Color Tuning in the Luminescence of Two Oligomers Derived from N,N′-(Naphthalenediyl)bis-phenylimine Containing Oligomers
Abstract
Two oligomers with different molecular weight were produced. Fluorescence properties were examined both in solutions and in the solid state. In the solid state, molecular weight turned out to be an important factor influencing the aggregation structures and therefore the emission of the compounds, in colour and intensity. The neat compounds emit two different colours, blue and yellow, with increasing the molecular weight, whereas both compounds are blue emitters in solution and dispersed in host polymers. Dissolved in a LC host polymer and in a PVK matrix, they displayed noteworthy photoluminescence quantum yields.
1. Introduction
Traditional light-emitting devices are solid-state based consisting of a thin emissive polymer film sandwiched between anode and cathode [1–4]. Most recently, due to ease of fabrication processes and wide area of applications, soft-matter and solution-based light-emitting devices have been largely explored. Among them are electrochemiluminescent cells (ECLs) [5] with fluorescent dye-doped organic solvents as emissive layer, organic light-emitting diodes (OLEDs) [6] with a liquid host doped with a guest fluorophore, light-emitting electrochemical cells (LECs) [7, 8] where the emission is provided by a doped ionic liquid, and finally, devices based on fluorophore-doped LC layers, giving polarized light emission [9–12].
The high degree of flexibility due to the nature of the emissive layers is the reason for the growing interest not only in lighting and photovoltaics, but also in chemo- and biosensors [13], as well as in most modern exotic applications such as PDLC panels and displays, smart windows, and colour filter glasses [14]. The basic concept is the production of emissive flexible layers by dissolving or dispersing dye molecules in an organic host matrix [1, 5, 7–12, 15–22] such as highly mouldable polymers, liquid semiconductors [22], polymer-containing electrolytes [7, 8], ionic liquids [18, 19], and liquid crystal (LC).
Efficient emitters, thermally and chemically stable and easily processable, are required both for traditional solid state and for soft-matter/solution-based applications.
Salen Schiff bases are usually excellent candidates for their ability to tune emission colour and represent a good choice for full-colour displays [23, 24]. However, some high-performance fluorophores exhibit poor solubility in the host phase, which strongly impedes their practical applications in many light-emitting devices. For this reason, the search for new low-cost and easily processable fluorophores is still in progress.
In the present work, we demonstrate a simple system consisting of emitting 4,4′ [(2,3 naphthalenediylbis (nitrilomethyllidene)]bis 1,3 benzenediol containing oligomers able to be layered as neat solid or as dopants for host matrixes. To date, 2,3-diaminonaphthalene derivatives are known for the emission properties [25–31]. The insertion of the bulky benzoyloxy groups in the oligomeric Schiff base derivatives was expected to provide good PL performance also in the solid state [32–37]. Flexible chain length turned out to be an important structural factor influencing the aggregation structures [38–45] and hence the emission of the compounds, both in colour and intensity. Very impressively, a dimer and a pentamer with the same fluorogenic core and terminal groups emit two different colours, blue and yellow, in dependence to the different molecular weight (MW) values. The fluorescence properties of the two oligomers were examined as neat solids, in solution and in addition to mouldable host matrixes. In the latter case, a remarkable PL performance was observed.
2. Experimental Section
The compound A12G [46] was obtained as described in previous works. QYPDLC-102 nematic LC polymer was purchased from Canaanchem. 2,3-diaminonaphthalene, PS (molecular weight 18700 Da), and PVK (molecular weight 1100 Da) were commercially supplied by Sigma Aldrich.
Optical observations on a Zeiss Axioscop polarizing microscope equipped with an FP90 Mettler heating stage were performed. Phase transition temperatures and related enthalpies were measured under nitrogen flow using the DSC scanning calorimeter Perkin Elmer Pyris 1 with a scanning rate of 10°C/min. The decomposition temperature (Td) was assumed as the temperature where the 5 wt.% weight loss was determined by thermogravimetric analysis under air flow with a Perkin Elmer TGA 4000. 1H NMR spectra were recorded with Bruker Advance II 400 MHz apparatus in 1,1,2,2-tetrachloroethane d2. UV-visible and fluorescence spectra were recorded, respectively, with Jasco F-530 (scan rate 200 nm·min−1) and with Jasco FP-750 (excitation wavelengths set at the absorption maxima of the samples, scan rate 125 nm·min−1). X-ray powder diffraction data were collected in reflection geometry with the diffractometric apparatus Empyrean (PANalytical) using Ni-filtered Cu Kα radiation (λ = 0.15418 nm).
Solid thin film samples were deposed on quartz slides by an SCS P6700 spin-coater apparatus operating in two steps (600 rpm for 60 s and 2000 rpm for 60 s). To obtain the PS or PVK blends, neat samples 10% by weight in PS or PVK were dissolved in o-dichlorobenzene and spinned. The samples were annealed for 10 min at 100°C. The films were dried at 60°C for 20 min in vacuum. Doped LC samples were obtained by dissolving NPh2 and NPh5 (1% by weight) and the commercial nematic LC polymer, using acetone as a solvent, removing the solvent at 100°C and deposing the mixture between quartz slides.
PLQYs of films have been measured on quartz substrates by a Fluorolog 3 spectrofluorometer (Horiba Jobin Instruments SA), within an integrating sphere equipped with an optical fibre connection. In solution, PLQYs were calculated in diluted solutions below absorbance 0.10, according to Melhuish [47].
2.1. Synthesis of NPh2 and NPh5
The synthesis of NPh2 and NPh5 was performed in solution following the same basic procedure (Scheme 1). A detailed description is reported for NPh2. In this case, 1 : 2 stoichiometric ratio diamine/dialdehyde (NPh/A12G) was employed. A total of 4.316 g (6.32 mmol) of dialdehyde A12G and 0.500 g (3.16 mmol) of 2,3-diaminonaphthalene was dissolved in 40 mL of boiling o-dichlorobenzene. The polymerization was carried out under a nitrogen atmosphere for 20 min at boiling temperature. The solution is then poured into n-hexane, and the precipitated oligomer washed twice in n-hexane and oven-dried at 100°C. Tm = 128°C; Ti = 205°C; Td = 303°C (in air). 1H NMR (400 MHz, TCE-d2, 25°C, ppm): 1.21 (s), 1.43 (m), 1.60 (s), 1.76 (s) (60 H tot.); 4.07 (s, 12H), 6.78 (d, 10H), 6.84 (m, 12H), 7.65 (m, 12H), 7.82 (s, 2H), 8.13 (m, 18H), 8.70 (s, 4H), 9.72 (s, 2H), 11.22 (s, 2H). Elemental analysis calculated (%) for C140H138N4O26: C, 73.34; H, 6.07; N, 2.44; found: C, 74.00; H, 6.16; N, 2.50.
The oligomer NPh5 was obtained with the same procedure employing 1 : 1.5 diamine/dialdehyde (NPh/A12G) stoichiometric ratio. Tm = 133°C; Ti = 215°C; Td = 305°C (in air). 1H NMR (400 MHz, TCE-d2, 25°C, ppm): 11.21 (s), 1.44 (m), 1.82 (m) (124 H tot.); 3.95 (s, 20 H), 6.85 (m, 48H), 7.55 (m, 22H), 8.05 (m, 44H), 8.76 (s, 10H), 9.86 (s, 2H), 11.21 (s, 2H). Elemental analysis calculated (%) for C290H282N10O50: C, 73.99; H, 6.04; N, 2.98; found: C, 74.01; H, 6.06; N, 3.00.
3. Results and Discussion
In our preliminary attempts to produce high MW polymers between the monomers in 1 : 1 stoichiometric ratio, the reaction continued for 3 h in boiling o-dichlorobenzene (o-DCB). The product resulted was poorly emissive and scarcely soluble in common organic solvents and in the LC host matrix. For this reason, we decided to produce low MW oligomers.
The compounds NPh2 and NPh5 (see Scheme 1) were synthetized by varying monomers ratio in the same milder reaction conditions. In particular, they were obtained by refluxing for 1 h the dialdehyde A12G and 2,3-diaminonaphthalene (NPh) in o-DCB using different stoichiometric excess of monomer A12G. Both dyes contain a potentially emissive Schiff base N,N-bis (salicylaldehyde)-2,3 diaminonaphthalene core [31] (in red in Scheme 1) and flexible (CH2)12 chains. The oligomers were synthesized with 1 : 2 and 1 : 1.5 mole ratio of the monomers (NPh/A12G). Under those conditions, both oligomers resulted in salicylaldehyde.
The emissive salen Schiff base site can undergo the ESIPT (excited-state intramolecular proton transfer) process. The fast proton transfer in ESIPT dyes causing nonradiative deactivation is known to provide strong emission in solution but weak emission in the solid state. In this case, referring to a more recent approach [48–50], the steric hindrance due to the benzoyloxy bulky groups was expected to restrict intramolecular rotation providing good emission performance also in the solid state.
On average, NPh2 contains two and NPh5 five NPh-derived moieties, as confirmed by 1H NMR spectra. In Figure 1, the NMR spectrum of NPh2 (blue curve) shows a signal at 9.72 ppm due to the free terminal aldehyde. The integration of that signal with respect to the one due to the iminic proton (8.70 ppm) is very close to 1 : 2 ratio.
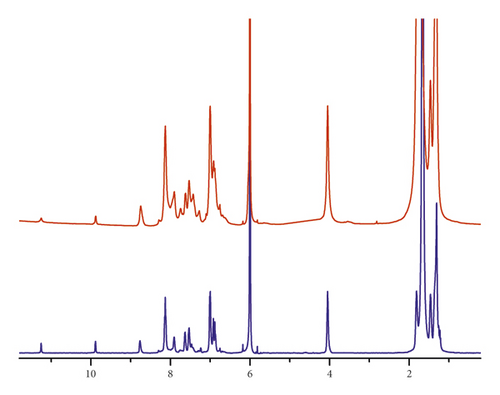
On this base, the spectrum identifies an oligomeric product derived from the condensation of two NPh and three A12G monomers. Moreover, the signal recorded at 4.07 ppm, due to O-CH2 protons, confirms the presence of 12 protons. The signal at 11.22 ppm is related with the terminal phenolic functions and is in the same ratio with the signal at 9.72 ppm. The signal of the phenolic protons intramolecularly bonded is absent in both the spectra. The NMR of a NPh5 sample shows a very similar pattern with broader signals. The ratio between the integral of the free terminal aldehydic protons (9.86 ppm) and the one related to the iminic proton (8.76 ppm) is about 1 : 5, indicating the condensation of five NPh and six A12G monomers, on average. Further confirmation is obtained from the integral of the O-CH2 proton signal (3.95 ppm) matching with about 23-24 protons. Once again, the integral for the terminal OH protons (11.21 ppm) has the same value as that for the aldehydic protons.
The combination of a rigid core and flexible (CH2)12 chains predicted a LC behaviour. By optical observation and supporting DSC and X-ray powder data, both oligomers were found to be semicrystalline. A very similar thermal behaviour and mesophase range stability were detected. Under polarized light, compound NPh2 melts to a marble nematic texture at 128°C and NPh5 at 133°C. Isotropization was recorded, respectively, at 205°C and 215°C, decomposition occurring above 300°C for both compounds. In the nematic phase, the dyes resulted emissive under an UV lamp at 365 nm.
NPh2 and NPh5 are soluble in common organic solvents and show blue emission (absorption and emission maxima reported in Table 1) with a poor solvatochromic effect depending on the polarity of the solvents. In natural light, the solutions are quite colourless with relevant Stoke’s shifts (about 130 nm) calculated from absorption to emission maxima in the same solvent. Due to the same emissive cores, photoluminescence quantum yields (PLQYs), measured in diluted acetone solutions, are very similar (respectively, 10% and 14%).
| Compound | λems (nm)a | λems (nm)b | State | λabs.f (nm)c | λem.f (nm)d | PLQY%e |
|---|---|---|---|---|---|---|
| NPh2 | 341 | 480 (PLQY = 10%) | Neat solid | 265 (342) | 488 | 45 ± 1 |
| PS-doped | 265 (342) | 483 | 24 ± 1 | |||
| PVK-doped | 291 (339) | 486 | 50 ± 1 | |||
| LC-doped | 350 | 484 | 70 ± 1 | |||
| NPh5 | 340 | 481 (PLQY = 14%) | Neat solid | 275 (346) | 275 (346) | 18 ± 1 |
| PS-doped | 260 (340) | 260 (340) | 16 ± 1 | |||
| PVK-doped | 290 (343) | 290 (343) | 54 ± 1 | |||
| LC-doped | 345 | 345 | 66 ± 1 | |||
- aWavelength of UV-visible absorbance maxima in acetone solution. bWavelength of emission maxima in acetone solution and PLQY in brackets. cWavelength of UV-visible absorbance maxima on the thin film obtained as specified in the fourth column. dWavelength of emission maxima on the thin film obtained as specified in the fourth column. ePL quantum yield on the thin film obtained as specified in the fourth column.
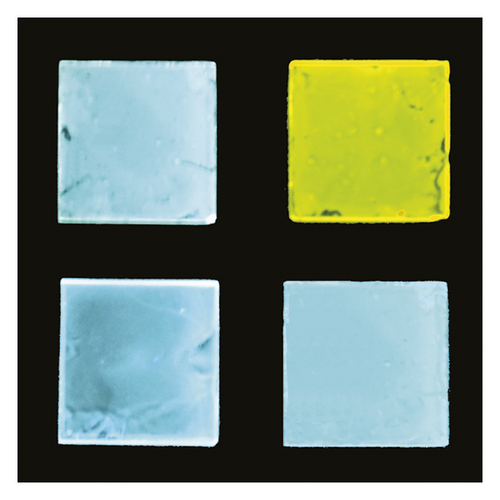
The photophysical characterization of the neat solid oligomers was performed on spin-coated thin films (the form typically employed as emissive layers in light-emitting devices). To our surprise, the different oligomers exhibit very different colours in natural light and moreover under a UV lamp. NPh2 and NPh5 neat samples, respectively, whitish and very pale-yellow in natural light, produce blue (maximum at 488 nm) and lime-yellow (the peak centred at 535 nm with a red-shifted shoulder) emission when excited at 340 nm (see Figure 2). Large (148 nm and 195 nm, respectively) Stoke’s shifts were measured, as a desirable hallmark able to improve emission colour purity and intensity in the light-emitting devices.

Photoluminescence tuning in dependence on MW has been investigated by scholars [51–53]. It was found that the length of the polymer chain plays an important role in the solid-state emission. The molecular architecture of the polymers facilitates the formation of more conformations with potentially strong intra- and/or interchain interactions leading to red-shifted and even decreased emissions [54, 55] to increasing MW parameters. Not unexpectedly, PLQY measured on the thin film of neat NPh5 (18%; see Table 1) is lower than the one of neat NPh2 thin films (45%) confirming structural factors influencing the aggregation structures.
In order to test PL performance in doped systems, the organic oligomers were added at 10% by weight in a nonconductive polymer, polystyrene (PS), and in a hole-transporting conductive polymer, poly-N-vinylcarbazole (PVK) host matrixes. Both the employed matrixes are flexible host polymers largely used in light-emitting devices [56–58] and in colour panels [59, 60]. All the doped blends obtained by spin-coating are colourless transparent glasses with blue emission under a UV lamp at 365 nm (lamp power 4 W/nm) or using a simple, cheap, and safe Wood’s lamp irradiating from 320 to 450 nm (lamp power 0.15 W/nm). Due to the different interactions with the host matrix, the samples in PS show lower PLQYs with respect to the neat solids. On the contrary, relevant values (over 50%; see Table 1) were recorded in PVK and again large Stoke’s shifts (about 150 nm). In Figure 3, the neat solid NPh2 and NPh5 samples (first line) are compared with NPh2 and NPh5 PVK blends (second line). This doping percent was optimal in previous work on organic dopants [41, 44, 61] taking into account the PL performance, the processability, and also the costs of the system.
Among the most fashionable and impressive applications, colourless/coloured layers have a potential as smart functional panels, optical fibres, and filter glasses [62]. In particular, a number of scientific articles and patents concern the use of blue light illumination to improve photosynthesis, promote cell growth, and achieve rapid cultivation growth [63].
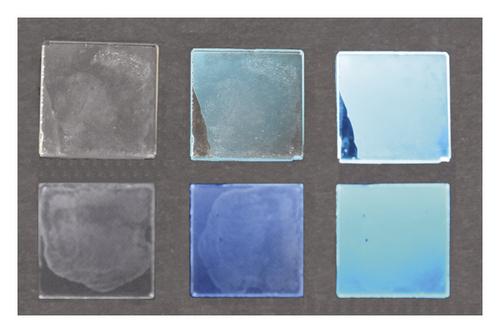
In Figure 4, it is shown the naked-eye effect of a common laboratory flask painted with NPh2-PS 10% doped blend, photographed in natural light (colourless) and under a commercial Wood’s lamp (blue). This simple model just shows the possible applications of the blends as filter glasses, through which the natural light is perceived unaltered. By lighting with a Wood’s lamp, the glass is still transparent, but the light is perceived blue. Even though they are more expensive, analogous more intense naked eye response has been detected for PVK blends. Interestingly, a cheap and low power Wood’s lamp is enough to produce the desired blue emission.


To date, the increasing interest for soft matter-based device technologies leads to new, stable, and highly efficient dopant dyes soluble in a specific liquid host known as luminescent liquid crystals (LLCs), highly desirable for light-emitting devices [63, 64]. NPh2 and NPh5 are luminescent nematic compounds with supramolecular organization and intrinsic light emission, employable also in LC polymeric blends. In our experiments, the two fluorophores have been dissolved at 1% by weight in a room temperature nematic polymer (QYPDLC-102, Ti = 113°C). The homogeneous doped LC materials were deposed between quartz slides (Figure 5). Optical observation confirms the complete dissolution of the dye in the LC matrix. The isotropization temperature of the matrix is not much altered due to the dopants. In the DSC curve, no other signal but the peak related to the isotropization can be detected, respectively, at 112°C and 111°C. The doped LC layers are quite colourless in natural light and blue emissive under a UV lamp at 365 nm or a Wood’s lamp. Although the solubility of NPh2 enables to increase concentration up to 10 times (5 times for NPh5), noteworthy PL performances were already provided in such diluted mixtures. PLQYs recorded on the nematic NPh2 and NPh5 doped layers reached the excellent value of 70% and 66%, respectively (see Table 1), with about 100 nm Stoke’s shifted emission in both cases. In Figure 5, the LC blends are photographed in natural light (colourless) and under a Wood’s lamp. Both oligomers produce a blue emission, that is very intense lights off and still perceivable natural light on. This feature makes the LC emissive layers employable in different lightning conditions and again using a low-power exciting light.
4. Conclusions
We obtained two oligomers containing a Schiff base N,N-bis (salicylaldehyde)-2,3 diaminonaphthalene emitting core with bulky terminal substituents and flexible chains. PL performances in the solid state resulted from medium to good. In dependence from MW, a tuning from blue to yellow in the fluorescence colour emission was observed in the neat solids. PLQY measurements for the lower MW compound provided the good 45% value. PLQYs for 10% PVK blends ranged over 50%. As diluted dopants of a nematic host polymer, PLQYs reached excellent values (from 65 to 70%). Thanks to the ability to change from a colourless to a blue emissive layer even by a low power excitation source, the doped blends revealed a potential as blue filter glass.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
This work was supported by the Italian Ministry of Education, University and Research (MIUR) (Piano Lauree Scientifiche “Scienza dei Materiali” 2016–2018).
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




