — | — | — | — |
| 49 | 2-Ethylphenol | 22.913 | 0.129 (B) | — | — | — | — |
| 50 | Trehalose | 22.914 | — | 0.324 | — | — | — |
| 51 | 2-Isopropyl-5-methyl-1-heptanol | 23.197 | — | — | 0.059 | — | — |
| 52 | 3-Methyl-2-butene-1-thiol | 23.208 | 0.120 | — | — | — | — |
| 53 | 2-Methylbutyl pentanoic acid ester | 23.225 | — | 0.251 | — | — | — |
| 54 | 4-Methoxy-1,3-benzenediamine | 23.300 | — | — | — | 0.216 (B) | — |
| 55 | 2-Methoxy-3-methyl-phenol | 23.306 | 0.236 (B) | — | — | — | — |
| 56 | 2-Methoxy-4-methylphenol | 23.676 | 0.444 (B) | 0.283 (B) | 0.271 (B) | 0.309 (B) | — |
| 57 | 2-Methoxy-5-methylphenol | 23.688 | — | 0.327 (B) | — | — | — |
| 58 | 3,6-Dimethyl-2,6-octadiene-4,5-diol | 24.144 | — | — | — | 0.129 | — |
| 59 | 1,4 : 3,6-Dianhydro-α-d-glucopyranose | 24.600 | 0.148 | 0.158 | 0.172 | 0.120 | — |
| 60 | trans-Cinnamaldehyde | 24.866 | — | — | — | 0.821 (B) | — |
| 61 | (1α, 2β, 5β, 6α)-Tricyclo[4.2.1.1(2,5)]deca-3,7-diene-9,10-dione | 24.883 | 0.446 | 0.325 | — | — | — |
| 62 | α-Methylene-benzeneacetaldehyde | 24.895 | — | — | 0.247 (B) | — | — |
| 63 | 2,4-Cyclopentadiene-1-ethanamine | 25.045 | 0.128 | — | 0.230 | — | — |
| 64 | 1,11-Dibromo-undecane | 25.062 | — | 0.101 | — | — | — |
| 65 | Pyrocatechol | 25.357 | 0.125 (B) | 0.287 | — | 0.93 (B) | — |
| 66 | 2,3-Anhydro-d-galactosan | 25.357 | — | — | 0.204 (B) | — | — |
| 67 | 2,3-Dihydrobenzofuran | 25.727 | 0.369 (B) | 0.382 (B) | 0.299 (B) | 0.255 (B) | — |
| 68 | 1-Methyl-1H-pyrrole-2(5H)-one | 26.131 | — | — | 0.203 | — | — |
| 69 | 2-Isopropoxyphenol | 26.183 | — | — | — | 0.226 (B) | — |
| 70 | 3-Methoxyphenol | 26.206 | 0.263 (B) | — | — | — | — |
| 71 | 5-Hydroxymethylfurfural | 26.229 | — | 0.252 | — | 0.389 | — |
| 72 | 4-Phenyl-2-butanone | 26.339 | 0.77 (B) | 1.13 (B) | 1.06 (B) | — | 0.177 (B) |
| 73 | 1-Methyl-4-amino-4,5(1H)-dihydro-1,2,4-triazole-5-one | 26.847 | — | — | — | 0.259 | — |
| 74 | Anisic aldehyde | 26.905 | 0.306 (B) | 0.301 (B) | 0.389 (B) | — | — |
| 75 | 2-Oxohexamethylenimine | 27.032 | — | 0.185 | — | — | — |
| 76 | 3-Methoxy-2-benzenediol | 27.454 | 0.401 (B) | 0.365 (B) | 0.251 (B) | 0.582 (B) | — |
| 77 | 3-Methoxybenzenethiol | 27.731 | 0.131 (B) | — | — | — | — |
| 78 | 1-Indanone | 28.043 | 0.279 (B) | — | — | 0.148 (B) | — |
| 80 | 2-Isopropyl-3-methoxypyrazine | 28.291 | — | — | — | 0.177 | — |
| 79 | 4-Ethyl-2-methoxyphenol | 28.291 | 0.292 (B) | 0.104 (B) | 0.071 (B) | — | — |
| 81 | 3-(4-Methylphenyl)-2-propenal | 28.441 | — | — | — | 0.206 (B) | — |
| 82 | 2,3-Dihydro-2-methyl-1H-inden-1-one | 28.447 | 0.223 | — | 0.056 (B) | — | — |
| 83 | α-Methylcinnamaldehyde | 28.557 | — | — | — | 0.127 (B) | — |
| 84 | 2-Methylnaphthalene | 28.621 | 0.285 (B) | — | — | — | — |
| 85 | 1-Azabicyclo[2.2.2]octane-4-methanol | 29.527 | — | 0.158 | — | — | — |
| 86 | (E)-2,4,4,7-Tetramethyl-5,7-octadien-3-ol | 29.666 | — | — | — | 0.179 | — |
| 87 | 4-Hydroxy-3-methoxystyrene | 30.099 | 2.343 (B) | 1.799 (B) | 1.512 (B) | 2.254 (B) | — |
| 88 | o-tert-Butyl phenol | 30.417 | — | — | — | 0.131 (B) | — |
| 89 | 3-Hydroxybenzaldehyde | 30.844 | — | — | — | 0.252 (B) | — |
| 90 | 2-Methoxybenzyl alcohol | 31.081 | — | — | — | 0.106 (B) | — |
| 91 | trans-3-Hexenedioic acid-bis(trimethylsilyl) ester | 31.411 | 0.127 | — | — | — | — |
| 92 | 2-exo-Chlorobicyclo[2.2.1]heptane-1-carbonyl chloride | 31.942 | 0.282 | — | 0.285 | — | — |
| 93 | 2-Ethyl-1H-pyrrolo[2,3-b]pyridine | 32.000 | — | — | — | 0.157 | — |
| 94 | 2,6-Dimethoxyphenol | 32.115 | 3.013 (B) | 2.939 (B) | 2.178 (B) | — | — |
| 95 | cis-4,5-Diethyl-1,2-dimethyl-cyclohexene | 32.190 | — | — | — | 4.085 | — |
| 96 | Eugenol | 32.358 | 0.517 (B) | 0.303 (B) | 0.262 (B) | — | — |
| 97 | 3-Allyl-6-methoxyphenol | 32.375 | — | — | — | 0.413 (B) | — |
| 98 | 3,4-Dimethoxyphenol | 32.566 | — | 0.112 (B) | — | 0.219 (B) | — |
| 99 | 3-Ethenyl-4-methyl-1H-pyrrole-2,5-dione | 32.849 | 0.205 | — | — | 0.149 | — |
| 100 | 3-Cyclohexene-1-acetaldehyde | 33.247 | 0.191 | — | 0.136 | — | — |
| 101 | 11-Methylene-tricyclo[4.3.1.1(2,5)]undecane | 33.247 | — | — | — | — | — |
| 102 | 2-Propyl-phenol | 33.848 | — | — | — | 0.1 (B) | — |
| 103 | 4-Hydroxybenzaldehyde | 33.975 | — | — | — | 0.225 (B) | — |
| 104 | Dichlorophenylsilane | 34.010 | — | — | 0.321 (B) | — | — |
| 105 | Phenylboronic acid | 34.016 | 0.406 (B) | 0.143 (B) | — | — | — |
| 106 | Vanillin | 34.408 | 0.8 (B) | 0.83 (B) | 0.708 (B) | 1.842 (B) | — |
| 107 | 4-(Methylthio)-benzaldehyde | 34.593 | 0.254 (B) | — | — | — | — |
| 108 | (E)-isoeugenol | 34.899 | 0.341 (B) | 0.18 (B) | 0.133 (B) | 0.31 (B) | — |
| 109 | o-Methoxy-benzenethiol | 35.084 | 0.125 (B) | — | — | 0.197 (B) | — |
| 110 | 2-Methoxy-1,4-benzenediol | 35.217 | — | — | — | 0.209 (B) | — |
| 111 | 2-Benzylidenemalonaldehyde | 35.217 | 0.204 (B) | — | — | — | — |
| 112 | 2-Vinylnaphthalene | 35.442 | 0.115 (B) | — | — | — | — |
| 113 | 3-Hydroxy-2-methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione | 36.083 | — | — | — | 0.237 | — |
| 114 | Biphenylene | 36.234 | 0.105 (B) | — | — | — | — |
| 115 | 2-Methoxy-4-(1-propen-1-yl)-phenol | 36.892 | 2.962 (B) | 2.126 (B) | 2.379 (B) | 3.254 (B) | — |
| 116 | 4-Hydroxy-2-methoxybenzaldehyde | 37.227 | 0.243 | 0.142 | — | 0.135 (B) | — |
| 117 | 2-Methoxy-4-propyl-phenol | 37.429 | 0.268 (B) | 0.242 (B) | 0.257 (B) | 0.454 (B) | — |
| 118 | 1,7-Dimethylpentacyclo[5.5.0(4,11).0(5,9).0(8,12)]dodecane-2,6-dione | 37.689 | — | — | 0.126 (B) | — | — |
| 119 | 2-Methoxy-6-[(4H-1,2,4-triazol-4-ylamino)methyl]-phenol | 37.689 | 0.107 | — | — | — | — |
| 120 | 4-Hydroxybenzylidene acetone | 37.707 | — | 0.101 | — | — | — |
| 121 | N-Phenylthioformamide | 37.712 | — | — | — | 0.126 (B) | — |
| 122 | 7-Ethylbenzo[b]thiophene | 38.053 | — | — | — | 0.153 | — |
| 123 | 5,6-Dimethyl-2-benzimidazolinone | 38.382 | — | — | — | 0.164 (B) | — |
| 124 | Cyclohexylmethylbenzene | 38.440 | 0.145 (B) | — | 0.161 (B) | — | — |
| 125 | 3,4-Dimethoxy-benzaldehyde | 38.573 | — | — | — | 0.712 (B) | — |
| 126 | 4′-(Methylthio)acetophenone | 38.660 | 0.370 | 0.298 (B) | 0.256 (B) | — | — |
| 127 | 4-(4-Methoxyphenyl)-2-butanone | 39.232 | 1.067 (B) | 1.161 (B) | 1.124 (B) | 0.137 | — |
| 128 | Pentadecane | 39.434 | — | — | 0.168 | — | — |
| 129 | Dibenzofuran | 39.451 | 0.186 (B) | 0.145 (B) | — | — | — |
| 130 | 2′,6′-Dihydroxyacetophenone, bis(trimethylsilyl) ether | 39.850 | 0.207 (B) | — | — | — | — |
| 131 | 1-Methyl-1-phenylmethoxy-1-silacyclohexane | 39.873 | — | 0.104 (B) | 0.111 (B) | — | — |
| 132 | 1,3,3-Trimethyl-2-(1-methylbut-1-en-3-on-1-yl)-1-cyclohexene | 40.173 | 0.273 | — | — | — | — |
| 133 | 1,2-Dimethoxy-4-(methoxymethyl)benzene | 40.196 | — | 0.178 (B) | — | — | — |
| 134 | 2,4-Di-tert-butylphenol | 40.202 | — | — | — | 0.287 (B) | — |
| 135 | 2-(2-Hydroxyhex-1-enyl)-3-methyl-5,6-dihydropyrazine | 40.260 | 0.138 | — | — | — | — |
| 136 | 5-(1,1-Dimethylethyl)-1,2,3-benzenetriol | 40.653 | 0.578 (B) | 0.34 (B) | 0.212 (B) | 0.684 (B) | — |
| 137 | Homovanillyl alcohol | 40.780 | 0.594 (B) | 0.507 (B) | 0.417 (B) | 1.119 (B) | — |
| 138 | [4-(1,1-Dimethylethyl)phenoxy]-acetate-methanol | 41.912 | — | — | — | — | 0.159 (B) |
| 139 | (S)-4,5,6,7,8,8a-Hexahydro-8aα-methylazulen-2(1H)-one | 42.137 | 0.109 | — | — | — | — |
| 140 | Acetic acid-2-propylphenyl ester | 42.160 | — | 0.114 (B) | — | — | — |
| 141 | 3-Nitrobenzaldehyde-(O-methyl oxime) | 42.420 | — | — | — | — | — |
| 142 | 2,3,5,6-Tetrafluoroanisole | 42.443 | 4.532 (B) | 3.741 (B) | 4.13 (B) | — | — |
| 143 | 3-tert-Butyl-4-hydroxyanisole | 42.547 | — | — | — | 5.641 (B) | — |
| 144 | 2,5-Dihydroxy-4-isopropyl-2,4,6-cycloheptatrien-1-one | 42.819 | — | — | — | 0.329 | — |
| 145 | α-Santalol | 42.848 | — | — | — | — | 0.511 (S) |
| 146 | 2,3-Dihydro-2,2-dimethyl-3,7-benzofurandiol | 42.888 | 0.359 (B) | 0.39 (B) | 0.394 (B) | — | — |
| 147 | 7-(1,1-Dimethylethyl)-3,4-dihydro-1(2H)-naphthalenone | 43.119 | — | — | — | — | 0.105 (B) |
| 148 | 3-Ethoxy-4-methoxybenzaldehyde | 43.183 | 0.324 (B) | — | 0.221 (B) | — | — |
| 149 | 3-Hydroxy-4-methoxybenzoic acid-methyl ester | 43.200 | — | 0.318 (B) | — | — | — |
| 150 | Ethyl vanillate | 43.211 | — | — | — | 0.279 (B) | — |
| 151 | α-Amino-3′-hydroxy-4′-methoxyacetophenone | 43.443 | 0.126 (B) | 0.143 (B) | — | — | — |
| 152 | (3S, 4R, 5R, 6R)-4,5-Bis(hydroxymethyl)-3,6-dimethylcyclohexene | 43.610 | — | — | 0.202 | — | — |
| 153 | Carbonic acid-2,3-dimethylphenyl methyl ester | 43.628 | 0.41 (B) | — | — | — | — |
| 154 | 3-(4-Methoxyphenyl)propionic Acid | 43.656 | — | 0.431 (B) | — | — | — |
| 155 | Hexadecane | 44.078 | 0.329 | 0.351 | 0.321 | 0.127 | — |
| 156 | 2,6-Dimethoxy-4-(2-propen-1-yl)-phenol | 44.274 | 0.785 (B) | 0.619 (B) | 0.521 (B) | 1.029 (B) | — |
| 157 | 2,6-Dimethyl-4-nitrophenol | 44.586 | 0.256 (B) | — | — | — | — |
| 158 | [1S-(1α, 4α, 7α)]-1,2,3,4,5,6,7,8-Octahydro-1,4,9,9-tetramethyl-4,7-methanoazulene | 44.592 | — | — | — | — | 0.406 (S) |
| 159 | 2-Ethyl-4-methyl-4,6-bis(1-methylethyl)-4H-1,3,2-dioxaborin | 44.592 | — | — | — | 0.139 | — |
| 160 | 8-Epi-γ-eudesmol | 44.633 | — | 0.261 (S) | 0.219 (S) | — | — |
| 161 | Methyl-2,6,6-trimethyl-3-oxo-1-cyclohexene-1-acrylate | 44.737 | — | — | 0.155 | — | — |
| 162 | 2′,6′-Dimethylacetanilide | 44.771 | — | 0.187 | — | — | — |
| 163 | [1S-(1α, 4aβ, 8aα)]-1,2,4a,5,8,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene | 45.152 | — | — | — | — | 0.141 (S) |
| 164 | [1R-(1α, 3aα, 7aα)]-1,2,3,6,7,7a-Hexahydro-2,2,4,7a-tetramethyl-1,3a-ethano-3aH-indene | 45.326 | — | 0.241 (B) | — | — | 0.149 (S) |
| 165 | Agarospirol | 45.551 | — | 0.238 (S) | 0.195 (S) | — | 0.413 (S) |
| 166 | Methyl 3-(bicyclo[2.2.1]hept-1-yl)-propenoate | 45.586 | — | — | — | 0.116 | — |
| 167 | Hinesol | 45.719 | 0.126 | — | — | — | 0.251 (S) |
| 168 | (1α, 6α, 7α)-1,5,5-Trimethyl-2-methylene-bicyclo[4.1.0]heptane-7-methanol | 45.736 | 0.121 (S) | — | — | — | — |
| 169 | (1R, 3aR, 4R, 7R)-1,2,3,3a,4,5,6,7-Octahydro-1,4-dimethyl-7-(1-methylethenyl)-azulene | 45.748 | — | — | 0.191 (S) | — | — |
| 170 | [1S-(1α, 4α, 7α)]-1,2,3,4,5,6,7,8-Octahydro-1,4-dimethyl-7-(1-methylethenyl)-azule | 45.776 | — | 0.222 (B) | — | — | — |
| 171 | Longifolene | 45.990 | — | — | — | — | 0.565 (S) |
| 172 | 10S,11S-Himachala-3(12),4-diene | 46.007 | 0.155 (S) | — | — | — | — |
| 173 | Neoisolongifolene | 46.042 | — | 0.262 (S) | 0.248 (S) | — | — |
| 174 | Ledol | 46.279 | — | — | 0.219 (S) | — | 0.519 (S) |
| 175 | β-Eudesmol | 46.325 | 0.150 | 0.343 (S) | — | — | — |
| 176 | Guaiol | 46.498 | — | — | 0.939 (S) | — | — |
| 177 | γ-Selinene | 46.504 | 0.589 (S) | 1.164 (S) | — | — | 1.868 (S) |
| 178 | 1-Bromooctadecane | 46.712 | — | — | — | — | — |
| 179 | 7,9-Dimethyl-hexadecane | 46.729 | 0.169 | — | 0.219 | — | — |
| 180 | (4-Methoxyphenyl)glycolic acid | 46.752 | — | — | — | 0.212 | — |
| 181 | 3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid | 46.880 | 0.629 (B) | 0.509 (B) | 0.517 (B) | 0.814 (B) | — |
| 182 | Dehydroaromadendrene | 47.301 | — | — | — | — | 0.442 (S) |
| 183 | 3,5-Dimethoxy-4-hydroxybenzaldehyde | 47.353 | 1.599 (B) | 1.78 (B) | 1.891 (B) | 2.92 (B) | — |
| 184 | 2,4,6-Trimethyl-pyridine | 47.428 | — | — | — | — | 0.383 |
| 185 | Camphene | 47.561 | — | — | — | — | 0.600 |
| 186 | Ethyl (3-pyridyl)carbamate N-oxide | 47.608 | — | 0.753 | — | — | — |
| 187 | Hexamethyl-benzene | 47.654 | 0.503 | — | 0.646 (B) | — | — |
| 188 | 1-(1,3a,4,5,6,7-Hexahydro-4-hydroxy-3,8-dimethyl-5-azulenyl)-ethanone | 47.896 | — | — | — | — | 0.142 (S) |
| 189 | 2-Allyl-1,4-dimethoxy-3-methyl-benzene | 48.029 | 0.249 (B) | — | 0.33 (B) | — | — |
| 190 | 2,5-Dibutyl-furan | 48.150 | — | — | — | 1.392 | — |
| 191 | Vanillylacetone | 48.208 | 1.036 (B) | 0.967 (B) | 0.425 (B) | — | — |
| 192 | [1S-(1α, 7α, 8aβ)]-1,2,3,5,6,7,8,8a-Octahydro-1,4-dimethyl-7-(1-methylethenyl)-azulene | 48.480 | — | — | — | — | 0.55 (S) |
| 193 | Dehydro-cyclolongifolene oxide | 48.509 | — | 0.717 (S) | 0.473 (B) | — | — |
| 194 | 4-Methoxymethyl-6-methyl-1H-pyrazolo[3,4-b]pyridin-3-ylamine | 48.572 | — | — | — | 0.283 | — |
| 195 | 1-Cyclohexyl-2-methoxy-benzene | 48.601 | — | — | 0.384 (B) | — | 0.72 (B) |
| 196 | N,N-Diethyl-2-benzoxazolamine | 48.613 | 0.781 (B) | — | — | — | — |
| 197 | Octahydro-2-(1-methylethylidene)-4,7-methano-1H-indene | 49.161 | — | — | — | — | 0.361 (B) |
| 198 | 4,6,6-Trimethyl-2-(3-methylbuta-1,3-dienyl)-3-oxatricyclo[5.1.0.0(2,4)]octane | 49.167 | — | — | 0.172 | — | — |
| 199 | 4-Methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-vinyl-4-methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-vinyl-cycloheptane | 49.219 | 0.121 | 0.181 | — | — | — |
| 200 | 3-Phenoxy-phenol | 49.467 | 0.325 | 0.154 (B) | 0.134 (B) | 0.248 (B) | — |
| 201 | (Z)-3,7-Dimethyl-1,3,6-octatriene | 49.537 | — | — | — | — | 0.406 |
| 202 | 1,7-Dimethyl-7-(4-methyl-3-pentenyl)-tricyclo[2.2.1.0(2,6)]heptane | 49.612 | — | 0.2 (S) | 0.192 (S) | — | — |
| 203 | 2-Acetate-1,3-dimethoxy-5-(1-propenyl)-benzene | 50.057 | 3.206 (B) | 2.309 (B) | — | — | — |
| 204 | 2,5-Dimethoxyterephthalic acid | 50.178 | — | — | 2.516 (B) | 3.780 | — |
| 205 | 2-(2-Furanylmethylene)-6-methyl-cyclohexanone | 50.270 | — | — | — | — | 0.255 |
| 206 | 4-Propylbiphenyl | 50.380 | 0.704 (B) | 0.711 (B) | — | — | — |
| 207 | 1-Ethyl-3-(phenylmethyl)-benzene | 50.386 | — | — | 0.745 (B) | — | — |
| 208 | N,N,S-Trimethyl-3-aminothiophenol | 50.415 | — | — | — | 0.684 (B) | — |
| 209 | Neocurdione | 50.438 | — | — | — | — | 0.161 (S) |
| 210 | endo-Borneol | 51.056 | — | — | — | — | 0.611 |
| 211 | 9-Fluorenone | 51.073 | 0.205 (B) | — | — | — | — |
| 212 | [1S-(1α, 3aβ, 4α, 8aβ, 9R∗)]-Decahydro-4,8,8-trimethyl-1,4-methanoazulene-9-methanol | 51.108 | — | 0.488 (S) | 0.295 (S) | — | — |
| 213 | Methyl α-hydroxy-4-methoxy-benzeneacetate | 51.605 | — | — | — | 0.101 (B) | — |
| 214 | 2,2′-Methylenebis[5-methyl-furan | 51.743 | — | — | — | — | 0.136 |
| 215 | 4-Hydroxy-2-methoxycinnamaldehyde | 51.882 | 0.937 (B) | 0.897 (B) | 0.78 (B) | — | — |
| 216 | 3-(4-Hydroxy-3-methoxyphenyl)-2-propenal | 52.072 | — | — | — | 2.664 (B) | — |
| 217 | Acetosyringone | 52.096 | 0.967 (B) | 0.829 (B) | 0.88 (B) | 1.499 (B) | — |
| 218 | syn-3,3,5,6,8,8-Hexamethyl-tricyclo[5.1.0.0(2,4)]oct-5-ene, | 52.333 | 1.530 | 1.979 | 1.762 | — | 0.624 |
| 219 | 2-Phenylethyl-1,1,2,2-d4-amine | 52.541 | — | — | 1.184 (B) | — | — |
| 220 | 2-Methyl-5-(1-methylethyl)-phenol | 52.552 | — | — | — | — | 1.377 (B) |
| 221 | 2′-Hydroxy-3,3-dimethyl-3-phenylpropanal | 52.558 | 0.762 (B) | — | — | — | — |
| 222 | 2-[3-Methoxyphenyl]-propionic acid | 52.581 | — | 1.048 (B) | — | — | — |
| 223 | 3-(2-Pentenyl)-1,2,4-cyclopentanetrione | 52.679 | — | — | — | 6.688 (B) | — |
| 224 | 7-(1,3-Dimethylbuta-1,3-dienyl)-1,6,6-trimethyl-3,8-dioxatricyclo[5.1.0.0(2,4)]octane | 52.951 | 0.340 | 0.621 | 0.732 | — | 0.491 |
| 225 | o-Mentha-1(7),8-dien-3-ol | 53.262 | — | — | — | — | 0.206 |
| 226 | 1,10b(2H)-Dihydropyrano[3,4,5-jk]fluorene | 53.488 | 0.153 (B) | — | — | — | — |
| 227 | Anthracene | 53.499 | — | — | — | 0.124 (B) | — |
| 228 | (1S, 6R, 9S)-5,5,9,10-Tetramethyltricyclo[7.3.0.0(1,6)]dodec-10(11)-ene | 53.609 | 0.121 | 0.457 | 0.414 | — | — |
| 229 | (Z)-3-Methyl-2-(2,4-pentadienyl)-2-cyclopenten-1-one | 53.632 | — | — | — | — | 0.559 |
| 230 | 1-Methoxy-4-methyl-2-(1-methylethyl)-benzene | 54.019 | — | — | — | — | 0.565 (B) |
| 231 | (1Z, 3aα, 7aβ)-1H-1-Ethylideneoctahydro-7a-methyl-indene | 54.089 | 0.466 (B) | 0.773 (B) | 0.531 (B) | — | — |
| 232 | 3-(Phenylmethoxy)-1-propanol | 54.233 | — | — | — | — | 0.238 (B) |
| 233 | trans-1,10-Dimethyl-2-methylene-decalin | 54.510 | — | — | — | — | 0.113 |
| 234 | 3,5-Dimethoxy-4-hydroxyphenylacetic acid | 54.649 | 1.383 (B) | 1.359 (B) | 1.391 (B) | 2.15 (B) | — |
| 235 | (1aR, 4S, 4aR, 7S, 7aR, 7bS)-Decahydro-1,1,4,7-tetramethyl-1H-cycloprop[e]azulene | 54.672 | — | — | — | — | 0.177 (S) |
| 236 | 2,7-Dimethyl-5-(1-methylethenyl)-1,8-nonadiene | 55.036 | — | — | — | — | 0.126 |
| 237 | Longifolenaldehyde | 55.261 | — | 0.12 (S) | — | — | — |
| 238 | (2R-cis)-1,2,3,4,4a,5,6,7-Octahydro-α,α,4a,8-tetramethyl-2-naphthalenemethanol | 55.377 | — | — | — | — | 0.409 (B) |
| 239 | (4aR, 5S)-4,4a,5,6,7,8-Hexahydro-4a,5-dimethyl-3-(1-methylethylidene)-2(3H)-naphthalenone | 55.850 | 0.459 (S) | 0.535 (S) | 0.609 (S) | — | 0.611 (S) |
| 240 | 1,2,4-Triethyl-benzene | 56.130 | — | — | — | — | 0.301 (B) |
| 241 | Isoaromadendrene epoxide | 56.145 | 0.114 (S) | — | 0.143 (S) | — | — |
| 242 | 2,3,4,5-Tetramethyl-tricyclo[3.2.1.02,7]oct-3-ene | 56.405 | — | — | — | — | 0.659 |
| 243 | Globulol | 56.584 | — | — | — | — | 0.344 (S) |
| 244 | Octadecane | 56.624 | 0.285 | 0.331 | 0.393 | 0.179 | — |
| 245 | 2,3-Dihydro-2,2-dimethyl-7-benzofuranol | 56.803 | — | — | — | — | 0.258 (B) |
| 246 | 1β, 2α-Dimethyl-3α, 5β-bis(1-methylethenyl)cyclohexane | 56.844 | — | — | 0.285 | — | — |
| 247 | 4,6-Dimethoxy-1-naphthaldehyde | 56.896 | 0.214 (B) | — | — | — | — |
| 248 | 2-Bromo-1,3-dimethoxy-benzene | 56.919 | — | 0.305 (B) | — | — | — |
| 249 | 1,3,5-Triethyl-benzene | 57.040 | — | — | — | — | 0.252 (B) |
| 250 | 7-Methyl-pentadecane | 57.144 | — | — | 0.189 | — | — |
| 251 | 2-Methyl-dodecane | 57.150 | 0.154 | — | — | — | — |
| 252 | Corymbolone | 57.225 | — | — | — | — | 0.164 (S) |
| 253 | 2,6,10,14-tetramethyl-Hexadecane | 57.225 | 0.130 | 0.285 | 0.151 | 0.298 | — |
| 254 | 1-(2-Thienyl)-1-heptanone | 57.520 | — | — | 0.500 | — | — |
| 255 | 1-(2-Thienyl)-1-hexanone | 57.543 | 0.391 | — | — | — | — |
| 256 | 2,4-Dimethylcyclopentane-1,3-dione | 57.601 | — | 0.475 | — | — | — |
| 257 | 1-(2-Thienyl)-ethanone | 57.612 | — | — | — | — | 1.207 |
| 258 | 1-(2,6-Dihydroxy-4-methoxyphenyl)-ethanone | 57.716 | — | — | — | 0.162 (B) | — |
| 259 | 5-(2-Thienyl)-4-pyrimidinamine | 57.832 | 1.086 | 1.513 | 2.402 | — | — |
| 260 | 1,3-Benzenedicarboxylic acid-4-methyl-1,3-dimethyl ester | 57.941 | — | — | — | — | 5.197 (B) |
| 261 | 3,4-Dimethoxy-benzaldehyde oxime | 58.051 | — | — | 0.196 (B) | — | — |
| 262 | 1,2-Dimethoxy-4-(1,2-dimethoxyethyl)benzene | 58.063 | 0.295 (B) | 0.233 (B) | — | — | — |
| 263 | 5-Methoxy-[1,2,4]triazolo[4,3-a]pyridine-3-thiol | 58.149 | — | — | — | 0.335 | — |
| 264 | Methyl-3-amino-4-methoxybenzoate | 58.248 | 0.192 (B) | 0.304 (B) | 0.29 (B) | — | — |
| 265 | Isomaltol | 58.271 | — | — | — | — | 0.623 |
| 266 | 9-Methyl-9-azabicyclo[4.2.1]nona-2,4-diene | 58.721 | 0.103 | — | — | — | 0.970 |
| 267 | Chlordimeform | 58.791 | — | 0.309 (B) | — | — | — |
| 268 | Nootkatone | 59.229 | — | — | — | — | 0.118 (S) |
| 269 | 4-(3-Methyl-2-butenyl)-phenol | 59.541 | — | — | — | — | 0.772 (B) |
| 270 | [2R-(2α, 4aα, 8aβ)]-1,2,3,4,4a,5,6,8a-Octahydro-4a,8-dimethyl-2-(1-methylethenyl)-naphthalene | 59.663 | — | 0.125 (S) | — | — | — |
| 271 | [1R-(1R∗, 4Z, 9S∗)]-4,11,11-trimethyl-8-methylene-bicyclo[7.2.0]undec-4-ene | 59.952 | — | — | — | — | 0.193 (S) |
| 272 | 2,3,4,5,6-Pentamethyl-benzoic acid | 60.200 | 0.221 (B) | 0.151 (B) | — | — | — |
| 273 | [1aR-(1aα, 7α, 7aα, 7bα)]-1a,2,3,5,6,7,7a,7b-Octahydro-1,1,7,7a-tetramethyl-1H-cyclopropa[a]naphthalene | 60.206 | — | — | — | — | 0.167 (S) |
| 274 | 4,5-Dihydro-6-(4-fluorophenyl)-pyridazin-3(2H)-one | 60.235 | — | — | — | — | — |
| 275 | N(1)-[(3-Methoxyphenyl)methyl]-1H-1,2,3,4-tetrazole-1,5-diamine | 60.367 | — | — | 0.17 (B) | — | — |
| 276 | 1-Ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-cyclohexane | 60.396 | — | — | — | — | 0.601 (S) |
| 277 | (E,E)-1,5-Dimethyl-8-(1-methylethylidene)-1,5-cyclodecadiene | 60.443 | — | 0.165 (S) | — | — | — |
| 278 | 6,7-Dimethyl-8-(1-methylethyl)-2,4(1H,3H)-pteridinedione | 60.708 | — | 0.105 | — | — | — |
| 279 | trans-Z-α-Bisabolene epoxide | 60.737 | — | — | — | — | 0.42 (S) |
| 280 | Aromadendrene oxide-(1) | 61.066 | — | — | 0.814 (S) | — | — |
| 281 | Cedrol | 61.072 | 0.376 (S) | — | — | — | — |
| 282 | 1-Hydroxy-6-(3-isopropenyl-cycloprop-1-enyl)-6-methyl-heptan-2-one | 61.153 | — | 1.008 | — | — | — |
| 283 | (1R, 7R, 8aS)-1,2,3,5,6,7,8,8a-Octahydro-1,8a-dimethyl-7-(1-methylethenyl)-naphthalene | 61.170 | — | — | — | — | 2.925 (S) |
| 284 | α-Farnesene | 61.546 | — | — | — | — | 0.429 |
| 285 | 2-Methylene-6,8,8-trimethyl-tricyclo[5.2.2.0(1,6)]undecan-3-ol | 61.592 | — | 0.156 | — | — | — |
| 286 | Thiocyanic acid-4-(dimethylamino)phenyl ester | 61.702 | — | — | — | — | 0.354 |
| 287 | [4aR-(4aα, 7α, 8aβ)]-Decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-naphthalene | 61.892 | — | — | — | — | 0.198 (S) |
| 288 | Decahydro-2,2,4,8-tetramethyl-4,8-methanoazulen-9-ol stereoisomer | 62.060 | — | — | — | — | 0.306 (S) |
| 289 | Diphenylmethane | 62.551 | — | — | — | 0.27 (B) | — |
| 290 | [1aR-(1aα, 4β, 4aβ, 7α, 7aβ, 7bα)]-Decahydro-1,1,4,7-tetramethyl-1H-cycloprop[e]azulen-4-ol | 62.568 | — | — | — | — | 0.205 (S) |
| 291 | Spiro(tricyclo[6.2.1.0(2,7)]undeca-2,4,6,9-tetraene-11,1′-cyclopropane | 62.568 | 0.198 (B) | — | — | — | — |
| 292 | 4-Methyl-1-[2,6,6-trimethyl-2-cyclohexen-1-yl]-1-penten-3-one, | 62.603 | — | 0.149 | — | — | — |
| 293 | [1S-(1α, 7α, 8aα)]-1,2,3,5,6,7,8,8a-Octahydro-1,8a-dimethyl-7-(1-methylethenyl)-naphthalene | 62.788 | — | — | — | — | 0.248 (S) |
| 294 | Caryophyllene | 63.007 | — | — | — | — | 0.172 (S) |
| 295 | Nonadecane | 63.059 | 0.129 | 0.134 | 0.125 | 0.145 | — |
| 296 | 7-Methoxy-3,4-dihydro-2[1H]-quinoxalinone | 63.233 | 0.241 (B) | 0.27 (B) | 0.367 (B) | — | — |
| 297 | 2-Allyl-1,4-dimethoxybenzene | 63.296 | — | — | — | — | 1.347 (B) |
| 298 | Dehydroxy-isocalamendiol | 63.493 | — | — | 0.241 (S) | — | — |
| 299 | 3,4,5,6-Tetramethyl-2,5-octadiene | 63.504 | 0.133 (S) | — | — | — | — |
| 300 | Megastigmatrienone | 63.539 | — | — | — | — | 0.914 |
| 301 | 6-(1-Hydroxymethylvinyl)-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-1H-naphthalen-2-one | 63.573 | — | 0.286 (B) | — | — | — |
| 302 | α-Ethyl-benzeneacetamide | 63.729 | — | — | — | — | 0.442 (B) |
| 303 | 8-Ethenyl-3,4,4a,5,6,7,8,8a-octahydro-5-methylene-2-naphthalenecarboxylic acid | 63.747 | — | 0.175 (B) | — | — | — |
| 304 | [1S-(1α, 2β, 4β)]-1-Ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane | 63.989 | — | — | — | — | 0.666 (S) |
| 305 | 1-Methylphenazine 5-oxide | 63.989 | — | — | — | 0.945 (B) | — |
| 306 | 3-Phenylbicyclo(3.2.2)nona-3,6-dien-2-one | 63.995 | — | — | 0.217 (B) | — | — |
| 307 | 1-(1-Hydroxybutyl)-2,5-dimethoxybenzene | 64.035 | 0.423 (B) | 0.49 (B) | — | — | — |
| 308 | Methyleugenol | 64.538 | 0.123 (B) | 0.315 (B) | 0.23 (B) | — | — |
| 309 | 2-Ethyl-3,4-dihydro-2H-1-benzothiopyran | 64.630 | — | — | — | — | 1.177 (B) |
| 310 | Acetic acid-cyano-hydroxyimino-methyl ester | 65.006 | — | — | — | — | 0.484 |
| 311 | N-Dimethylaminomethylene-anthranilic acid | 65.012 | 0.252 (S) | 0.26 (S) | 0.165 (S) | — | — |
| 312 | 2-Methyl-9-(prop-1-en-3-ol-2-yl)-bicyclo[4.4.0]dec-2-ene-4-ol | 65.243 | — | — | 1.25 (S) | — | — |
| 313 | (1aR, 4aR, 7R, 7aR, 7bS)-Decahydro-1,1,7-trimethyl-4-methylene-1H-cycloprop[e]azulene | 65.254 | 0.514 (B) | 1.064 (B) | — | — | — |
| 314 | [4aR-(4aα, 5α, 8aα)]-4a,5,6,7,8,8a-Hexahydro-3,4a,5-trimethyl-naphtho[2,3-b]furan-9(4H)-one | 65.381 | — | — | — | — | 4.963 (S) |
| 315 | 7,7,8,8-Tetracyanoquinodimethane | 65.716 | 0.137 (B) | — | — | — | — |
| 316 | 4-(1,3,3-Trimethyl-bicyclo[4.1.0]hept-2-yl)-but-3-en-2-one | 65.780 | — | 0.233 | — | — | — |
| 317 | Alloaromadendrene oxide | 65.820 | — | — | — | — | 0.543 (S) |
| 318 | γ-Elemene | 66.207 | — | — | — | — | 0.835 (S) |
| 319 | 1-(2,4,6-Trimethylphenyl)-3-(2-propynyl)-thiourea | 66.698 | 0.68 (B) | 1.52 (B) | 1.68 (B) | — | 5.466 (B) |
| 320 | 3,4-Dimethylphenyl trifluoro-acetate | 66.883 | — | — | 0.209 (B) | — | — |
| 321 | 12-Azabicyclo[9.2.2]pentadeca-1(13),11,14-trien-13-ylamine | 66.901 | 0.205 | 0.276 | — | — | 1.177 |
| 322 | 1-Butyl-1H-pyrrole | 67.062 | 0.213 | 0.288 | 0.174 | — | — |
| 323 | (3aα, 8β, 8aα)-5,6-1,2,3,3a,8,8a-Hexahydro-2,2,8-trimethyl-azulenedimethanol | 67.259 | — | — | — | — | 0.631 (S) |
| 324 | (2R, 5S, 10R)-6,10-Dimethyl-2-(1-methylethenyl)-spiro[4.5]dec-6-en-8-one | 67.386 | 0.326 (S) | — | — | — | — |
| 325 | N-Salicylidene-N′-salicyloylhydrazine | 67.496 | — | 0.597 (B) | — | — | — |
| 326 | 3,5-Dimethyl-benzenamine | 67.507 | — | — | — | — | 0.633 (B) |
| 327 | 2-Allyl-3-ethoxy-4-methoxyphenol | 67.709 | — | — | 1.709 (B) | — | — |
| 328 | 1,2-Dimethoxy-4-(3-methoxy-1-propenyl)benzene | 67.732 | 1.572 (B) | — | — | — | — |
| 329 | Levomenol | 67.778 | — | — | — | — | 0.305 (S) |
| 330 | n-Hexadecanoic acid | 67.807 | — | — | — | 1.701 | — |
| 331 | 2,2,8,8-Tetramethyl-3,6-nonadien-5-one | 67.958 | — | — | — | — | 0.428 |
| 332 | 3,5-Dimethoxy-4-hydroxycinnamaldehyde | 68.119 | 0.123 (B) | 1.723 (B) | — | 3.472 (B) | — |
| 333 | 1-Hydroxy-6-methylphenazine | 68.529 | 1.19 (B) | 1.897 (B) | 1.555 (B) | — | — |
| 334 | Desaspidinol | 68.847 | — | — | — | 3.559 (B) | — |
| 335 | 2-Chloro-4-cyclohexyl-phenol | 68.882 | 0.177 (B) | — | 0.302 (B) | — | — |
| 336 | 5-Ethyl-1,2,3,4-tetrahydro-naphthalene | 68.899 | — | — | — | — | 0.779 (B) |
| 337 | Heptadecane | 69.361 | — | — | — | 0.195 | — |
| 338 | Humulane-1,6-dien-3-ol | 69.384 | — | — | — | — | 0.784 (S) |
| 339 | 8,8-Dimethyl-9-methylene-1,5-cycloundecadiene | 69.777 | — | — | — | — | 0.465 (S) |
| 340 | 2,4-Dichloro-1-nitrobenzene | 70.083 | — | — | — | — | 0.429 (B) |
| 341 | 1-(2-Benzyloxyethyl)cyclohexene | 70.453 | — | — | 0.369 (B) | — | — |
| 342 | Caryophyllene oxide | 70.476 | — | — | — | — | 2.082 (S) |
| 343 | Aromadendrene oxide-(2) | 70.476 | 0.272 (S) | — | — | — | — |
| 344 | Diepicedrene-1-oxide | 70.545 | — | 0.368 (S) | — | — | — |
| 345 | 3,4-Dihydro-3,3,6,8-tetramethylnaphthalen-1(2H)-one | 70.580 | — | — | — | 0.115 (B) | — |
| 346 | 1-Methyl-2,4-bis(1-methylethenyl)-cyclohexane | 71.031 | — | — | — | — | 0.248 (S) |
| 347 | [1aR-(1aα, 4aα, 7β, 7aβ, 7bα)]-Decahydro-1,1,7-trimethyl-4-methylene-1H-cycloprop[e]azulen-7-ol | 71.140 | — | — | — | — | 0.512 (S) |
| 348 | 3-Hydroxy-2-methyl-4-[4-t-butyl]-butanal | 72.481 | 0.242 | — | — | — | — |
| 349 | 1-(1-Hydroxy-3-methoxy-2-naphthyl)ethanone | 72.498 | — | — | — | — | 1.586 (B) |
| 350 | 2-(Butenyl)-5-(1,1-dimethylethyl)-1,3-dimethyl-benzene | 72.544 | — | 0.346 (B) | — | — | — |
| 351 | (1R, 2R, 6S, 7S, 8S) -1-Methyl-8-(1-methylethyl)-tricyclo[4.4.0.02,7]dec-3-ene-3-methanol | 72.885 | — | — | 0.359 (S) | — | — |
| 352 | 4-Hydroxy-4a,5-dimethyl-3-methylene-3a,4,4a,5,6,7,9,9a-octahydro-3H-naphtho[2,3-b]furan-2-one | 72.896 | 0.194 (B) | — | — | — | — |
| 353 | 1,2,3,4-Tetrahydro-6-nitronaphthalene | 72.931 | — | — | — | — | 0.888 (B) |
| 354 | 1-(3,3-Dimethyl-1-yl)-2,2-dimethylcyclopropane-3-carboxylic acid | 72.960 | — | 0.324 | — | — | — |
| 355 | N-(p-Methoxy-trans-styryl)-formamide | 73.272 | — | — | — | — | 0.546 (B) |
| 356 | 8,9-Dehydro-9-formyl-cycloisolongifolene | 73.705 | 0.548 (S) | — | 0.691 (S) | — | — |
| 357 | 2-tert-Butyl-quinoxaline 4-oxide | 73.745 | — | — | — | — | 1.237 (B) |
| 358 | β-Vatirenene | 73.763 | — | 0.803 (S) | — | — | — |
| 359 | Octadecanal | 73.919 | — | — | — | 0.144 | — |
| 360 | [1S-(1α, 3aβ, 4α, 7aβ)]-Octahydro-1,7a-dimethyl-4-(1-methylethenyl)-1,4-methano-1H-indene | 74.872 | — | — | — | — | 0.368 (S) |
| 361 | 1-(1-Hydroxyethyl)-1-(diethylphosphonyl)-2-methylene-cyclopropane | 74.878 | — | 0.109 | — | — | — |
| 362 | 1-Nonadecene | 75.265 | — | — | — | 0.149 | — |
| 363 | (E,E)-3,7-Dimethyl-10-(1-methylethylidene)-3,7-cyclodecadien-1-one | 75.692 | — | — | — | — | 0.232 (S) |
| 364 | Alloaromadendrene | 76.195 | — | — | — | — | 0.488 (S) |
| 365 | Heneicosane | 76.235 | — | 0.148 | 0.177 | 0.127 | — |
| 366 | 2,3-Dihydro-7-hydroxy-2,2-dimethyl-4H-1-benzopyran-4-one | 76.859 | — | — | — | — | 0.277 (B) |
| 367 | 2-Butyl-5-hexyloctahydro-1H-indene | 77.344 | — | — | — | — | 0.253 |
| 368 | 2,2′ : 5′,2″-Terthiophene | 77.777 | — | — | — | — | 0.228 |
| 369 | 1-Methyl-4-(2-methyloxiranyl)-7-oxabicyclo[4.1.0]heptane | 78.355 | — | — | — | — | 0.248 (B) |
| 370 | 4,4-Dimethyl-1-phenyl-1-penten-3-one | 78.563 | — | — | — | — | 0.199 (B) |
| 371 | 1,5-Diphenyl-1-penten-3-one | 78.603 | 0.16 (B) | 0.354 (B) | 0.139 (B) | — | — |
| 372 | (1-Methylbutyl)-benzene | 79.424 | — | — | — | — | 0.266 (B) |
| 373 | Stearic acid | 79.504 | — | — | — | 0.198 | — |
| 374 | [1S-(1α, 2α, 3aβ, 4α, 8aβ, 9R∗)]-Decahydro-1,5,5,8a-tetramethyl-1,2,4-methenoazulene | 79.666 | — | — | — | — | 0.186 (S) |
| 375 | Z-8-Methyl-9-tetradecenoic acid | 79.799 | — | 0.105 | — | — | — |
| 376 | 1,2,3,4-Tetrahydro-1,5,7-trimethylnaphthalene | 79.903 | — | — | — | — | 0.426 (B) |
| 377 | N-Phenyl-2-naphthylamine | 80.746 | 0.121 (B) | — | 0.186 (B) | — | — |
| 378 | Z-5-Nonadecene | 81.024 | 0.196 | — | 0.181 | 0.230 | — |
| 379 | Cyclopentadecane | 81.047 | — | 0.188 | — | — | — |
| 380 | Diaveridine | 81.105 | — | — | — | — | 0.108 (B) |
| 381 | (Z)-3-Tridecen-1-yne | 81.428 | — | — | — | — | 0.322 |
| 382 | Ambrosin | 82.277 | — | — | — | — | 0.102 (S) |
| 383 | 2-Decanone O-methyl oxime | 82.329 | 0.104 | 0.115 | 0.092 | — | — |
| 384 | N,N-Dimethyldecanamide | 82.352 | — | — | — | 0.140 | — |
| 385 | [1aR-(1aα, 4aβ, 8aS∗)]-1,1a,5,6,7,8-Hexahydro-4a,8,8-trimethyl-cyclopropa[d]naphthalen-2(4aH)-one | 82.710 | — | — | — | — | 0.124 (S) |
| 386 | Murolan-3,9(11)-diene-10-peroxy | 83.248 | — | — | — | — | 0.267 (S) |
| 387 | Chromone derivative | 85.396 | 0.109 (C) | — | 0.08 (C) | — | — |
| 388 | Chromone derivative | 85.818 | 0.132 (C) | 0.217 (C) | 0.151 (C) | — | — |
| 389 | Chromone derivative | 86.783 | 2.245 (C) | 1.812 (C) | 1.697 (C) | 3.633 (C) | — |
| 390 | Chromone derivative | 87.187 | 0.104 | — | — | 0.112 | — |
| 391 | Chromone derivative | 87.539 | — | — | — | — | 0.331 (C) |
| 392 | Chromone derivative | 88.724 | 0.159 | — | — | — | — |
| 393 | Chromone derivative | 88.880 | 0.118 | — | 0.123 | — | — |
| 394 | Chromone derivative | 88.891 | — | — | — | — | — |
| 395 | Chromone derivative | 88.897 | — | 0.152 (C) | — | 0.175 | — |
| 396 | Chromone derivative | 89.209 | 0.14 (C) | 0.662 (C) | 0.345 (C) | — | 0.334 (C) |
| 397 | Chromone derivative | 89.515 | — | — | 0.064 (C) | 0.14 (C) | — |
| 398 | Chromone derivative | 89.769 | — | — | 0.221 (C) | — | — |
| 399 | Chromone derivative | 89.787 | — | 0.125 (B) | — | — | — |
| 400 | Chromone derivative | 89.844 | 0.286 | — | — | — | — |
| 401 | Chromone derivative | 89.867 | — | 0.108 (B) | — | — | — |
| 402 | Chromone derivative | 90.595 | — | — | 3.447 (C) | — | — |
| 403 | Chromone derivative | 90.601 | 1.127 (C) | — | — | — | — |
| 404 | Chromone derivative | 90.630 | — | — | — | 0.179 (C) | — |
| 405 | Chromone derivative | 90.641 | — | 3.239 (C) | — | — | — |
| 406 | Chromone derivative | 90.676 | — | — | — | — | 7.398 (C) |
| 407 | Chromone derivative | 91.000 | — | — | 0.197 (C) | — | — |
| 408 | Chromone derivative | 91.000 | 0.121 (C) | — | — | — | — |
| 409 | Chromone derivative | 91.017 | — | 0.148 (C) | — | — | — |
| 410 | Chromone derivative | 91.485 | — | — | 3.502 (C) | — | — |
| 411 | Chromone derivative | 91.485 | 1.32 (C) | — | — | — | — |
| 412 | Chromone derivative | 91.571 | — | — | — | — | 0.542 (C) |
| 413 | Chromone derivative | 91.571 | — | 4.593 (C) | — | — | — |
| 414 | Chromone derivative | 91.629 | — | — | — | 0.105 (C) | — |
| 415 | Chromone derivative | 91.710 | — | — | 0.649 (C) | — | — |
| 416 | Chromone derivative | 91.716 | 0.132 (C) | — | — | — | — |
| 417 | Chromone derivative | 91.727 | — | — | — | — | 1.405 (C) |
| 418 | Chromone derivative | 91.745 | — | 0.561 (C) | — | — | — |
| 419 | Chromone derivative | 91.803 | — | — | 0.592 (C) | — | — |
| 420 | Chromone derivative | 91.808 | 0.481 (C) | — | — | — | — |
| 421 | Chromone derivative | 91.837 | — | — | — | 0.411 | — |
| 423 | Chromone derivative | 91.837 | — | 0.539 (C) | — | — | — |
| 422 | Chromone derivative | 91.953 | — | — | 0.88 (C) | — | — |
| 424 | Chromone derivative | 91.953 | 0.434 (C) | — | — | — | — |
| 425 | Chromone derivative | 91.987 | — | 0.796 (C) | — | — | — |
| 426 | Chromone derivative | 92.571 | — | — | — | — | 0.143 (C) |
| 427 | Chromone derivative | 92.894 | — | 0.254 (B) | — | — | — |
| 428 | Chromone derivative | 92.935 | — | — | — | — | 1.293 (C) |
| 429 | Chromone derivative | 92.958 | — | — | 0.221 (C) | — | — |
| 430 | Chromone derivative | 92.969 | — | 0.485 (B) | — | — | — |
| 431 | Chromone derivative | 93.339 | — | — | — | — | 0.388 (C) |
| 432 | Chromone derivative | 93.784 | — | — | — | — | 0.6 (C) |
| 433 | Chromone derivative | 94.136 | — | — | — | — | 0.14 (C) |
| 434 | Chromone derivative | 94.356 | — | — | 0.731 | — | — |
| 435 | Chromone derivative | 94.361 | — | — | — | — | 0.125 (C) |
| 436 | Chromone derivative | 94.361 | 0.596 (C) | — | — | — | — |
| 437 | Chromone derivative | 94.390 | — | 0.832 (C) | — | — | — |
| 438 | Chromone derivative | 94.795 | — | — | 0.323 (C) | — | — |
| 439 | Chromone derivative | 94.800 | 0.187 (C) | — | — | — | — |
| 440 | Chromone derivative | 94.812 | — | 0.371 (C) | — | — | — |
| 441 | Chromone derivative | 95.228 | — | — | — | — | 1.254 (C) |
| 442 | Chromone derivative | 95.228 | — | — | 0.596 (C) | — | — |
| 443 | Chromone derivative | 95.257 | — | 0.614 (C) | — | — | — |
| 444 | Chromone derivative | 95.268 | 0.165 (C) | — | — | — | — |
| 445 | Chromone derivative | 95.609 | — | — | — | — | 0.217 (C) |
| 446 | Chromone derivative | 95.748 | 0.243 (C) | — | — | — | — |
| 447 | Chromone derivative | 95.754 | — | — | 0.237 (C) | — | — |
| 448 | Chromone derivative | 95.765 | — | — | — | 0.478 (B) | — |
| 449 | Chromone derivative | 95.771 | — | 0.206 (C) | — | — | — |
| 450 | Chromone derivative | 95.834 | — | — | — | — | 0.414 (C) |
| 451 | Chromone derivative | 95.904 | 0.291 (C) | — | — | — | — |
| 452 | Chromone derivative | 95.921 | — | — | — | 0.678 (B) | — |
| 453 | Chromone derivative | 95.967 | 0.713 (C) | — | — | — | — |
| 454 | Chromone derivative | 95.973 | — | — | 3.243 (C) | — | — |
| 455 | Chromone derivative | 96.019 | — | 2.703 (C) | — | — | — |
| 456 | Chromone derivative | 96.088 | — | — | — | — | 7.2 (C) |
| 457 | Chromone derivative | 96.308 | — | — | 0.178 (C) | — | — |
| 458 | Chromone derivative | 96.308 | 0.192 (C) | — | — | — | — |
| 459 | Chromone derivative | 96.319 | — | — | — | 0.177 (B) | — |
| 460 | Chromone derivative | 96.343 | — | 0.281 (C) | — | — | — |
| 461 | Chromone derivative | 96.372 | — | — | — | — | 0.498 (C) |
| 462 | Chromone derivative | 96.395 | — | — | 0.267 (C) | — | — |
| 463 | Chromone derivative | 96.400 | 0.313 (C) | — | — | — | — |
| 464 | Chromone derivative | 96.412 | — | — | — | 0.381 | — |
| 465 | Chromone derivative | 96.418 | — | 0.43 (B) | — | — | — |
| 466 | Chromone derivative | 96.556 | — | 0.138 (B) | — | — | — |
| 467 | Chromone derivative | 96.632 | — | — | 0.058 (C) | — | — |
| 468 | Chromone derivative | 96.637 | 0.312 (C) | — | — | — | — |
| 469 | Chromone derivative | 96.649 | — | — | — | 0.236 (B) | — |
| 470 | Chromone derivative | 96.655 | — | 0.16 (B) | — | — | — |
| 471 | Chromone derivative | 96.718 | — | — | — | — | 0.332 (C) |
| 472 | Chromone derivative | 96.753 | — | 0.206 | — | — | — |
| 473 | Chromone derivative | 97.088 | — | — | — | — | 0.464 (C) |
| 474 | Chromone derivative | 97.319 | — | — | — | — | 0.176 (C) |
| 475 | Chromone derivative | 97.469 | — | — | — | — | 0.171 (C) |
| 476 | Chromone derivative | 97.700 | — | — | — | — | 0.469 (C) |
| 477 | Chromone derivative | 98.341 | — | — | — | — | 0.863 (C) |
| 478 | Chromone derivative | 98.399 | — | 0.103 | — | — | — |
| 479 | Chromone derivative | 98.982 | — | — | — | — | 0.104 (C) |
| 480 | Chromone derivative | 99.242 | — | — | — | — | 0.374 (C) |
| 481 | Chromone derivative | 99.566 | — | — | — | — | 0.264 (C) |
| 482 | Chromone derivative | 99.751 | — | — | — | — | 0.192 (C) |
| 483 | Chromone derivative | 100.034 | — | — | — | — | 0.281 (C) |
| 484 | Chromone derivative | 100.346 | — | — | — | — | 0.577 (C) |
| Total | 97.620 | 97.164 | 99.859 | 97.040 | 96.079 | ||
- B: aromatic compound; C: chromone derivative; S: sesquiterpenes; —: not detected.
2.4. LPS-Stimulated TNF-α and IL-1α Release in RAW264.7 Cells
2.4.1. Isolation and Culture of RAW264.7 Cells
RAW264.7 cells in logarithmic growth phase were washed twice with phosphate-buffered saline (PBS) and inoculated in 96-well plates at a density of 1 × 104 cells per well, and 100 μL of cell suspension was added to each well. Three compound wells were set in each group and cultured at 37°C in 5% CO2 for 24 h.
2.4.2. Measurement of TNF-α and IL-1α Production
The cells were incubated with 1 ng/mL LPS in the presence of indomethacin, AAW, BCDA, and AWIT (20, 40 and 80 μg/mL) and cultured at 37°C and 5% CO2 for 24 h. Then, the levels of TNF-α and IL-1α in the cell-free culture supernatant were determined by ELISA kits. Briefly, 10 μL of supernatant was mixed with an equal volume of reagents A and B [1 : 1 (v/v)] in a 96-well flat-bottom plate. The absorbance at 540 nm was measured after 10 min using an ELISA reader. The amounts of TNF-α and IL-1α were calculated from a standard curve created using known concentrations of standards.
3. Results and Discussion
3.1. GC-MS Analysis
n-Hexane, methanol, DMSO, and CH2Cl2 were used to collect the chemical constituents of incense smoke from agarwood. The GC-MS peaks of incense smoke samples collected using CH2Cl2 were the most intense among the peaks obtained using the above solvents. Therefore, CH2Cl2 was selected to dissolve the chemical constituents of smoke samples (agarwood and AS). Finally, 484 compounds in total (Table 1 and Figure 1) were identified from the incense smoke samples (AAW, BCDA, AWIT, and AS) and the samples obtained by CH2Cl2 extraction of sticks from AWIT. The numbers of compounds identified in incense smoke from AAW, BCDA, AWIT, AS, and EAWIT were 167, 158, 141, 127, and 131, respectively. Aromatics and chromone derivatives were the main chemical constituents in AAW, BCDA, and AWIT; among all chemical constituents, aromatics represented 69.617, 55.038, and 60.483%, and chromone derivatives represented 9.252, 17.725, and 16.946%, respectively.
The chemical constituents of incense smoke may be quantifiable. Therefore, the chemical constituents of incense smoke from agarwood produced by AWIT were compared with the corresponding constituents from agarwood produced by AAW and BCDA. A total of 61 compounds in the AWIT sample, representing 54.837%, were also found in the AAW and BCDA samples. The major compounds (relative content >1%) were phenylethylene (7.973%); ethylbenzene (6.384%); 2,3,5,6-tetrafluoroanisole (4.130%); 5-(2-thienyl)-4-pyrimidinamine (2.402%); 2-methoxy-4-(1-propen-1-yl)-phenol (2.379%); 2,6-dimethoxyphenol (2.178%); syn-3,3,5,6,8,8-hexamethyl-tricyclo[5.1.0.0(2,4)]oct-5-ene (1.762%); 1-(2,4,6-trimethylphenyl)-3-(2-propynyl)-thiourea (1.680%); phenylacetylene (1.612%); 1-hydroxy-6-methylphenazine (1.555%); 4-hydroxy-3-methoxystyrene(1.512%) 3,5-dimethoxy-4-hydroxyphenylacetic acid (1.391%); 2,2,4,6,6-pentamethylheptane (1.152%); 4-(4-methoxyphenyl)-2-butanone (1.124%); 4-phenyl-2-butanone (1.060%); and benzaldehyde (1.008%). Moreover, chromone derivatives and sesquiterpenes are the main components responsible for pharmacodynamic effects [23–25]. In this experiment, 21 compounds, representing 16.946%, were identified as chromone derivatives according to the peaks at m/z 91, 121, 137, 107, 160, 176, 190, 220, 250, 266, 280, 282, 296, 310, 312, 326, 328, and 342 [22], and 16 compounds were identified as sesquiterpenes, representing 6.768%. In short, aromatic compounds were the main chemical constituents of incense smoke from agarwood, including AWIT, AAW, and BCDA samples.
To identify whether agarwood (AAW, AWIT, and BCDA) contained chemical constituents of AS, incense smoke produced from AS was tested by the same method. No sesquiterpenes were detected among the chemical constituents of the smoke, and chromones only represented 4.569%, which was less than the contents in AWIT (16.946%), AAW (9.252%), and BCDA (17.725%). Finally, 29 compounds, representing 32.627%, were also found in AWIT, AAW, and BCDA. The main compounds (relative amount >1%) were phenylethylene (5.132%); 2-methoxy-4-(1-propen-1-yl)-phenol (3.254%); 3,5-dimethoxy-4-hydroxybenzaldehyde (2.920%); 4-hydroxy-3-methoxystyrene (2.254%); 3,5-dimethoxy-4-hydroxyphenylacetic acid (2.150%); vanillin (1.842%); acetosyringone (1.499%); guaiacol (1.362%); ethylbenzene (1.313%); benzaldehyde (1.146%); homovanillyl alcohol (1.119%); 2,6-dimethoxy-4-(2-propen-1-yl)-phenol (1.029%); and phenylacetylene (1.013%). These components may be from the residue of A. sinensis, a sticky powder, making agarwood powder bind, used in the preparation of sticks of AS (making sticks from pure AS alone is difficult, so the addition of a sticky powder is necessary) or agarwood in A. sinensis (AS can form agarwood in the process of storage).
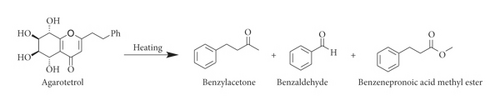
The data for incense smoke from agarwood (AAW, AWIT, and BCDA) showed that low-molecular-weight aromatic compounds (LACs) represented more than 55% of the total constituents. Michiho Ito et al. reported that chromone derivatives could be converted and produce the pleasant smell of agarwood through the generation of LACs in the process of heating [26,27] (Scheme 1). Chromone derivatives are among the main chemical constituents of agarwood. They can generate unique and different LACs at high temperature (when burned). As a result, many LACs were detected in the agarwood smoke. To verify the results, an extraction experiment of sticks from AWIT was carried out at room temperature (to avoid high temperature). The results showed that chromone derivatives, sesquiterpenes, and aromatics were the main chemical constituents, representing 26.547, 26.767, and 26.941% of the total constituents, respectively. Few chemical constituents of EAWIT were observed before 40 min (tR), as shown in Figure 1, while there was far higher number of peaks after 58 min (tR), which is indicative of chromone derivatives and sesquiterpenes. Interestingly, the chemical constituents of incense smoke showed the opposite trend in Figure 1. The results indicated that high-molecular-weight compounds might be cracked into low-molecular-weight compounds at high temperature. In other words, some chromone derivatives and sesquiterpenes might be converted into low-molecular-weight compounds, which is consistent with the reported literature [26, 27]. Therefore, low-molecular-weight compounds accounted for a high percentage of the incense smoke obtained from agarwood during burning. Moreover, some studies suggested that the inhalation of some LACs had a sedative or hypnotic effect on mice and that benzylacetone in particular reduces mouse locomotor activity [28–30]. Hence, inhalation of the pleasant aroma generated by agarwood during heating could lead to pharmacological effects.
3.2. Effect of Chemical Constituents on TNF-α and IL-1α Release in LPS-Stimulated RAW264.7 Cells
As shown in Tables 2 and 3, normal inactivated RAW264.7 cells produced low amounts of TNF-α and IL-1α after 24 h of incubation at 37°C, and exposure to LPS induced higher amounts of TNF-α and IL-1α. In contrast, under indomethacin treatment, AAW, BCDA, and AWIT produced a concentration-dependent decrease at concentrations of 20, 40, and 80 μg/mL. The TNF-α and IL-1α levels of model group were significantly higher than those of the normal group (P < 0.05 or P < 0.01). The incense components of AAW, BCDA, AWIT, and indomethacin significantly reduced TNF-α and IL-1α levels (P < 0.05, P < 0.01, or P < 0.001), showing better anti-inflammatory effects. These results showed that the anti-inflammatory activities of AAW, AWIT, and indomethacin were comparable and superior to that of BCDA.
| Drug/dose | 80 μg/mL | 40 μg/mL | 20 μg/mL | |
|---|---|---|---|---|
| Normal | 189.09 ± 15.25 | |||
| Model | 236.09 ± 18.79 ∗∗ | |||
| Indomethacin | 152.39 ± 16.67### | 169.14 ± 18.23### | 200.19 ± 19.42# | |
| AAW | 157.69 ± 15.98### | 181.79 ± 19.45## | 209.19 ± 21.03# | |
| BCDA | 165.09 ± 16.12### | 194.84 ± 17.67## | 213.04 ± 22.43# | |
| AWIT | 154.89 ± 17.13### | 187.84 ± 18.37## | 212.84 ± 19.35# |
- Note. This result is the average of three parallel experiments. ∗∗P < 0.01 vs normal; ###P < 0.001, ##P < 0.01, #P < 0.05 vs model.
| Durg/dose | 80 μg/mL | 40 μg/mL | 20 μg/mL | |
|---|---|---|---|---|
| Normal | 15.00 ± 1.78 | |||
| Model | 20.43 ± 2.32 ∗ | |||
| Indomethacin | 10.38 ± 2.12## | 12.52 ± 2.65## | 17.87 ± 1.85# | |
| AAW | 10.78 ± 2.56## | 15.72 ± 2.57# | 19.10 ± 2.58 | |
| BCDA | 15.05 ± 1.74# | 18.99 ± 2.97 | 22.19 ± 2.94 | |
| AWIT | 10.88 ± 1.97## | 12.15 ± 2.72## | 22.52 ± 2.93 |
- Note. This result is the average of three parallel experiments. ∗P < 0.05 vs normal; ##P < 0.01, #P < 0.05 vs model.
4. Conclusions
The chemical constituents of incense smoke from AAW, BCDA, AWIT, AS, and EAWIT were analyzed by GC-MS, and 484 compounds were identified. Aromatic compounds were the main chemical constituents of incense smoke from AAW, BCDA, and AWIT. A total of 61 aromatic compounds from AWIT, representing 54.837%, were also found in AAW and BCDA. All experimental data suggested that aromatic compounds were the main chemical constituents in agarwood smoke and that some chromone derivatives could be cracked into LACs at high temperature. Furthermore, agarwood incense smoke showed anti-inflammatory activities by inhibiting lipopolysaccharide- (LPS-) induced TNF-α and IL-1α release in RAW264.7 cells.
Conflicts of Interest
The authors declare that they have no conflicts of interest in any form.
Authors’ Contributions
De-Qian Peng, Zhang-Xin Yu contributed equally to this work.
Acknowledgments
This research work was financially supported by the Natural Science Foundation of Hainan Province (no. 217291), the Science and Technology Programs from Hainan Province of China (no. ZDKJ2016004), and the Major Science and Technology Innovation Project of the Chinese Academy of Medical Sciences (no. 2016-I2M-2-003). The authors are grateful to Hai-Yan Wang (an English teacher) of Qiqihar University and Ass. Prof. Lu-Jia Mao of Hainan Medical University for revising the manuscript.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




