Corrigendum to “Evaluation of Complication Rates after Breast Surgery Using Acellular Dermal Matrix: Median Follow-Up of Three Years”
In the article titled “Evaluation of Complication Rates after Breast Surgery Using Acellular Dermal Matrix: Median Follow-Up of Three Years” [1], there were errors, which should be corrected as follows.
(1) The title should be changed to “Evaluation of complication rates after breast surgery using two different kinds of Acellular Dermal Matrix and one Bovine Pericardium Collagen Membrane: median follow-up of three years.”
(2) Tables 1, 2, and 4 along with their legends should be corrected as below.
(3) In the “Material and Methods,” the sentence reading “Sixteen patients had a history of radiotherapy prior to ADM implementation; see Table 3” should be changed to “Sixteen patients had a history of radiotherapy prior to ADM implementation; see Table 2.”
(4) In the “Discussion,” the sentence reading “Other limitations of our study include the relative brevity of follow-up with a mean time period of 36 months” should be changed to “Other limitations of our study include the relative brevity of follow-up with a median time period of 36 months.”
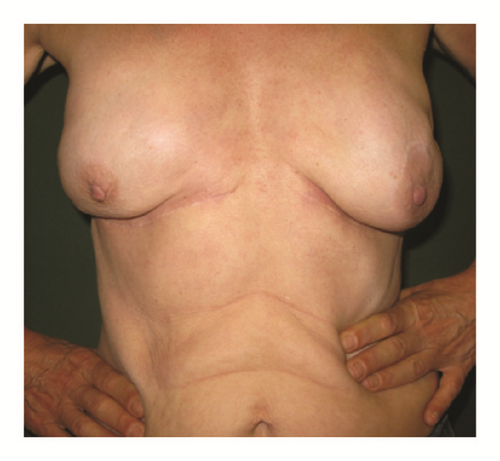
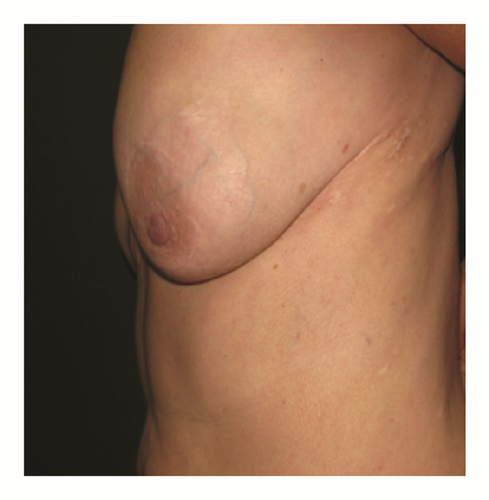
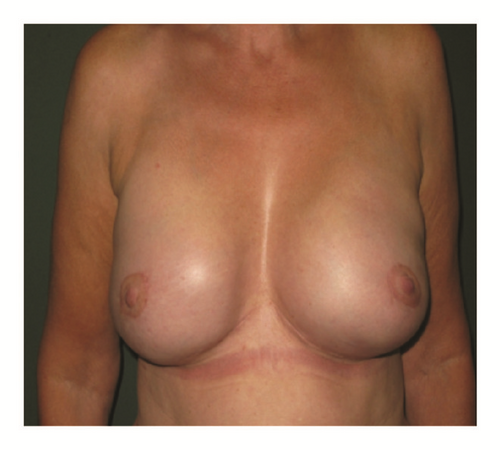
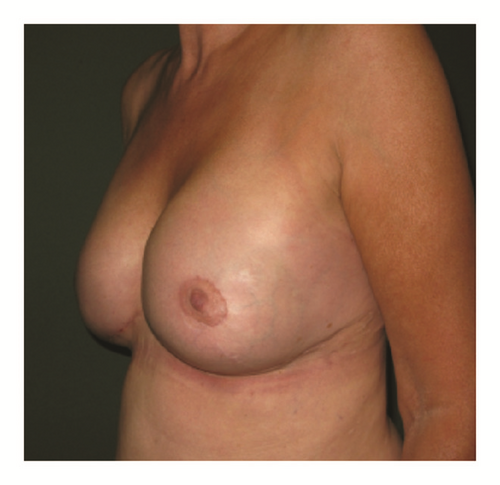
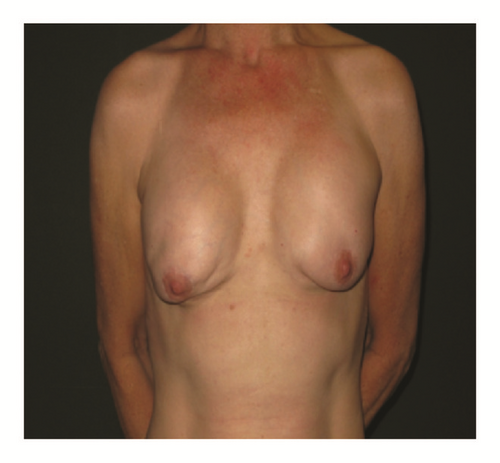
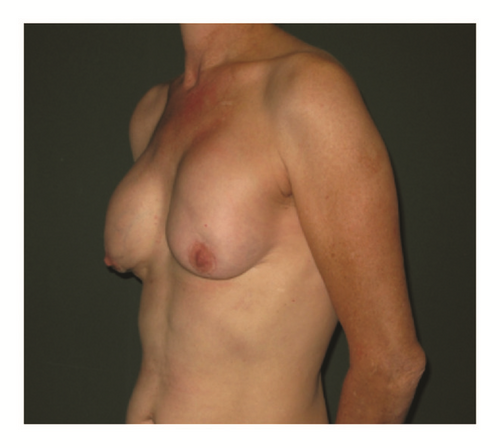
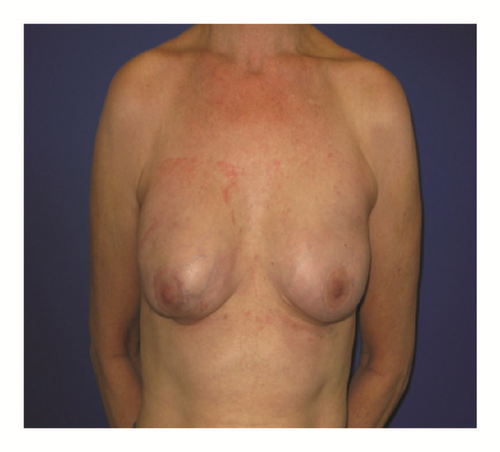
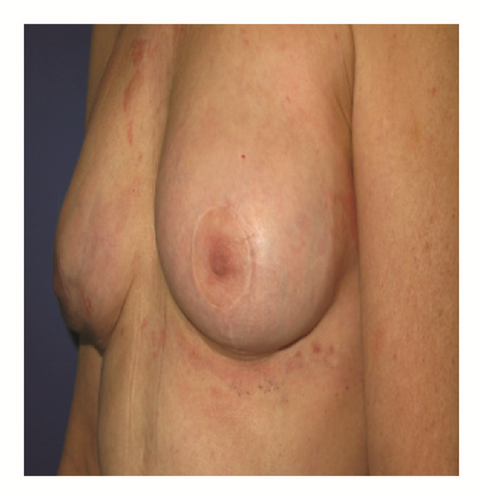
(5) The legends of Figures 3 and 4 should be corrected where “capsular contracture and” should be added to the legend of Figure 3 and “and capsular contracture, followed by” should be added to the legend of Figure 4. The corrected figures are as below.
| ADM | Product | Number of ADM implants used | Number of treated patients | Median patient age (years) | Median follow-up time (months) |
|---|---|---|---|---|---|
| HADM | Epiflex® / DIZG | 15 | 12 | 46 (36-76) | 40 (20-50) |
| PADM | Strattice® / LifeCell | 21 | 16 | 56 (44-66) | 43 (30-54) |
| BADM | Tutomesh® / rti surgical | 16 | 13 | 53 (33-74) | 20 (12-31) |
| total | all ADMs | 52 | 41 | 52 (33-76) | 36 (12-54) |
- Listing of matrix, name of ADM-product and its fabricant, amount of breast reconstructions (BR)/augmentations with usage of ADMs, number of patients treated with ADMs, median patient age and range in years, and median follow-up time and range in months of the given patient collective, which received a breast reconstruction/augmentation with ADM; HADM: human ADM; PADM: porcine ADM; and BADM: bovine ADM.
| Oncologic indication | Aesthetic indication | ||||||
|---|---|---|---|---|---|---|---|
| ADM | Product | BR with ADM (n) | BR with no capsular contracture ∗ | BR with capsular contracture ∗∗ | Primary augmentation ∗ ∗∗∗ | Secondary augmentation after capsular contracture ∗ | Secondary augmentation after aesthetic-related complications ∗ ∗∗∗∗ |
| HADM | Epiflex® / DIZG | 15 | 1 | 3 | 9 | 2 | 0 |
| PADM | Strattice® / LifeCell | 21 | 6 | 8 | 5 | 2 | 0 |
| BADM | Tutomesh® / rti surgical | 16 | 2 | 5 | 3 | 2 | 4 |
- Listing of kind of matrix used, name of product, number of breast reconstructions with certain ADMs, and indications for ADM usage (breast reconstruction (BR) with no capsular contracture, BR with capsular contracture, primary augmentation, secondary augmentation after capsular contracture, or secondary augmentation after aesthetic-related complications including IMF-loss and insufficient implant coverage with breast tissue). Oncologic patients made up 27% of the HADM group, 67% of the PADM group, and 44% of the BADM group; HADM: human ADM, PADM: porcine ADM, and BADM: bovine ADM; ∗ denotes no history of radiotherapy; ∗∗ denote history of radiotherapy; ∗∗∗ denote primary augmentation in cases with large breasts; ∗∗∗∗ denote loss of IMF.
| ADM | HADM (Epiflex® / DIZG), PADM (Strattice® / LifeCell), BADM (Tutomesh® / rti surgical) | |
|---|---|---|
| Breasts (total) | 52 | |
| Median follow-up time (in months) | 36 (12-54) | |
| Complications (total) | 9 (17%) | |
| Short-term complications | Skin necrosis | 2 (4%) |
| Seroma | 1 (2%) | |
| Haematoma | 0 (0%) | |
| Infection | 2 (4%) | |
| RBS ∗ | 3 (6%) | |
| Long-term complications | Capsular contracture ∗∗ | 3 (6%) |
| Implant malposition | 0 (0%) | |
| Implant loss | 1 (2%) |
- Short-term (skin necrosis, seroma, haematoma, infection, and Red Breast Syndrome (RBS)) and long-term complications (capsular contracture, implant malposition, and implant loss) for all breasts with usage of ADMs (human ADM (HADM), porcine ADM (PADM), and bovine ADM (BADM)), median follow-up time for all patients, and total complications of all breasts being reconstructed with ADMs; ∗ denotes being excluded from overall complications and requiring further medical treatment; ∗∗ denote >Baker-St. II.